Back to Journals » Clinical, Cosmetic and Investigational Dentistry » Volume 16
“Compression Necrosis” – A Cause of Concern for Early Implant Failure? Case Report and Review of Literature
Authors Ramesh R , Sasi A, Mohamed SC , Joseph SP
Received 8 December 2023
Accepted for publication 28 February 2024
Published 7 March 2024 Volume 2024:16 Pages 43—52
DOI https://doi.org/10.2147/CCIDE.S453798
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Christopher E. Okunseri
Roshni Ramesh,* Anoop Sasi,* Shahana C Mohamed, Sonia P Joseph
Department of Periodontology, Government Dental College, Thiruvananthapuram, Kerala, India
*These authors contributed equally to this work
Correspondence: Roshni Ramesh, Department of Periodontology, Government Dental College, Thiruvananthapuram, Kerala, India, Email [email protected]
Purpose: Compression necrosis refers to bone tissue damage that can occur when excessive pressure or force is applied to surrounding bone during implant placement. This pressure can compromise blood supply to the bone, leading to necrosis. Compression necrosis is a concern, because it can affect the stability and long-term success of dental implant.
Patients and Methods: This case report highlights a case of early bone loss and implant failure possibly due to compression necrosis. Clinical data, photographs, radiographs, blood examination report and histology were presented to document the early failure of an implant placed in the mandibular left posterior region of a 33-year-old female patient.
Results: Radiograph taken six weeks after implant placement showed severe angular defect. Therefore, the implant was surgically removed. Histological examination of the area showed bony trabeculae with an absence of osteoblastic riming, suggestive of necrotic bone.
Conclusion: Using excessive torque values when placing implants in dense bones can increase the risk of implant failure due to bone over compression. Dental professionals must follow the manufacturer’s instructions and employ quality surgical techniques during implant placement into dense cortical bone to minimise risks.
Keywords: bone loss, dental implants, implant failure, necrosis
Introduction
Newer developments in implant systems and surgical techniques ever since the landmark research on successful osseointegration by Branemark in 1969 have markedly improved the reliability of dental implants. This has led to increased interest in the replacement of missing tooth or teeth for functional and aesthetic reasons. Newer implant protocols, such as the use of short-length implants, immediate loading implants, and implant placement in poor-quality bone, have placed clinicians with many biomechanical challenges.1–4 These protocols expose the implant to prospective mechanical stresses before the completion of biological integration and hence pose a risk of micromovements during the healing process.5 Excessive micromovement is detrimental to osseointegration and is among the most common causes of implant failure. The best way to control micromovements is to place implants with a high primary stability. Primary stability stems from the mechanical interaction between the implant and bony walls during implant insertion.6 Various factors affect implant stability including the surgical protocol, implant macro design, quality of bone and insertion torque.7
A study by Gaya et al concluded that surgical technique influences the primary stability when implants are placed in poor quality bone.8 Site preparation in any surgical technique is critical for implant survival.9 Osteotomy procedure (drilling) is a highly delicate process involving many fundamental related factors that can affect crestal bone stability and implant osseointegration.10 Implants can be inserted using conventional or undersized drilling techniques. Underpreparation technique may cause excessive compression of cortical bone leading to microfractures.11 However, a study by Huang et al in an animal model evaluated the effects of different surgical drilling protocols on implant stability and osseointegration and concluded that undersized drilling protocol is biologically and mechanically superior in low-density bone.12 Careful selection of drilling sequence is recommended based on bone density to obtain adequate primary stability and to preserve the crestal bone morphology.13 Wrong choice of surgical technique can result in early or late implant failure.14
The second factor that can affect primary stability is the quality of bone. Lekholm and Zarb classified bone according to the amount of trabecular and cortical bone on a scale ranging from I to IV.15 Type IV bone has thin cortical plate around a main layer of trabecular bone. Primary stability is lowest in type IV bone and implants placed in type III or IV bones experienced higher failure rates (4.27–8.06%) compared to type I or II bones.16
