Back to Journals » International Journal of General Medicine » Volume 17
Correlations of Aflatoxin Exposure from Cooking Oil and Dietary Foods During Pregnancy with Birth Weight and Gestational Age at Birth in Guangxi, China
Authors Zhong Y , Lu H, Lu X, He Z, Jiang Y, Chen J, Liabsuetrakul T
Received 7 December 2023
Accepted for publication 2 March 2024
Published 27 March 2024 Volume 2024:17 Pages 1171—1184
DOI https://doi.org/10.2147/IJGM.S453839
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Woon-Man Kung
Yanxu Zhong,1,2 Huan Lu,3 Xiaodan Lu,4 Zhini He,4 Yuyan Jiang,1 Jie Chen,1 Tippawan Liabsuetrakul2
1Food Safety Monitoring and Evaluation Department, Guangxi Zhuang Autonomous Region Centre for Disease Control and Prevention, Nanning, Guangxi Region, 530000, People’s Republic of China; 2Department of Epidemiology, Faculty of Medicine, Prince of Songkla University, Hat Yai, Songkhla, 90110, Thailand; 3Infectious Diseases Department, the Fourth People’s Hospital of Nanning, Nanning, Guangxi Region, 530000, People’s Republic of China; 4Food Safety and Health Research Center, School of Public Health, Southern Medical University, Guangzhou, Guangdong, 510515, People’s Republic of China
Correspondence: Tippawan Liabsuetrakul, Department of Epidemiology, Faculty of Medicine, Prince of Songkla University, Hat Yai, Songkhla, 90110, Thailand, Tel +66 74 451165, Fax +66 74 281166, Email [email protected]
Background: Cooking oil and dietary foods are easily contaminated by aflatoxins (AFs) in Guangxi, China where low birth weight and preterm birth were prevalent. However, there are no data on AF exposure in pregnant women or their impact on newborn birth outcomes. This study aims to measure the levels and correlations of AFs in cooking oil, estimated dietary intake (EDI) of AFs in dietary foods, and serum AFB1 albumin adducts (AFB1-alb) with newborn birthweight and gestational age at birth.
Methods: A prospective study was conducted among 126 pregnant women in Guangxi, China. All recruited women were interviewed for demographic data and behavior and obstetric information and then followed up until giving birth. AF measurements were obtained from cooking oil, dietary foods, maternal serum, and cord blood and the correlations of AF levels with newborn birthweight and gestational age at birth were tested using correlation analysis.
Results: The median EDI of AFs in cooking oil was 2.61 ng/kg.bw/day and in dietary foods 2.95 ng/kg.bw/day. High positive correlations among EDI of aflatoxin B1 (AFB1) from cooking oil and dietary foods were found (r > 0.7). Low positive correlations of AFB1-alb in maternal serum and cord blood and both EDI of AFB1 in both cooking oil and dietary foods were shown (r ≈0.3). Significant correlations between AF levels in both cooking oil and dietary foods with birth weight were found, but very low negative correlations (r = - 0.244 ~ − 0.285). AFB1 levels in foods, maternal serum and cord blood levels were high in pregnant women with newborn low birth weight and preterm birth.
Conclusion: The EDIs of AFB1 from both cooking oil and dietary foods were significantly correlated with AFB1-alb in maternal serum and cord blood. Negative correlations of AFs from cooking oils and foods with newborn birth weight should be paid more attention.
Keywords: aflatoxins, EDI, birthweight, gestational age at birth, AFB1 albumin adducts
Introduction
Aflatoxins (AFs) are poisonous carcinogens and mutagens which are well-known to be a leading cause of liver cancer1 and commonly contaminate staple foods such as maize, groundnuts, and peanut oil.2 AFs are most prevalent in areas where the climate is wet and hot, with the greatest health risk in developing countries located in tropical regions. Food insufficiency and lack of diversity substantially contribute to the susceptibility of individuals chronically exposed to AFs.3 Maize, rice, and peanuts are the main contaminated foods in Asia with incidences of contamination of AFs varying from 5.4% to 91%.4
China is one of the countries with the most serious AF contamination, where high contamination with AFs were found in corn,5 peanuts, and peanut products including peanut oil.6–9 Aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G1 (AFG1), and aflatoxin G2 (AFG2) were commonly detected in foods, including cooking oil.8,10 Guangxi, a province in the southwest of China, was found in a recent study to be the province most seriously contaminated by AFs in China,7 where the cooking oil is a common food contaminated by AFs, especially AFB1. Two earlier studies in China detected AFB1 in the cooking oil specimens of which the median value of AFB1 in homemade peanut oil was much higher than in commercial cooking oil.6,7 Apart from cooking oil, AF contamination has also been detected in other dietary foods of which the estimated dietary intake (EDI) is now acceptably used to estimate the potential AF exposure in the human body through dietary exposure.11
The EDI of AFs from dietary foods has been reported to be low in high-income countries such as 0.93 to 2.45 ng/kg.bw/day in the European Union,12 2.7 ng/kg.bw/day in the United States,13 and 0.004 ng/kg.bw/day in Japan,14 and high in lower middle-income countries such as 0.02 to 427.8 ng/kg.bw/day in Indonesia15 and 35.0 to 43.7 ng/kg.bw/day in Vietnam.16 Likewise, in China, the EDI of AFs from dietary foods is high, ranging from 15.0 to 27.2 ng/kg.bw/day, of which the highest EDI was found in children aged 2–6 years.8 A literature review, searched from 1970 to 2015, published in 201717 found only one cross-sectional human study in Kenya reporting significantly lower newborn birth in AF positive mothers. The levels of AFs in serum are measured using the aflatoxins albumin adduct such as AFB1 albumin adduct (AFB1-alb), which can remain in the blood for more than 2 months, reflecting the past 2–3 months of dietary aflatoxin intake.18 A study found higher levels AFB1-alb in pregnant women who had chronic exposure of AFs19 which was significantly associated with newborn low birth weight (LBW), small head circumference, and preterm birth (PB).20–22 A previous study in a county of Guangxi, in the Southwest of China, found higher prevalences of LBW (10.4%) and PB (11.1%)23 compared to the average rate of LBW (6.1%) and PB (7.0%) in China.24 Furthermore, most of individuals living in that county use peanut oil for cooking, which is easily contaminated by AFB1in previous study.8
To date, the relationship of AFs from different exposures and serum levels has not been clearly identified and there have been no studies on the correlation of AF EDI in dietary foods and serum AF levels in pregnant women with their newborn’s birth weight and gestational age at birth. Therefore, the aim of this study was to measure the levels and correlations of AFs in cooking oil, estimated dietary intake (EDI) of AFs in dietary foods, and serum AFB1 albumin adducts (AFB1-alb) with newborn birthweight and gestational age at birth in a county of Guangxi, Southwest China.
