Back to Journals » International Medical Case Reports Journal » Volume 17
Coronary Artery Fistula and Severe Coronary Artery Stenosis: A Case Report and an Insight for Potential Pathogenesis of Coronary Artery Atherosclerosis
Received 19 October 2023
Accepted for publication 18 March 2024
Published 26 March 2024 Volume 2024:17 Pages 227—233
DOI https://doi.org/10.2147/IMCRJ.S442878
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Vinay Kumar
Jinchun Liu,1 Zhijun Yu,2,3 Guohua Wang2
1Department of Medicine, Henan Vocational College of Nursing, Anyang, Henan Province, 455000, People’s Republic of China; 2Department of Neurophysiology and Neuropharmacology, Institute of Special Environmental Medicine and Co-Innovation Center of Neuroregeneration, Nantong University, Nantong, Jiangsu, 226019, People’s Republic of China; 3Department of Thoracic Surgery, Nantong Second People’s Hospital, Nantong, Jiangsu, 226001, People’s Republic of China
Correspondence: Guohua Wang, Email [email protected]
Abstract: Coronary artery fistulae (CAF) are a rare anomaly characterized by abnormal connections between a coronary artery and a cardiac chamber or a great vessel, with most patients remaining asymptomatic. Despite being predisposed to severe complications like heart failure, patients with CAF infrequently experience severe stenosis in the coronary artery. This study delineates a case involving a 46-year-old male presenting with a fistula bridging the right coronary artery (RCA) and right atrium (RA), manifesting a pronounced 99% stenosis at the right extremity of the coronary artery proximal to the fistula. Concurrently, the individual exhibits six conventional risk factors: age over 40, male gender, hypertension, diabetes, smoking, and hypertriglyceridemia. Following pharmaceutical intervention, the patient was discharged and subjected to extended follow-up. This case highlights the dual processes of “accelerating damage” and “retarding renewal” in the progression of atherosclerosis. Factors such as shear stress, smoking, and hypertension are posited to expedite endothelial cell damage, while aging and diabetes may impede the renewal and repair of these cells. Together with the concept of secondary atherosclerotic plaque healing, this case prompts the introduction of a “Double Endothelial Healings” hypothesis, proposing a potential pathogenetic mechanism for coronary artery atherosclerosis.
Keywords: coronary artery fistulae, atherosclerosis, pathogenesis, plaque healing, endothelial injury
Introduction
Coronary artery fistulae (CAF) represent a subset of rare cardiac anomalies, with an estimated prevalence of 0.002% within the general populace, which escalates to approximately 5% among patients subjected to coronary angiography.1 CAF are either congenital or acquired anomalies where the epicardial coronary arteries communicate with a cardiac chamber (coronary-cameral fistulae) or the great vessels of the heart such as pulmonary veins or arteries.1 Typically discovered incidentally, most CAF, seldom manifest with symptoms.2 The reported cases of CAF are mostly due to rare complications, such as heart failure, ischemia, and arrhythmia. Among the reported complications, about 30% of CAF cases are associated with coronary atherosclerosis in adults.3 However, the relationship between CAF and coronary atherosclerosis has not yet been elucidated.
Atherosclerosis, characterized by the accumulation of cells, lipids, and debris within the vascular intima, stands as the primary etiological factor behind cardiovascular diseases, which impose a significant burden on global healthcare systems. Accounting for approximately 31% of deaths worldwide, cardiovascular diseases underscore the critical impact of atherosclerosis.4 The genesis of atherosclerosis is multifactorial, with oxidative stress, inflammation, hypertension, and hyperlipidemia playing pivotal roles, culminating in the deposition of low-density lipoprotein cholesterol within the walls of medium and large arteries.4 Despite extensive research, the pathogenesis of atherosclerosis remains incompletely understood. Insights from specific patient populations, particularly those presenting with unique clinical features, are imperative for advancing our understanding of the mechanisms underlying atherosclerosis.