Yet another factor affecting primary stability is the implant macro design. Several researchers have reported how the design and properties of implant threads impact implant stability especially in low-density bone.17,18 Abrahamsson et al studied early stages of osseointegration using implants with different geometry and different osteotomy protocols and found that implant placement in undersized osteotomy sites resulted in increased remodeling of the cortical bone during the early healing process.19 An in vitro study by Antonelli et al comparing the primary stability of two different implant macro designs concluded that magneto dynamic geometry showed better performance in low-density bone.20 Dayan et al in an ex-vivo study to find out the differences in primary stability between straight and tapered implants in type I and type III bones showed that implant shape with a thread cutting and forming design did not affect the primary stability.21 Falco et al suggested that for cases with poor bone quality, large thread implant designs are highly desirable.22 A randomized controlled trial by Gehrke et al to evaluate the influence of dental implant macro geometry on early stability found that modifications of implant macro geometry produced significant reduction in the insertion torque compared to conventional implant design.23 Haseeb et al compared the implant stability and insertion torque of two different macro geometry in different bone densities and concluded that lower insertion torques of new implant types help in reducing failure.24 Gandhi et al evaluated how two different implant designs responded to conventional osteotomy drilling and found that tapered pro implant design offered a better primary stability when osseodensification was used.25
Insertion torque is defined as the amount of torque required to place the implant into the prepared osteotomy site.26 Literature suggests that high insertion torque is an indicator of three-dimensional primary stability even though there is no consensus regarding minimal torque needed for implant success. A torque value in the range of 20–40Ncm is used by most clinicians. Animal experiments showed the highest rate of osseointegration when an insertion torque of 30–35 Ncm was applied.27 Souza et al studied the relation between insertion torque and implant stability and concluded that there is a positive correlation between insertion torque and implant stability quotient.28 A clinical study showed that increasing the peak insertion torque significantly improved the survival rates of immediate loaded implants.29 Trisi et al in a study concluded that implants placed with a torque above 100Ncm were always below the threshold risk for micromovements and hence have less chance of failure.30 Similarly, other authors have shown that an insertion torque of up to 150Ncm did not produce any deleterious effects.31 Khayat et al in a study using tapered implants concluded that a high insertion torque up to 176Ncm did not affect osseointegration.32 The above-mentioned results have prompted clinicians using the newer mechanically challenging protocols to use high insertion torques for implant placement.
However, some researchers are concerned that a high insertion torque may be responsible for a phenomenon called “osseous pressure necrosis”.33,34 Although frequently used, this concept has not been clearly explained in the literature. However, previously published consensus reports suggest that compression necrosis is a possible risk factor for peri-implant disease.35,36 This phenomenon is defined as excessive compression (pressure) of the bone created during implant insertion. Compression of bone beyond physiological limits may cause ischaemia, leading to bone necrosis. Excessive torque is also thought to produce high levels of strain in the adjacent bone. However, it is generally accepted that compression necrosis is limited to the cortical bone.37 Pressure necrosis during implant placement can create non-vital bone around the implant and even lead to temporary neurosensory impairment if the implant is placed near the mandibular canal. Some authors believe that when the strain on the bone exceeds a certain tolerable limit, it will cause irreversible damage in the form of plastic deformation and microcracks, ultimately leading to early bone loss and implant failure.11
This report highlights a case of early bone loss and implant failure possibly due to compression necrosis.
Materials and Methods
Clinical Presentation
A 33-year-old female patient reported to the Department of Periodontology on 10th of April 2023 for the replacement of a missing tooth with an implant in relation to the mandibular left posterior region. The patient had already undergone implant surgery in the mandibular right posterior area 1 year back (Figure 1). The patient was a non-smoker and non-diabetic (Table 1) and had no other relevant medical history. A written informed consent was obtained from the patient for implant placement.
 |
Table 1 Blood Examination Report Obtained 4 Weeks After Implant Placement |
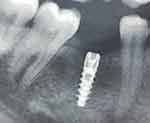 |
Figure 1 Radiograph of implant placed in relation to #30. |
A two-stage implant placement was planned in relation to tooth #19. Cone beam computed tomography (CBCT) scan showed adequate height and width of mandible in relation to tooth #19 with an available mesiodistal width of 11.03 mm, a buccolingual width of 4.2 mm and an apicocoronal length of 12.81 mm (Figure 2).