Materials and Methods
Study Design and Study Setting
A prospective cohort study was conducted from December 2021 to May 2022 in a county in Eastern Guangxi province with a humid subtropical climate and the largest population in Guangxi at over 2 million. In this county, high rates of low birth weight (LBW) in 10.4% and preterm birth (PB) in 11.1% were reported between 2016 and 2020,23 and most of the citizens consume homemade-peanut oil of which 50% of tested samples had AFB1 exceeding the allowable limit (20 μg/kg).6 The study county hospital was chosen to be the study site as it is the biggest county hospital in Guangxi province with more than 5000 newborns delivered each year. Both rural and urban pregnant women go there for antenatal care and delivery.
Study Participants and Sample Size
Singleton pregnant women at 28 weeks or more of gestation living in study county who come for antenatal care and planned to give birth at the study hospital were included. Those having one or more known medical diseases before their pregnancy such as hypertension, diabetes, autoimmune diseases, neurological disorders or verbal communication disorders such as dumbness, deafness, or mental retardation were excluded. To determine the sample size for the main objective for a correlation study, we used the correlation of AFs between serum and breastmilk levels from a previous study (r = 0.42).25 Based on α significance level of 0.05, β Type II error of 0.1, and estimated 10% of missing values, at least 61 women were needed of which we additionally considered enough women for classification of LBW based on birth weight, or PB based on gestational age. To reduce the confounding effect of gestational age on BW and PB, we selected gestational ages matched within 3 weeks to achieve a sufficient sample size for each group.
Variables and Their Measurements
According to the objective of this study, AF measurements were obtained from four sources, namely cooking oil, dietary foods, maternal serum, and cord blood. AFs in cooking oil were measured from actual testing of aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G1 (AFG1), aflatoxin G2 (AFG2), and aflatoxin total (AFT), and EDI calculations. For dietary foods, only the EDI calculation was used. Actual testing for AFs was done for maternal serum and cord blood samples. Only AFB1 and AFT were estimated for EDI from cooking oil and dietary foods. Only AFB1-alb was measured from maternal serum and cord blood samples. Apart from the AFs, newborn birthweight (g) was measured using Electronic Counting Scale EBSA-20 (Zhongshan jinli electronic weighing equipment CO., Ltd) and gestational age (weeks) was defined based on the date from last menstrual period (LMP) recorded in medical card. For pregnant women who had uncertain LMP, the ultrasound was used for estimation.
The demographic data and behavior and obstetric information of all participants were collected. The demographic factors collected were age group, ethnicity, residency, education, occupation, and family income. The behavioral factors recorded were physical exercise during pregnancy and passive smoking (exposure to workplace or home, workplace and home or no exposure during pregnancy) and the obstetric details were history of hepatitis B virus (HBV) defined as diagnosis of HBV infection before pregnancy, pregnancy complications, and medication intake (mainly due to pregnancy complications) and supplementations (folic acid, calcium, iron, and vitamin D) during pregnancy.
Data Collection
All eligible participants were approached during routine antenatal care visit and informed of the goals of the study and signed an informed consent form if they agreed to participate. After that face-to-face interviews were obtained from the participants using a constructed questionnaire on demographic characteristics and behavior and obstetric information as well as a food frequency questionnaire26 for AFs EDI exposure calculations. Then 5 mL of maternal serum during pregnancy was collected from each participant. All participants were requested to bring a 250-mL sample of the cooking oil they normally used at home to give to the researchers. When the participants came to give birth at the hospital, a cord blood sample of 3mL was collected after baby born and before delivery of placenta.
Maternal venous blood and cord blood samples collected were centrifuged at the hospital facility. The resulting upper serum portion was carefully separated and were transported daily to the setting county Centre for Disease Control and Prevention (CDC) using a transferal box at −20 degrees Celsius (°C) and were stored in a −80 degrees Celsius (°C) freezer for AFB1-alb testing. Cooking oil samples were stored in a shaded area at 23°C for testing the levels of AFB1, AFB2, AFG1 and AFG2.
Data Management and Statistical Analysis
The data management for the levels of AFs in cooking oil and dietary foods by actual testing and EDI calculation as well as other participants’ information was described as the following.