It is well-established that CAF can cause severe haemodynamic disturbances in blood flow within the coronary arteries. This haemodynamic disturbance, often referred to as shear stress, is a key player in the pathogenesis of coronary artery atherosclerosis.5 Consequently, it is plausible to inquire whether there’s a heightened incidence of atherosclerosis accompanied by severe stenosis in coronary arteries affected by CAF. Furthermore, beyond hemodynamic perturbations, it is imperative to consider additional factors that may contribute to the pathogenesis of coronary atherosclerosis. Is it possible to identify a case that amalgamates these diverse factors, providing critical insights into their collective impact on atherosclerosis? This report details a case of coronary artery fistula concomitant with severe coronary artery atherosclerosis, offering invaluable perspectives on the pathogenesis of coronary atherosclerosis. Subsequently, we introduce the “Double Endothelial Healings” hypothesis as a framework to interpret the phenomena observed.
Case Presentation
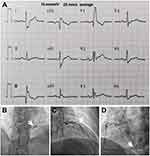
A 46-year-old male farmer was referred to our institution at Anyang People’s Hospital (Henan Province, China) on October 25, 2020, presenting with “paroxysmal chest tightness, chest pain for 3 days”. Written informed consent for the publication of patient data and figures was obtained from the patient. Three days prior to admission, the patient experienced compression-like chest tightness in the precordial region of unknown etiology, intermittently accompanied by chest pain radiating to the left scapular region. These primary symptoms were accompanied by palpitations, fatigue, and shortness of breath, alleviated by rest for several minutes. Over the preceding three days, symptom recurrence was noted, irrespective of the patient’s activity levels. The symptoms exacerbated a day before admission. The patient had a 20-year history of hypertension managed with oral antihypertensive medications, with no history of diabetes, stroke, surgical trauma, blood transfusion, or known allergies. He had a 25-year history of smoking (30 cigarettes a day). Familial history revealed maternal hypertension. On physical examination, his pulse rate was recorded at 85 beats per minute with a blood pressure of 140/63 mm Hg. Laboratory evaluations revealed fasting blood glucose of 8.14 mmol/L, total cholesterol 3.86 mmol/L, triglycerides 4.37 mmol/L, low-density lipoprotein (LDL) 2.466 mmol/L, and high-density lipoprotein (HDL) 0.636 mmol/L, ascertained from fasting blood tests conducted the following day post-admission. Initial electrocardiography (ECG) showcased a complete right bundle branch block (RBBB) (Figure 1A). The provisional diagnosis included coronary atherosclerotic heart disease, stable angina, arrhythmia, and complete RBBB. Coronary angiography executed on October 28, 2020, unveiled scattered plaques in the right coronary artery with 20–30% stenotic lesions in the middle and distal segments. Notably, a conspicuous fistula was observed at the terminal segment of the posterior branch of the left ventricle, terminating in the left atrium (Figure 1B). Additionally, a diffuse-type lesion, demonstrating 50–60% stenosis, was identified in the mid-segment of the left anterior descending artery (LAD) (Figure 1C). A significant lesion, exhibiting 99% stenosis, was located in the proximal segment of the posterior branch of the left ventricle, proximal to the fistula that connects the right coronary artery with the right atrium (Figure 1D). Echocardiography demonstrated bilateral carotid intima-media thickening and mixed plaques on the posterior wall of the right carotid bulb, measuring between 11.2 to 2.1 mm. Elevated fasting and postprandial blood glucose levels, alongside a high glycosylated hemoglobin level, led to a diagnosis of type 2 diabetes mellitus, managed with oral metformin. Hypertriglyceridemia was diagnosed post-admission and managed with routine atorvastatin administration. Other standard treatments comprised aspirin, metoprolol, and irbesartan. The application of optical coherence tomography (OCT) would have enriched our case report by providing intricate details regarding plaque characteristics and endothelial healing processes; however, OCT was not available during the patient’s evaluation. Symptomatic relief was achieved, facilitating the patient’s discharge on the seventh day following admission. Seventeen months post-discharge, the patient reported a satisfactory clinical status, with no episodes of discomfort, chest pain, or chest tightness during routine daily activities and exercise. Written informed consent was obtained from the patient for the publication of the case details.