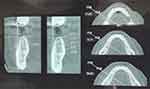 |
Figure 2 Pre-operative CBCT #19 region. |
Case Management
Thorough professional mechanical plaque removal, followed by subgingival instrumentation, was performed. In two weeks recall visit, an implant of 3.5D × 11.5 L was planned in relation to tooth #19. As the bone quality of the site was presumed to be D2-D3, an undersized drilling technique was decided upon. Sequential drilling was done using 800–1200 rpm speed and 35–50Ncm torque starting with a pilot drill (2mm). Osteotomy site was enlarged up to a drill size of 3.2mm following which an implant of 3.5D × 11.5 L was placed manually with a torque of 35–50Ncm using torque wrench with ratchet until primary stability was achieved. The surgical site was closed passively and completely using a 3–0 nonabsorbable silk suture (Figure 3).
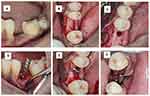 |
Figure 3 Implant Surgery (A) pre-operative view (B) Incision placed and flap reflected (C) After osteotomy (D) Parallel pin placed (E) Implant placed (F) Sutures given. |
Results
The sutures were removed one week after implant surgery. Healing in the first week was uneventful and the patient reported no discomfort during the study period. The patient was recalled three weeks later, during which she complained of intermittent dull pain at the implant site. Clinical healing was complete with no signs of inflammation or infection. The patient was advised to rinse with warm saline and report if the symptoms worsened.
Six weeks after the implant placement, the patient reported pain. Intraoral periapical radiography (IOPAR) was taken which revealed severe bone loss extending to half of the implant length (Figure 4). Antibiotics (Amoxicillin 500 mg thrice daily and Metronidazole 250 mg thrice daily for five days) were prescribed, and the patient was asked to review after five days. On the fifth day, the surgical site was reopened after getting patient consent (Figure 5). A biopsy of the surgical site was performed, and a small sequestrum of bone was retrieved, there was 50% bone loss relative to the implant, and the implant was mobile. The implant was removed using a fixture removal kit (Figure 6). Biopsy specimen was preserved in 10% formalin and sent for histopathological examination.
 |
Figure 4 Radiographs (A) immediate post operative (B) 6 weeks post operative radiograph showing angular defect around implant. |
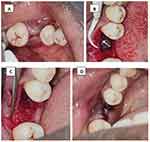 |
Figure 5 Implant site reopened (A) Incision placed (B) flap reflected and implant exposed. Note the granulation tissue around implant. (C) Implant removed (D) flap closed and sutures placed. |
 |
Figure 6 Fixture removal kit. |
Histopathological Examination
The bone biopsy specimen was composed of a single hard tissue of size 0.3 × 0.15 × 0.05 cm non-viable bony sequestrum. Serial sections of haematoxylin and eosin-stained decalcified hard tissue showed bony trabeculae with an absence of osteoblastic riming, suggestive of necrotic bone (Figure 7). There were no signs of viable bone throughout the specimen and the lacunae were devoid of osteocytes.