AF Testing
AF testing from the cooking oil samples was conducted in the Guangxi CDC laboratory and analyzed using high-performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS) following the China National Food Safety Standard GB 5009.22–2016.27 The limits of detection (LOD) of the 4 aflatoxins (AFB1, AFB2, AFG1, AFG2) tested were all 0.030 μg/kg while the limits of quantification (LOQ) were all 0.1 μg/kg. According to the method recommended by the Global Environment Monitoring System-Food Contamination Monitoring and Assessment Program (GEMS/Food), all non-detected results were replaced with zero and LOD to produce the lower bound estimate (LB) and the upper bound estimate (UB), respectively.28 Cooking oil with 5 g were placed into a 50 mL centrifuge tube, 15 mL of 84% acetonitrile, mixed and centrifuge for 30 min. The resulting supernatant was combined with a solution containing polysorbate-20 and phosphate buffer saline. The mixture underwent purification by passing through immunoaffinity columns. Subsequently, the eluate obtained from the columns was evaporated to dryness using a stream of nitrogen gas. The purified extract was dissolved in methanol and then subjected to filtration. Quantification of the filtrate was performed using HPLC-MS/MS with an electrospray ion source in multi-reaction monitoring (MRM) mode. Chromatographic separation was achieved using a C18 column with a mobile phase consisting of a mixture of acetonitrile, methanol, and ammonium acetate.
AF EDI Calculation
The Estimated Dietary Intakes (EDIs) of Aflatoxins (AFs) from both cooking oil and dietary foods were determined using the following formula: 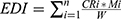
Here, the EDI represents the estimated daily dietary AF intake (ng/kg.bw/day), where CRi is the daily consumption rate of the i-th food item (g/person/day), Mi is the value level of AFs in each food category (ng/kg), and W is the body weight (kg) calculated based on the mean weight of participants before pregnancy, routinely self-reported and recorded at the first antenatal care visit and before delivery.
The dietary EDI is the cumulative sum of the EDI from cooking oil and the EDI from other dietary foods. The consumption rates (CRi) or dietary foods were obtained through a questionnaire. Notably, for the cooking oil, the values for Mi were derived from the test results from our study. Conversely, the values for Mi of dietary foods were based on 5-year monitoring data from a previous report.7
AFB1-Alb Testing
An AFB1-alb ELISA kit (Shanghai Xinyu Biotechnology Co., Ltd.) was used to detect the AFB1-alb content in the serum samples, which testing method was used in a previous study.29 The absorbance (OD) of the samples was measured at a wavelength of 450 nm using an enzyme-plate analyzer. The percentage absorbance value was determined by dividing the OD value of the standard solution by the OD value of the 0 ng/mL standard solution, and then multiplying the result by 100%. The curve was constructed by plotting the logarithm (log) of the standard concentrations of Aflatoxin B1 albumin adduct on the X-axis and the corresponding percentage absorbance values on the Y-axis. Based on the constructed standard curve, the reciprocal values of the aflatoxin B1 albumin adduct concentrations were obtained. By taking the reciprocal, the concentration of AFB1-alb in the sample could be determined in ng/mL. The detection limit of this kit was specified as 0.05 ng/mL. All non-detected results (less than 0.05ng/mL) were replaced with 1/2 detection limitation.
Participant Information
The age was collected in years and then categorized into age groups, which were divided into <20, 20–34, and ≥35 years. Ethnicity was categorized into Han and other, while residency was categorized into urban and rural. Education was divided into three levels, middle school and above, high school, and college and above, while occupation was categorized into four types, government staff, farmer or worker, self-employed, and other. Family incomes were converted from Chinese Yuan (CNY) into United States Dollars (USD), and divided into four levels ≤450, 450–750, 750 −1500 USD/month, and >1500 USD/month.
All data were analyzed using R software version 4.1.2 (The R Foundation for Statistical Computing 2020, Vienna, Austria). Mean ± standard deviation or median with interquartile range for continuous data or percentage for the categorical data were used for the characteristics of the participants. Correlations of exposures and outcomes were analyzed using the corrplot and ggcorrplot packages. Correlations are presented using correlation coefficients (r) and interpreted as very high positive (negative) correlations with r 0.90 to 1.00 (−0.90 to −1.00); high positive (negative) correlations with r 0.70 to 00.90 (−0.70 to −0.90); moderate positive (negative) correlations with r 0.50 to 0.70 (−0.50 to −0.70); low positive (negative) correlations with r 0.30 to 0.50 (−0.30 to −0.50); or very low positive (negative) correlations with r 0.00 to 0.30 (0.00 to −0.30).30 The levels of AFB1-alb in the maternal serum and cord blood were analyzed and represented by the ggplot2 and ggpubr packages. A p-value less than 0.05 was considered statistically significant.