Discussion
CAF predominantly present without symptoms in the majority of affected individuals.1 Despite their rarity, a subset of patients may develop severe complications, including heart failure, ischemia, and arrhythmias. Notably, approximately 30% of adult cases of CAF are associated with coronary atherosclerosis,3 yet the precise nature of the relationship between CAF and coronary atherosclerosis remains unclear. In certain instances, CAF and coronary atherosclerosis seem to occur as distinct and independent phenomena, as illustrated in the case reported by Wandwi et al,6 and in another case by Brunetti et al,7 where occlusions in the left circumflex and right coronary arteries coexisted with a fistula connecting the left main and diagonal branch, draining into the pulmonary artery, without apparent interrelation. Conversely, there are instances where CAF and coronary atherosclerosis coexist within the same coronary artery, suggesting a potential interconnection between these two conditions.8
In some cases, CAF may exacerbate CAD conditions, as demonstrated in a case reported by Mahboob Ali,1 where a patient presented with NSTEMI but exhibited no severe stenosis on coronary angiography. In this scenario, the coronary artery steal phenomenon through CAF was posited as the sole explanation for the observed ischemia. CAF has been implicated in causing reversible perfusion defects of the corresponding wall through anomalous flow, leading to ischemia.9 The primary pathophysiological concern in coronary fistulae is often attributed to the coronary steal phenomenon.1,10 Simultaneously, the presence of severe coronary stenosis may also promote the development of CAF by creating a gradient between a high-pressure system and a low-pressure one.11 This complex interplay highlights the need for further investigation into the mechanisms linking CAF and coronary atherosclerosis.
In exceptional instances, a causal link between CAF and coronary atherosclerosis has been posited, as exemplified by the case under discussion. This particular case showcased a severe manifestation of coronary heart disease (CHD), providing valuable insights that may catalyze further investigation into the pathogenetic mechanisms of coronary atherosclerosis. A significant pressure gradient between high-pressure and low-pressure systems induced anomalous flow or hemodynamic disturbances. Among these disturbances, shear stress, especially arising from disturbed and/or non-laminar blood flow, is identified as a critical factor in the development of atherosclerotic plaques.5 This phenomenon underscores the necessity of examining the role of shear stress in the progression of coronary atherosclerosis. The present case contributes to the ongoing discourse regarding the complex interplay between CAF and coronary atherosclerosis, potentially unveiling novel pathophysiological insights and therapeutic avenues.
Coronary atherosclerosis is a progressive ailment predominantly observed in elderly males.12 The etiology of coronary atherosclerosis remains elusive, although numerous traditional risk factors for CHD have been delineated, encompassing diabetes, age exceeding 40 years, male gender, familial history of premature CHD, elevated blood pressure and LDL levels, presence of microalbuminuria, and obstructive sleep apnea.5 Besides his age and gender, the patient in the present case exhibited a prolonged history of smoking and hypertension prior to admission. Post-admission diagnoses included type 2 diabetes and hypertriglyceridemia, thereby aligning the patient with six pivotal risk factors for coronary atherosclerosis, amongst which diabetes is deemed a paramount concern relative to CHD.3 The patient manifested diffuse-type atherosclerotic plaques within the mid left anterior descending artery (LAD) – a common site for coronary atherosclerosis – displaying approximately 50–60% stenosis, a comprehensible finding given the rarity of premature coronary heart disease in clinical settings.13 Nonetheless, the identification of a 99% stenosis at the terminal segment of the right coronary artery (precisely anterior to the fistula) is scarcely observed, especially in a 46-year-old individual. This case accentuates the contributory role of blood flow shear stress in the inception and progression of coronary atherosclerotic plaque. The 99% stenosis was pinpointed at a high-velocity blood flow juncture, a locale seldom associated with severe stenosis even amidst the presence of myriad classic risk factors under conventional circumstances. Emerging evidence increasingly supports the notion that laminar blood flow and sustained high shear stress play a pivotal role in modulating the expression of endothelial cell genes and proteins, which serve protective functions against atherosclerosis. Conversely, disturbed blood flow, characterized by low and oscillating shear stress, is known to upregulate the expression of proatherosclerotic genes and proteins, thereby fostering the development of atherosclerosis.14 In the case at hand, the significant hemodynamic stress exerted on endothelial cells is believed to markedly contribute to the initiation and progression of coronary atherosclerotic plaques. The literature indicates that the primary pathological event in atherosclerosis involves the accumulation of fatty streaks within the vascular wall, particularly at arterial bifurcations where the disruption of laminar blood flow occurs.15 This disruption is a critical factor in the pathogenesis of atherosclerotic lesions, highlighting the intricate relationship between hemodynamic forces and vascular health.