Discussion
Implants can fail for various reasons, but when the reason is unknown, we can only speculate on the cause of failure. In this report, we present a case of an implant inserted in mandibular left posterior region which failed for no obvious reason. A study by Staedt et al showed that early dental implant failure occurs more often in posteriorly placed implants than in anterior region.38
Bone overheating during osteotomy is a common cause of early implant loss.39,40 However, in this case, the osteotomy site was prepared using copious amounts of saline irrigation. In addition, the hard tissue specimen obtained during surgical re-entry was necrotic. Hence, we can assume that the possible reason for this failure was non-inflammatory. The second possible reason for early implant failure is the over compression of the bone during implant placement which results in localised pressure necrosis. This usually occurs when a cortical component of the bone is present in the crestal region (D1 or D2 bone). When excessive stresses and strains occur in bone beyond physiologic limits, it can have deleterious effects on local microcirculation and bone cellular responses leading to bone compression necrosis.37 Studies report an extensive area of apoptotic osteocytes, tissue damage and finally peri-implant bone loss.41,42 A study by Toia has shown that the high stress generated on implant insertion can be avoided if a crestal bone drill is used.43 The bone in the present case was of quality D2, pretapping was not done preceding implant placement, which might have led to excessive pressure transmitted to adjacent bone and a non-inflammatory necrosis of the bone.44
Another possible reason for the failure in this case was the activation of a latent infection by surgical trauma, even if no pathology was observed during implant placement. However, in this case, it was highly unlikely to be the primary reason for the failure, as no apparent infection was noted in the area. Undiagnosed systemic illnesses can also affect local healing. In this case, the patient’s blood investigation results were normal; hence, systemic influence could not have been the reason for implant failure (Table 1). Contamination of the implants before placement can cause infection and implant failure. In this case, the patient had no signs of infection; therefore, there was a low possibility of implant contamination.
Although it was impossible to ascertain the true cause of the implant failure in this case, the most probable reason could be compression necrosis. A significant finding in this case was that there were no obvious signs of infection from the implant placement to implant failure. A study on early implant failure concluded that aseptic necrosis is the main cause of non-infectious trauma to the bone which can occur from either over compression or overheating.45 Yet another possible reason for the occurrence of compression necrosis in this case may be the undersized drilling technique employed for implant insertion.11 Implant insertion into the underprepared osteotomy site might have led to excessive compression of cortical bone leading to failure.
In the present case, histopathology revealed aseptic necrosis of the bone close to the implant, suggesting that compression of the surrounding bone may be the reason for implant failure. There were no signs of viable bone throughout the specimen and the lacunae were devoid of osteocytes (Figure 7). Over compression of the bone, ultimately resulting in bone necrosis, usually occurs during the early healing phase (within one month after implant placement). In this case, the implant failed within two months of insertion. Bone over compression can also occur if implants are placed in dense bone, such as type I or type II bone43 or D1 or D2 bone.11 The extreme density and reduced vascularity of the crestal cortical bone make it an ideal candidate for necrosis when compressed during implant placement. Implants placed with high torque can lead to over compression of the surrounding bone. A torque value of up to 35Ncm is considered safe for dense bones. Another contributing factor to early implant failure is the overheating of the bone which can initiate necrosis around the implant. Bone necrosis can occur when temperature reaches 47°C for ≥ one minute.46 However, a study conducted by Chacon et al showed that twist drills did not generate heat above 47°C for a depth of even 15 mm and after twenty-five uses, if the drill was designed to include a relief angle.47 Another cause for early implant failure is necrosis of bone graft placed around implant due to lack of adequate blood supply to the area.48
Various methods have been suggested to prevent implant failures due to over compression.37
These include:
- Precise surgical techniques.
- Adequate irrigation.
- Avoiding insertion of implants at torque values beyond manufacturer’s recommendation.
- Reversal of implant by a one-quarter turn after implant insertion minimizing the stress on the adjacent bone.
- Pre-tapping into dense bone prior to implant placement precludes the use of high torque values.
Conclusion
The advent of new implant systems has significantly increased the success rate of dental implants. However, failures can occur, which demand immediate implant removal. Many a times the reasons for this failure are unknown. This case report demonstrates an unusual early implant failure that might have occurred because of over compression of the surrounding bone. Using excessive torque values when placing implants into dense bones can increase the risk of implant failure owing to bone over compression. The thickness and quality of the crestal bone must be assessed in advance to preparation of implant osteotomy site for successful implantation. Adequate steps must be taken to follow the manufacturer’s instructions and to employ high-quality surgical techniques during implant placement into the dense cortical bone to minimise the risk of early implant failure.
Abbreviations
CBCT, Cone beam computed tomography; IOPAR, Intra oral periapical radiograph.
Consent for Publication
The authors certify that they obtained written informed consent from the patient before treatment. In the consent form, the patient was informed of the available treatment options. The patient provided written informed consent for publication of the case report. The patient understood that due efforts would be made to conceal her identity and that her name would not be published. Institutional approval was not required to publish the case details.