Results
The socio-demographic characteristics and behavioral and obstetric factors of the 126 study participants are shown in Table 1. The majority of participants lived in a rural area, were aged between 20–30 years, worked as a farmer or worker, and had a family income between 450 and 1500 USD/month. Most of them had regular physical exercise, and more than 65.0% were exposed to passive smoking in at least one location. One-third of them reported having previous pregnancy complications and 22.2% were taking medications during their pregnancy. Folic acid and calcium supplementation were prescribed in 91.3% and 73.0% of the participants, respectively.
 |
Table 1 Characteristics of the Study Participants |
The estimated dietary intakes (EDIs) of aflatoxin B1 (AFB1) and aflatoxin total (AFT) from the cooking oil and total EDI are presented in Table 2. The levels of AFB1 and AFT were not normally distributed. AFB1 was the main EDI exposure of AFs in cooking oil and total diet with median values of 2.61 ng/kg.bw/day and 2.95 ng/kg.bw/day, respectively. The lower and upper medians of AFT levels in cooking oil were 2.74 and 2.80 and in the total diet were 3.03 and 3.11 ng/kg.bw/day, repetitively. Only 44 samples (37.0%) had aflatoxin B2 (AFB2) with a mean value of 1.48 ug/kg and a zero median value. AFG1 and AFG2 were non-detectable in all cooking oil samples.
 |
Table 2 Estimated Dietary Intake to AFs from Dietary Foods Categorized by Cooking Oil and Other Foods |
The correlations among the measured AFB1 levels and measured AFT and EDI of AFB1 and AFT in cooking oil and dietary foods, and maternal serum and cord blood AFB1 albumin adducts (AFB1-alb), newborn birthweight and gestation age at birth are shown in Figure 1. Very high positive correlations were found between measured AFB1 and AFT in only cooking oil and among AFB1and AFT measured by EDI regardless of cooking oil and dietary foods, with correlation coefficients (r) of more than 0.900. High positive correlations were found for AFB1-alb measured from serum and cord blood (r = 0.822). Both measured AFB1 and AFT in cooking oil had high correlations with the EDI of both AFB1 and AFT in cooking oil and dietary foods, with correlation coefficients ranging from 0.744 to 0.755. Moderate positive correlations were found between birth weight and gestational age (r = 0.649). Very low negative correlations were found between birth weight and any AF measurements in both cooking oil and dietary foods (r = - 0.244~ −0.285).
The levels of AFB1 in cooking oil, AFB1-alb in maternal serum, and cord serum AFB1-alb, categorized by low birth weight (LBW), normal birth weight (NBW), preterm birth (PB), and non-preterm birth (NPB), are shown in Table 3. Three samples of maternal serum (2.38%) were non-detectable of AFB1-alb while all cord blood sample were detectable Their mean and median values in both cooking oil AFB1and maternal serum AFB1-alb were higher in both the LBW (mean and median of cooking oil: 14.33 ug/kg and 5.70 ug/kg; mean and median of maternal serum: 4.55 ng/mL and 4.96 ng/mL) and PB (mean and median of cooking oil: 14.11 ug/kg and 6.18 ug/kg; mean and median of maternal serum: 4.51 ng/mL and 4.92 ng/mL) groups compared to those in the NBW (mean and median of cooking oil: 9.88 ug/kg and 4.78 ug/kg; mean and median of maternal serum: 4.14 ng/mL and 3.85 ng/mL) and NPB (mean and median of cooking oil: 10.03 ug/kg and 4.00 ug/kg; mean and median of maternal serum: 4.19 ng/mL and 3.73 ng/mL) groups. The mean and median values for cord blood serum AFB1-alb in LBW (mean: 3.46 ng/mL and median 2.48 ng/mL) similar to the NBW (mean: 3.69 ng/mL and median 2.13 ng/mL) group. Figure 2 presents the changes of AFB1-alb measured in maternal serum before delivery and cord blood serum after delivery followed up from the same women in the NBW and LBW groups. Lower cord blood levels compared to maternal serum were observed in both groups but more in the LBW group (Figure 2A) than the NBW (Figure 2B) group. Figure 3 presents the changes of AFB1-alb measured in maternal serum before delivery and cord blood serum after delivery followed up from the same women in the NPB and PB groups. Lower cord blood levels compared to maternal serum were observed in both groups but more in the NPB group (Figure 3B) than the PB (Figure 3A) group.
 |
Table 3 AFB1 Level from Foods, AFB1 Albumin Adducts from Maternal Serum and Cord Blood Between LBW and NBW, PB and NPB |
Discussion
The pregnant women in Guangxi, China study cohort were exposed to aflatoxins, particularly aflatoxin B1 (AFB1) and aflatoxin total (AFT) which were consistently detected from different sources including the estimated dietary intake (EDI) of dietary foods, mainly from cooking oil, and maternal serum and cord blood. High significantly positive correlations between AFB1 and AFT levels from cooking oil and the EDI of cooking oil and dietary foods were observed. Although low positive correlations of EDI of foods with AFB1-alb levels in both maternal serum and cord blood or very low correlations with birth weight and gestational age at birth were found, the levels of AFB1 in maternal serum and cord blood were not negligible. The AFB1 albumin adducts (AFB1-alb) in maternal serum and cord blood of the low birth weight (LBW) group were higher than those in the normal birth weight (NBW) group as well as those in the preterm birth (PB) group was higher than in the non-preterm birth (NPB) group. The changes of AFB1-alb measured in maternal serum before delivery and cord blood serum after delivery followed up from the same women were more frequently detected in the LBW and NPB groups.