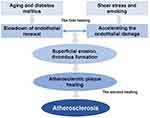
In healthy individuals, a dynamic equilibrium is maintained within the endothelial system, wherein the loss and regeneration of endothelial cells are balanced. This equilibrium can be disrupted through two primary pathways: the acceleration of endothelial cell damage or the deceleration of endothelial cell renewal and repair. The patient in this case displayed six risk factors for coronary heart disease, namely age, male gender, smoking, hypertension, type 2 diabetes, and hyperlipidemia. Shear stress, smoking, and hypertension were identified as accelerators of endothelial cell damage,16 while aging and diabetes were associated with a reduced pace of vascular cell regeneration post-injury.17 Research posits that individuals with diabetes may exhibit impaired revascularization of ischemic tissues due to aberrant production of circulating provascular progenitor cells, a phenomenon termed “regenerative cell exhaustion”.17 Specifically, those with a prolonged history of type 2 diabetes demonstrate decreased frequencies of circulating proangiogenic ALDHhiCD34+ progenitor cells. Such changes in circulating cell phenotypes are believed to contribute to the progressive decline in vascular repair capacity.18 This phenomenon underscores the complex interplay between diabetes and vascular health, highlighting the critical role of progenitor cells in maintaining vascular integrity and the potential impact of diabetes on these reparative processes.
The disruption of the balance between injury and repair leads to the loss of punctate and flaky endothelial cells.19 This inability of the endothelial system to maintain its integrity, referred to as endothelial damage, is characterized as “superficial erosion” of an atherosclerotic plaque.20 Superficial erosion exposes thrombogenic components of the plaque, such as the necrotic core, tissue factor, and collagen, initiating platelet activation, aggregation, and subsequent thrombus formation.21 This process not only damages the endothelial barrier but also provokes proinflammatory responses and a prethrombotic state.22 This Recent advancements in imaging techniques have bolstered this notion, shedding light on a process dubbed “coronary plaque healing”.23 Investigations into healed plaques revealed that these lesions harbored multi-layered lipids and necrotic cores, indicating recurrent episodes of thrombosis and healing.19 OCT images depicted healed plaques with heterogeneous multilayers rich in signals, with optical signal intensity differing from the underlying plaques, thereby exhibiting a characteristic onion-like appearance.24 This appearance has led medical researchers to further investigate the impact of thrombotic events on the development of atherosclerotic plaques in vivo. These thrombotic events initiate a subsequent repair process, known as plaque healing, which stabilizes the eroded site and results in a healed plaque characterized by distinct layers of organized collagen. This process contributes to the progression of coronary atherosclerosis.21 Thus, a pathogenic trajectory for atherosclerosis is proposed, encompassing the failure of endothelial repair, exposure of plaque components, activation and aggregation of platelets, thrombosis, activation of endogenous fibrinolysis, thrombolysis, formation of granulation tissue, and ultimately, reendothelialization and plaque healing.23
We hereby propose a hypothesis termed “Double Endothelial Healings” to elucidate the genesis and progression of atherosclerotic plaque (Figure 2), in which both the process of “acceleration of endothelial damage” and the process of “deceleration of endothelial renewal” play critical roles in the development and progression of atherosclerotic plaque. The first facet of “Endothelial healing” pertains to the restoration of the arterial endothelial system compromised by shear stress, smoking, and hypertension, all of which accelerate the endothelial damage, or compromised by aging and diabetes, all of which hamper the pace of healing. The objective of this initial “Endothelial healing” phase is to uphold the dynamic equilibrium of the endothelial system. Should the endothelial system falter in maintaining its integrity, the secondary “Endothelial healing” phase is activated to address the recurrent thrombosis ensuing from the exposure of subendothelial matrix proteoglycans after superficial erosion or plaque rupture. This secondary healing leads to the stabilization of the eroded site and culminates in a healed plaque, delineated by distinct layers of organized collagen. The iterative “Double Endothelial Healings” process thus contributes to the edification of coronary atherosclerosis.