Funding
The study was conducted without external funding.
Disclosure
The authors declare that they have no competing interests for this work.
References
1. Zupancic Cepic L, Frank M, Reisinger A, Pahr D, Zechner W, Schedle A. Biomechanical finite element analysis of short-implant-supported, 3-unit, fixed CAD/CAM prostheses in the posterior mandible. Int J Implant Dent. 2022;8(1):8. doi:10.1186/s40729-022-00404-8
2. Horita S, Sugiura T, Yamamoto K, Murakami K, Imai Y, Kirita T. Biomechanical analysis of immediately loaded implants according to the all-on-four. Concept J Prosthodont Res. 2017;61(2):123–132. doi:10.1016/j.jpor.2016.08.002
3. Lung H, Hsu J-T, Wu AY-J, Huang H-L. Biomechanical effects of diameters of implant body and implant platform in bone strain around an immediately loaded dental implant with platform switching concept. Applied Sciences. 2019;9(10):1998.
4. Azcarate-Velázquez F, Castillo-Oyagüe R, Oliveros-López LG, et al. Influence of bone quality on the mechanical interaction between implant and bone: a finite element analysis. J Dent. 2019;88:103161. doi:10.1016/j.jdent.2019.06.008
5. Fernández-Olavarria A, Gutiérrez-Corrales A, González-Martín M, Torres-Lagares D, Torres-Carranza E, Serrera-Figallo M. Influence of different drilling protocols and bone density on the insertion torque of dental implants. Med Oral Patol Oral Cir Bucal. 2023;28(4):e385–e394. doi:10.4317/medoral.25804
6. Fanali S, Tumedei M, Pignatelli P, et al. Implant primary stability with an osteocondensation drilling protocol in different density polyurethane blocks. Comput Methods Biomech Biomed Engin. 2021;24(1):14–20. doi:10.1080/10255842.2020.1806251
7. Palaskar JN, Joshi N, Shah PM, Gullapalli P, Vinay V. Influence of different implant placement techniques to improve primary implant stability in low-density bone: a systematic review. J Indian Prosthodont Soc. 2020;20(1):11–16. doi:10.4103/jips.jips_244_18
8. Olmedo-Gaya MV, Romero-Olid MN, Ocaña-Peinado FM, et al. Influence of different surgical techniques on primary implant stability in the posterior maxilla: a randomized controlled clinical trial. Clin Oral Invest. 2023;27(7):3499–3508. doi:10.1007/s00784-023-04962-y
9. Tomasi C, Derks J. Etiology, occurrence, and consequences of implant loss. Periodontol. 2000;88(1):13–35. doi:10.1111/prd.12408
10. Ga K, Ge R. Biological mechanisms underlying complications related to implant site preparation. Periodontol. 2022;88(1):52–63. doi:10.1111/prd.12410
11. Stocchero M, Toia M, Cecchinato D, Becktor JP, Coelho PG, Biomechanical JR. Biologic, and clinical outcomes of undersized implant surgical preparation: a systematic review. Int J Oral Maxillofac Implants. 2016;31(6):1247–1263. doi:10.11607/jomi.5340
12. Huang HM, Chee TJ, Lew WZ, Feng SW. Modified surgical drilling protocols influence osseointegration performance and predict value of implant stability parameters during implant healing process. Clin Oral Investig. 2020;24(10):3445–3455. doi:10.1007/s00784-020-03215-6
13. Antonacci D, Del Fabbro M, Bollero P, Stocchero M, Jinno Y, Canullo L. Clinical effects of conventional and underprepared drilling preparation of the implant site based on bone density: a systematic review and meta-regression. J Prosthodont Res. 2023;67(1):23–34. doi:10.2186/jpr.JPR_D_21_00275
14. Zucchelli G, Wang HL, Chambrone L. Complications and treatment errors in periodontal and implant therapy. Periodontol. 2000;92(1):9–12. doi:10.1111/prd.12442
15. Lekholm U, Zarb GA, Albrektsson T. Patient Selection and Preparation. Tissue Integrated Prostheses. Chicago: Quintessence Publishing Co. Inc; 1985:199–209.