Most related studies reported the EDI of AFs mostly from corn, rice, peanuts, and other foods;31–33 however, there have been few studies on the EDI of AFs from cooking oil or peanut oil.34 A study conducted in Egypt showed that the EDIs of AFB1 in vegetable oils ranged from 0.00047 to 0.30 ng/kg.bw/day,35 which was much lower than our study. Another study in Pakistan on the EDI of AFT in sunflower oil found that its values among females aged 16–32 years were lower than our findings.36 From the findings of a systematic review on the AFB1 and AFT in common dietary foods, the EDIs of AFB1 or AFT in our study were lower than in studies conducted in low- and middle-income countries, namely India, Vietnam, Ghana, Malawi, Nigeria, Tanzania, Togo, Iran, and Qatar, but higher than in studies conducted in high-income countries.37 This implies that the AFs were detected from foods mostly from low- and middle-income rather than high-income countries, and the sources of food contaminations depended on the types of foods commonly consumed in tropical areas of the country.15,37
In Guangxi province, China, our study setting, we found the food most affected by AFB1-contamination was homemade peanut oil.38 The mean EDI values of AFB1 from cooking oil in our study in 2021 were consistent with a previous study in Guangxi among the general population in 2020,7 but lower than a study conducted in 2017.6 This may be due to the implementation of government policies in Guangxi province to control the homemade peanut oil manufacture procedure included strengthened the supervision, evaluation, and monitoring of AFB1 when the media reported higher AFB1 detected in homemade peanut oil.39 The EDI value in our setting was also 50% lower than the findings of another study conducted in the same year in Shandong province, China which is a well-known area for high peanut oil consumption.40 However, the EDI of AFT from cooking oil among the pregnant women in our study was four times higher than the national level in China but two times lower than that of Guangdong province.8
Very high positive correlations were shown between measured AFB1 and AFT in only cooking oil and among AFB1and AFT measured by EDI regardless of cooking oil and dietary foods. It may be because the cooking oil was the main source of AFB1 and AFT contamination in dietary exposure, which is consistent with a previous study that found cooking oil contamination was more serious than contamination in other foods.8 Another study found that serious AFB1 contamination was usually from bulk cooking oil, known as homemade peanut oil, especially in the South of China.41 However, to date, there have been no studies examining the correlations between the EDI values of AFs in dietary sources and AFB1-alb levels in the serum in pregnant women, including their newborns’ cord blood. There has been only one study which reported a significant association between the daily consumption of local milk and AF levels in breast milk.25
A high positive correlation between AFB1-alb levels in maternal serum and cord blood was found in our study participants, which was consistent with the findings from a previous study conducted in the United Arab Emirates,42 even though this study measured aflatoxin M1 (AFM1) levels, not AFB1-alb levels as in our study. AFM1 is a common biological marker but likely to reflect exposure only for the last 24–48 hours, while the albumin adduct of AFs such as AFB1-alb reflect 2–3 months of chronic exposure and are more reliable for aflatoxin measurements.43 Another study conducted in Gambia reported moderate correlations, but the AF-alb levels in maternal and cord blood were measured using different units and method of measurement,2 not AFB1-alb thus a comparison could not be directly made. However, different methods of AF measurements reflect maternal AF exposure and its transportation across the placenta to the fetus. Our study showed low positive correlations between the EDIs of AFB1 and AFT from both cooking oil and dietary foods with AFB1-alb blood levels, and these correlations between AFs in foods and maternal serum level were consistent with the findings from a study conducted in Timor in 2019.44 Such lower maternal serum levels compared to EDI levels could be explained by the influence of rumen microbiota in the human body on the ability of mycotoxin degradation and individual susceptibility reflecting AF metabolism.1
There were few studies on the correlations of AF levels in cooking oil and dietary foods and serum or cord blood with newborn birth weight and gestational age at birth which showed inconsistent findings. Our study found significant but very low negative correlations between newborn birth weight and the EDI of AF values in cooking oil and dietary foods were found in our study women, which were similar to the findings of a study which found that AF exposure through food affected children’s growth.19 No significant correlations between newborn weight or gestational age and AFB1-alb levels in either maternal or cord blood may be explained through the levels of AFs,45 since previous studies having AF levels in maternal serum less than 5 ng/mL showed no significant associations in Tanzania,45 Nepal,46 or a report from China.47 In contrast, levels of AFs in maternal serum greater than 5ng/mL were negatively associated with birth weight in studies conducted in the United Arab Emirates20 and Ghana.22 However, although the maternal serum AFB1-alb level of our study was marginal at around 5ng/mL, the effects of AF exposure should not be overlooked.
AF detection in maternal serum and cord blood confirmed the biological mechanism from mother to fetus. Lower levels in cord blood than maternal serum was similar to a finding among Gambian pregnant women,2 which may be because of the interval between maternal serum and cord blood testing. We could not find an explanation for higher levels of AFB1 in cooking oil, AFB1-alb in maternal serum, and cord blood seen in both the LBW and PB groups compared to the NBW and NPB groups. However, we hypothesized that the mediation of plasma insulin-like growth factor-1 (IGF-1) for LBW could be due to the relationship between plasma insulin-like growth factor-1 (IGF-1) and LBW48,49 and aflatoxin and plasma insulin-like growth factor 1 levels,50,51 not PB.52
Our study highlights the comprehensive correlations between of AFs from cooking oil and dietary foods with maternal serum, cord blood and newborn birth weight and gestational age at birth which have not been previously published. The levels of AFs tested from cooking oil that the study women consumed were used to calculate the EDIs of AFs for dietary foods, which are more accurate than mean or median from previous estimated charts. A prospective follow-up study design including stratification of LBW and NBW groups could make us to explore the potential relationships of AF levels in maternal serum and cord blood and adverse birth outcomes.