In this report, we have presented a case characterized by the presence of coronary artery fistulae in conjunction with six traditional risk factors for CHD, resulting in severe coronary stenosis proximal to the fistula. This case prompts a deeper examination of the pathogenesis of coronary atherosclerosis. Despite the complexity and partially understood nature of the onset and progression of coronary atherosclerosis, a wealth of clinical and basic research has provided valuable insights into this condition. The “Double Endothelial Healings” hypothesis, inspired by the clinical observations from this case, suggests a novel perspective on the development of coronary atherosclerosis. However, it is important to acknowledge the limitations inherent in formulating a hypothesis based on a single case and supplementary literature, which may be considered a relatively weak foundation. A more rigorous approach, including a quantitative analysis of flow disorders, would be essential to provide a solid base of evidence to support our hypothesis. The use of OCT for in vivo plaque evaluation would have significantly enhanced our ability to substantiate our observations. The hypothesis regarding the pathogenesis of coronary atherosclerosis, as proposed in this case, is built upon the preliminary evidence presented. It necessitates further comprehensive clinical and basic research for validation, refinement, and detailed exploration.
Conclusion
This case, featuring a coronary fistula alongside significant risk factors for coronary artery atherosclerosis, serves as a catalyst for proposing the “Double Endothelial Healings” hypothesis. This hypothesis aims to shed light on the potential mechanisms underlying the pathogenesis of coronary artery atherosclerosis, inviting further investigation into this critical area of cardiovascular research.
Consent
The authors confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from Anyang People’s Hospital (Henan Province, China) and written consent has been obtained from the patient.
Acknowledgments
We thank Dr. Ming Liu (Heart Center, Anyang People Hospital, Anyang, Henan Province 455000) for his help in original data collection and writing assistance.
Funding
This study was supported by the National Natural Science Foundation of China (grant 82171190), and Key scientific research projects of colleges and universities in Henan Province (22B310001).
Disclosure
No conflict of interest is declared.
References
1. Ali M, Kassem KM, Osei K, et al. Coronary artery fistulae. J Thromb Thrombolysis. 2019;48(2):345–351. doi:10.1007/s11239-019-01897-8
2. Maknojia A, Pride Y, Ghatak A, et al. Fistula between the first obtuse marginal branch of the left circumflex and the left ventricular cavity: a rare anomaly. Cureus. 2021;13(2):e13316. doi:10.7759/cureus.13316
3. Turek Ł, Polewczyk A, Janion M, et al. Coronary artery fistula and premature coronary atherosclerosis. Cardiol J. 2019;26(3):296–297. doi:10.5603/cj.2019.0059
4. Chan YH, Ramji DP. Atherosclerosis: pathogenesis and key cellular processes, current and emerging therapies, key challenges, and future research directions. Methods Mol Biol. 2022;2419:3–19. doi:10.1007/978-1-0716-1924-7_1
5. Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 2005;85(1):9–23. doi:10.1038/labinvest.3700215
6. Wandwi WB, Mitsui N, Sueda T, et al. Coronary artery fistula to bronchial artery on contralateral side of coronary atherosclerosis and myocardial insufficiency. A case report. Angiology. 1996;47(2):211–213. doi:10.1177/000331979604700214
7. Brunetti ND, Cuculo A, Campanale EG, et al. Left coronary to pulmonary artery fistula in a patient with severe coronary atherosclerosis. Eur J Cardiothorac Surg. 2011;40(4):e152. doi:10.1016/j.ejcts.2011.05.040
8. Balanescu S, Sangiorgi G, Medda M, et al. Successful concomitant treatment of a coronary-to-pulmonary artery fistula and a left anterior descending artery stenosis using a single covered stent graft: a case report and literature review. J Interv Cardiol. 2002;15(3):209–213. doi:10.1111/j.1540-8183.2002.tb01059.x
9. Rubini G, Ettorre GC, Sebastiani M, et al. alutazione del significato emodinamico delle fistole arterovenose coronariche: integrazione diagnostica tra angiografia coronarica e scintigrafia miocardica da sforzo/riposo [Evaluation of hemodynamic significance of arteriovenous coronary fistulas: diagnostic integration of coronary angiography and stress/rest myocardial scintigraphy]. Radiol Med. 2000;100(6):453–458. Italian.