There were some limitations to the study. First, the consumption of dietary foods was assessed using questionnaires, which may have introduced recall bias. However, this bias was likely minimal as the data collection was collected concerning the previous 7 to 10 months diet, and the participants’ lifestyles regarding the use of cooking oils tended to be consistent. Second, we used median values from published monitoring data in Guangxi for EDI calculations of other foods because the Guangxi Centre for Disease Control and Prevention has tested dietary foods for over 5 years. Third, we included pregnant women with gestational age at least 28 weeks but did not record the actual gestational age when the maternal serum was collected which may have affected the relationship between maternal AF levels and cord blood due to the time interval from AF testing from collecting the maternal serum and cord blood at delivery. However, this bias was expected to be minimal as the maximum period until delivery was less than 3 months. Lastly, in this paper, we presented only the correlations of AF levels in different sources, not the factors associated with the levels since these levels would be analyzed and published elsewhere.
Conclusion
The concentrations of EDI of AFB1 and AFT from both cooking oil and dietary foods from pregnant women in a province in Guangxi, China showed significant correlations with AFB1-alb in maternal serum and cord blood as well as birth weight. The higher the EDI of AF values in cooking oil and dietary foods, the lower the newborn birth weight was found. Pregnant women with LBW newborns had higher levels of AFB1-alb in the maternal serum and cord blood than those with NBW. Future research on the effects of AFs on adverse pregnancy outcomes and interventions for reducing AF exposure in pregnant women should be done.
Ethics Statement
The study was approved by the institutional ethics committee of the Faculty of Medicine, Prince of Songkla University, Songkhla (REC.64-404-18-1), adhering to the principles of the Declaration of Helsinki.
Acknowledgments
We acknowledge the great work of all the staff from the study hospital and study county Centre for Disease Control and Prevention of Guangxi Zhuang Autonomous Region, China who facilitated the study and supported the data collection in the study hospital. We also thank Mr. Dave Patterson of the International Affairs Office, Faculty of Medicine, Prince of Songkla University for his English editing.
Author Contributions
YZ, HL, XL, ZH, YJ, JC, and TL made a significant contribution to the work reported in conceptualizing study, study design, execution, and planning the study data collection. YZ, HL, XL, ZH, YJ, and JC were responsible for data collection and quality of data collection and specimens tested. YZ and TL conducted data management and analysis and substantially wrote and revised manuscript. All authors substantially critically reviewed, agreed on the journal to which the article submitted, and collectively reviewed and approved all versions of the manuscript before initial submission, during revisions, and upon acceptance of the final version for publication. Any noteworthy modifications introduced during the proofing stage were also collectively acknowledged and agreed upon. All authors agree to take responsibility and be accountable for the contents of the article.
Funding
This study was supported by the Guangxi Medical and Health Appropriate Technology Development and Promotion Application Project (S2022047), and through a scholarship from the TUYF Charitable Trust: Research Capacity through Education and Networking on Epidemiology in Asia, the Department of Epidemiology, Faculty of Medicine, Prince of Songkla University, Songkhla, Thailand (Grant number 5/2022).
Disclosure
The authors declare that they have no competing financial interests or personal relationships that could have influenced this paper, and all of them have agreed to submit the manuscript to this journal.
References
1. Hussein HS, Brasel JM. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology. 2001;167(2):101–134. doi:10.1016/s0300-483x(01)00471-1
2. Turner PC, Collinson AC, Cheung YB, et al. Aflatoxin exposure in utero causes growth faltering in Gambian infants. Int J Epidemiol. 2007;36(5):1119–1125. doi:10.1093/ije/dym122
3. Wu F, Guclu H. Aflatoxin regulations in a network of global maize trade. PLoS One. 2012;7(9):e45151. doi:10.1371/journal.pone.0045151
4. Benkerroum N. Aflatoxins: producing-Molds, Structure, Health Issues and Incidence in Southeast Asian and Sub-Saharan African Countries. Int J Environ Res Public Health. 2020;17(4). doi:10.3390/ijerph17041215
5. Li FQ, Yoshizawa T, Kawamura O, Luo XY, Li YW. Aflatoxins and fumonisins in corn from the high-incidence area for human hepatocellular carcinoma in Guangxi, China. J Agric Food Chem. 2001;49(8):4122–4126. doi:10.1021/jf010143k
6. Cheng HY, Zhong YX, Chen J, et al. Dietary exposure assessment of aflatoxin B1 in edible vegetable oil consumed by Guangxi residents. Appl Preventive Med. 2017;23(6):451–454.
7. Shi MM, Liang J, Zhao P, Zhong YX, Jiang YY. Dietary exposure assessment of aflatoxins of residents in Guangxi. Chin J Food Hyg. 2020;32(4):432–436. doi:10.13590/j.cjfh.2020.04.016
8. Qin M, Liang J, Yang D, et al. Spatial analysis of dietary exposure of aflatoxins in peanuts and peanut oil in different areas of China. Food Res Int. 2021;140:109899. doi:10.1016/j.foodres.2020.109899
9. Lien KW, Wang X, Pan MH, Ling MP. Assessing aflatoxin exposure risk from peanuts and peanut products imported to Taiwan. Toxins. 2019;11(2):E80. doi:10.3390/toxins11020080
10. Ding X, Wu L, Li P, et al. Risk assessment on dietary exposure to Aflatoxin B₁ in post-harvest peanuts in the Yangtze river ecological region. Toxins. 2015;7(10):4157–4174. doi:10.3390/toxins7104157
11. Joint FAO/WHO Expert Committee on Food Additives. Meeting (71st: 2009: Geneva S, Joint FAO/WHO Expert Committee on Food Additives. Meeting (71st: 2009: Geneva S, Safety IP on C. Evaluation of Certain Food Additives: seventy-First Meeting of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organization; 2010. Available from: https://apps.who.int/iris/handle/10665/44266.
12. Opinion of the Scientific Panel on contaminants in the food chain [CONTAM] related heptachlor as an undesirable substance in animal feed | European Food Safety Authority. Available from: https://www.efsa.europa.eu/en/efsajournal/pub/478.
13. Humphrey JH. Summary of Evaluations Performed by the Joint FAO who Expert Committee on Food Additives (Jecfa). Nutr Rev. 2000;58(3):90. doi:10.1111/j.1753-4887.2000.tb01846.x
14. Sugita-Konishi Y, Sato T, Saito S, et al. Exposure to aflatoxins in Japan: risk assessment for aflatoxin B1. Food Additives and Contaminants: Part A. 2010;27(3):365–372. doi:10.1080/19440040903317497
15. Nugraha A, Khotimah K, Rietjens IMCM. Risk assessment of aflatoxin B1 exposure from maize and peanut consumption in Indonesia using the margin of exposure and liver cancer risk estimation approaches. Food and Chemical Toxicology. 2018;113:134–144. doi:10.1016/j.fct.2018.01.036
16. Huong BTM, Tuyen LD, Tuan DH, Brimer L, Dalsgaard A. Dietary exposure to aflatoxin B1, ochratoxin A and fuminisins of adults in Lao Cai province, Viet Nam: a total dietary study approach. Food and Chemical Toxicology. 2016;98:127–133. doi:10.1016/j.fct.2016.10.012
17. Smith LE, Prendergast AJ, Turner PC, Humphrey JH, Stoltzfus RJ. Aflatoxin Exposure During Pregnancy, Maternal Anemia, and Adverse Birth Outcomes. Am J Trop Med Hyg. 2017;96(4):770–776. doi:10.4269/ajtmh.16-0730
18. Leroy JL, Sununtnasuk C, García‐Guerra A, Wang JS. Low level aflatoxin exposure associated with greater linear growth in southern Mexico: a longitudinal study. Maternal and Child Nutrition. 2018;14(4):e12619. doi:10.1111/mcn.12619
19. Alamu EO, Gondwe T, Akello J, Maziya-Dixon B, Mukanga M. Relationship between serum aflatoxin concentrations and the nutritional status of children aged 6–24 months from Zambia. Int J Food Sci Nutr. 2020;71(5):593–603. doi:10.1080/09637486.2019.1689547
20. Lauer JM, Duggan CP, Ausman LM, et al. Maternal aflatoxin exposure during pregnancy and adverse birth outcomes in Uganda. Matern Child Nutr. 2019;15(2):e12701. doi:10.1111/mcn.12701
21. Abdulrazzaq YM, Osman N, Ibrahim A. Fetal exposure to aflatoxins in the United Arab Emirates. Ann Trop Paediatr. 2002;22(1):3–9. doi:10.1179/027249302125000094
22. Shuaib FMB, Jolly PE, Ehiri JE, et al. Association between birth outcomes and aflatoxin B1 biomarker blood levels in pregnant women in Kumasi, Ghana. Trop Med Int Health. 2010;15(2):160–167. doi:10.1111/j.1365-3156.2009.02435.x
23. Zhang XM, Zhao S, Chen SC, et al. Analysis of related factors of adverse birth outcomes of living newborns from 2016 to 2020 in Guiping county, China. Chin J Dis Control Prev. 2022;26(9):1050–1056. doi:10.16462/j.cnki.zhjbkz.2022.09.011
24. Chen Y, Li G, Ruan Y, Zou L, Wang X, Zhang W. An epidemiological survey on low birth weight infants in China and analysis of outcomes of full-term low birth weight infants. BMC Pregnancy Childbirth. 2013;13:242. doi:10.1186/1471-2393-13-242
25. Mahdavi R, Nikniaz L, Arefhosseini SR, Vahed Jabbari M. Determination of aflatoxin M(1) in breast milk samples in Tabriz-Iran. Matern Child Health J. 2010;14(1):141–145. doi:10.1007/s10995-008-0439-9
26. Zhang H, Qiu X, Zhong C, et al. Reproducibility and relative validity of a semi-quantitative food frequency questionnaire for Chinese pregnant women. Nutr J. 2015;14:56. doi:10.1186/s12937-015-0044-x
27. NHFPC (National Health and Family Planning Commission of the People’s Republic of China), CFDA (National Health and Family Planning Commission of the People’s Republic of China). Determination of Aflatoxins Groups B and G in Foods. Beijing, China: China Standard Press.; 2016.
28. GEMS-Food-EURO - Reliable Evaluation of Low-Level Contamination of Food.pdf. Available from: http://toolbox.foodcomp.info/References/LOD/GEMS-Food-EURO%20%20-%20%20Reliable%20Evaluation%20of%20Low-Level%20Contamination%20of%20Food.pdf.