10. Sunkara A, Chebrolu LH, Chang SM, et al. Coronary artery fistula. Methodist DeBakey Cardiovasc J. 2017;13(2):78–80. doi:10.14797/mdcj-13-2-78
11. Castedo E, Oteo JF, Burgos R, et al. Coronary artery fistula as a bypass of a left anterior descending coronary artery stenosis. Ann Thorac Surg. 1997;64(6):1813–1814. doi:10.1016/S0003-4975(97)00932-6
12. Negi S, Anand A. Atherosclerotic coronary heart disease-epidemiology, classification and management. Cardiovasc Hematol Disord Drug Targets. 2010;10:257–261. doi:10.2174/187152910793743832
13. Shah N, Kelly AM, Cox N, et al. Myocardial infarction in the ”young”: risk factors, presentation, management and prognosis. Heart Lung Circ. 2016;25:955–960. doi:10.1016/j.hlc.2016.04.015
14. Chiu -J-J, Usami S, Chien S. Vascular endothelial responses to altered shear stress: pathologic implications for atherosclerosis. Ann Med. 2009;41(1):19–28. doi:10.1080/07853890802186921
15. Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364(3):226–235. doi:10.1056/NEJMoa1002358
16. Tang EW, Wong CK, Herbison P. Global Registry of Acute Coronary Events (GRACE) hospital discharge risk score amortality post acute coronary syndrome accurately predicts long-term. Am Heart J. 2007;153:29–35. doi:10.1016/j.ahj.2006.10.004
17. Terenzi DC, Trac JZ, Teoh H, et al. Vascular regenerative cell exhaustion in diabetes: translational opportunities to mitigate cardiometabolic risk. Trends Mol Med. 2019;25:640–655. doi:10.1016/j.molmed.2019.03.006
18. Terenzi DC, Al-Omran M, Quan A, et al. Circulating pro-vascular progenitor cell depletion during type 2 diabetes: translational insights into the prevention of ischemic complications in diabetes. JACC. 2019;4:98–112. doi:10.1016/j.jacbts.2018.10.005
19. Fracassi F, Crea F, Sugiyama T, et al. Healed culprit plaques in patients with acute coronary syndromes. J Am Coll Cardiol. 2019;73(18):2253–2263. doi:10.1016/j.jacc.2018.10.093
20. Durand E, Scoazec A, Lafont A, et al. In vivo induction of endothelial apoptosis leads to vessel thrombosis and endothelial denudation: a clue to the understanding of the mechanisms of thrombotic plaque erosion. Circulation. 2004;109:2503–2506. doi:10.1161/01.Cir.0000130172.62481.90
21. Vergallo R, Crea F, Jarcho JA. Atherosclerotic plaque healing. New Engl J Med. 2020;383(9):846–857. doi:10.1056/NEJMra2000317
22. Vergallo R, Porto I, D’Amario D, et al. Coronary atherosclerotic phenotype and plaque healing in patients with recurrent acute coronary syndromes compared with patients with long-term clinical stability: an In Vivo Optical Coherence Tomography Study. JAMA Cardiol. 2019;4(4):321–329. doi:10.1001/jamacardio.2019.0275
23. Chiti A. Atherosclerotic plaque healing. New Engl J Med. 2021;384:293–294. doi:10.1056/NEJMc2033613
24. Shimokado A, Matsuo Y, Kubo T, et al. In vivo optical coherence tomography imaging and histopathology of healed coronary plaques. Atherosclerosis. 2018;275:35–42. doi:10.1016/j.atherosclerosis.2018.05.025
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.