29. Lu H, Liu F, Zhu Q, et al. Aflatoxin B1 can be complexed with oxidised tea polyphenols and the absorption of the complexed aflatoxin B1 is inhibited in rats. J Sci Food Agric. 2017;97(6):1910–1915. doi:10.1002/jsfa.7994
30. Mukaka M. A guide to appropriate use of Correlation coefficient in medical research. Malawi Med J. 2012;24(3):69–71.
31. Panrapee I, Phakpoom K, Thanapoom M, Nampeung A, Warapa M. Exposure to aflatoxin B1 in Thailand by consumption of brown and color rice. Mycotoxin Res. 2016;32(1):19–25. doi:10.1007/s12550-015-0236-4
32. Do TH, Tran SC, Le CD, et al. Dietary exposure and health risk characterization of aflatoxin B1, ochratoxin A, fumonisin B1, and zearalenone in food from different provinces in Northern Vietnam. Food Control. 2020;112:107108. doi:10.1016/j.foodcont.2020.107108
33. Arzandeh S, Selamat J, Lioe H. Aflatoxin in raw peanut kernels marketed in Malaysia. Journal of Food and Drug Analysis. 2020;18(1). doi:10.38212/2224-6614.2222
34. da Silva JVB, de Oliveira CAF, Ramalho LNZ. Effects of prenatal exposure to aflatoxin B1: a review. Molecules. 2021;26(23):7312. doi:10.3390/molecules26237312
35. Kholif OT, Sebaei AS, Eissa FI, Elhamalawy OH. Determination of aflatoxins in edible vegetable oils from Egyptian market: method development, validation, and health risk assessment. J Food Composition Analysis. 2022;105:104192. doi:10.1016/j.jfca.2021.104192
36. Iqbal SZ, Waqas M, Razis AFA, Usman S, Ali NB, Asi MR. Variation of aflatoxin levels in stored edible seed and oil samples and risk assessment in the local population. Toxins. 2022;14(9):642. doi:10.3390/toxins14090642
37. Bhardwaj K, Meneely JP, Haughey SA, et al. Risk assessments for the dietary intake aflatoxins in food: a systematic review (2016–2022). Food Control. 2023;149:109687. doi:10.1016/j.foodcont.2023.109687
38. Yang B, Zhang XJ, Wang W, et al. Investigation of aflatoxin B1 and cyclopiazonic acid in bulk peanut oil in China. China Oils Fats. 2020;45(9):34–37+53. doi:10.12166/j.zgyz.1003-7969/2020.09.007
39. The regulations on the management of small food workshops and food vendors in Guangxi Zhuang autonomous region, which effect on July 1, 2017. Available from: https://www.gxrd.gov.cn/html/art155614.html.
40. Liang J, Ning M, Guan S, et al. Risk assessment of multiple-mycotoxin exposure for consumers of chestnuts in Shandong Province markets in China. Food Addit Contam Part a Chem Anal Control Expo Risk Assess. 2021;38(12):2137–2150. doi:10.1080/19440049.2021.1970240
41. Qi N, Yu H, Yang C, Gong X, Liu Y, Zhu Y. Aflatoxin B1 in peanut oil from Western Guangdong, China, during 2016–2017. Food Addit Contam Part B. 2019;12(1):45–51. doi:10.1080/19393210.2018.1544173
42. Abdulrazzaq YM, Osman N, Yousif ZM, Trad O. Morbidity in neonates of mothers who have ingested aflatoxins. Ann Trop Paediatrics. 2004;24(2):145–151. doi:10.1179/027249304225013420
43. Eaton DL, Groopman JD. The Toxicology of Aflatoxins: Human Health, Veterinary, and Agricultural Significance. Elsevier; 2013.
44. de Almeida L, Williams R, Soares DM, Nesbitt H, Wright G, Erskine W. Aflatoxin levels in maize and peanut and blood in women and children: the case of Timor-Leste. Sci Rep. 2019;9(1):13158. doi:10.1038/s41598-019-49584-1
45. Passarelli S, Bromage S, Darling AM, et al. Aflatoxin exposure in utero and birth and growth outcomes in Tanzania. Matern Child Nutr. 2020;16(2):e12917. doi:10.1111/mcn.12917
46. Andrews-Trevino JY, Webb P, Shively G, et al. Relatively low maternal aflatoxin exposure is associated with small-for-gestational-age but not with other birth outcomes in a prospective birth cohort study of Nepalese infants. J Nutr. 2019;149(10):1818–1825. doi:10.1093/jn/nxz122
47. Ye Y. AFB1 Exposure in Pregnant Women in Guangxi Zhuang Autonomous Region and Bioinformatics Analysis of Human Liver Cells by AFB1. D. Guangi Medical University; 2019.
48. de Jong M, Cranendonk A, Twisk JWR, van Weissenbruch MM. IGF-I and relation to growth in infancy and early childhood in very-low-birth-weight infants and term born infants. PLoS One. 2017;12(2):e0171650. doi:10.1371/journal.pone.0171650
49. Fall CH, Pandit AN, Law CM, et al. Size at birth and plasma insulin-like growth factor-1 concentrations. Arch Dis Child. 1995;73(4):287–293. doi:10.1136/adc.73.4.287
50. Castelino JM, Routledge MN, Wilson S, et al. Aflatoxin exposure is inversely associated with IGF1 and IGFBP3 levels in vitro and in Kenyan schoolchildren. Mol Nutr Food Res. 2015;59(3):574–581. doi:10.1002/mnfr.201300619
51. Tessema M, De Groote H, Brouwer ID, et al. Exposure to aflatoxins and fumonisins and linear growth of children in rural Ethiopia: a longitudinal study. Public Health Nutr. 2021;24(12):3662–3673. doi:10.1017/S1368980021000422
52. Hellström A, Sigurdsson J, Löfqvist C, Hellgren G, Kistner A. The IGF system and longitudinal growth in preterm infants in relation to gestational age, birth weight and gender. Growth Horm IGF Res. 2020;51:46–57. doi:10.1016/j.ghir.2020.02.002
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