16. Chrcanovic BR, Albrektsson T, Wennerberg A. Bone quality and quantity and dental implant failure: a systematic review and meta-analysis. Int J Prosthodont. 2017;30(3):219–237. doi:10.11607/ijp.5142
17. Romanos G, Damouras M, Veis AA, Hess P, Schwarz F, Brandt S. Comparison of histomorphometry and microradiography of different implant designs to assess primary implant stability. Clin Implant Dent Relat Res. 2020;22(3):373–379. doi:10.1111/cid.12915
18. Silva GAF, Faot F, Possebon APDR, da Silva WJ, Del Bel Cury AA. Effect of macrogeometry and bone type on insertion torque, primary stability, surface topography damage and titanium release of dental implants during surgical insertion into artificial bone. J Mech Behav Biomed Mater. 2021;119:104515. doi:10.1016/j.jmbbm.2021.104515
19. Abrahamsson I, Carcuac O, Berglund T. Influence of implant geometry and osteotomy design on early bone healing: a pre-clinical in vivo study. Clin Oral Implants Res. 2021;32(10):1190–1199. doi:10.1111/clr.13816
20. Antonelli A, Barone S, Attanasio F, et al. Effect of implant macro-design and magnetodynamic surgical preparation on primary implant stability: an in vitro investigation. Dentistry J. 2023;11(10):227. doi:10.3390/dj11100227
21. Dayan C, Geckili O, Bural C. The influence of implant shape on primary stability of implants with a thread cutting and forming design: an ex vivo study. J Oral Implantol. 2019;45(3):181–185. doi:10.1563/aaid-joi-D-18-00158
22. Falco A, Berardini M, Trisi P. Correlation between implant geometry, implant surface, insertion torque, and primary stability: in vitro biomechanical analysis. Int J Oral Maxillofac Implants. 2018;33(4):824–830. doi:10.11607/jomi.6285
23. Gehrke SA, Cortellari GC, de Oliveira Fernandes GV, et al. Randomized clinical trial comparing insertion torque and implant stability of two different implant macrogeometries in the initial periods of osseointegration. Medicina. 2023;59(1):168. doi:10.3390/medicina59010168
24. Rajendra K, Manual L, Kochhar AS, Haseeb SA, Dubey D, Dang GS. Comparative evaluation of implant stability, insertion torque, and implant macrogeometry in different bone densities using resonance frequency analysis. J Contemp Dent Pract. 2021;22(6):665–668. doi:10.5005/jp-journals-10024-3118
25. Gandhi Y, Padhye N. Comparison of insertion torque, implant stability quotient and removal torque, in two different implant designs with and without osseodensification. - An ex vivo bench top study. J Oral Biol Craniofac Res. 2023;13(2):249–252. doi:10.1016/j.jobcr.2023.02.004
26. Cehreli MC, Karasoy D, Akea K, Eckert SE. Meta-analysis of methods used to assess implant stability. Int J Oral Maxillofac Implants. 2009;24:1015–1032.
27. Rea M, Botticelli D, Ricci S, Soldini C, González GG, Lang NP. Influence of immediate loading on healing of implants installed with different insertion torques – an experimental study in dogs. Clin Oral Implants Res. 2015;26(1):90–95. doi:10.1111/clr.12305
28. Do Vale Souza JP, de Moraes Melo Neto CL, Piacenza LT, et al. Relation between insertion torque and implant stability quotient: a clinical study. Eur J Dent. 2021;15(4):618–623. doi:10.1055/s-0041-1725575
29. Ottoni JM, Oliveira ZF, Mansini R, Cabral AM. Correlation between placement torque and survival of single-tooth implants. Int J Oral Maxillofac Implants. 2005;20(5):769–776.
30. Trisi P, Perfetti G, Baldoni E, Berardi D, Colagiovanni M, Scogna G. Implant micromotion is related to peak insertion torque and bone density. Clin Oral Implants Res. 2009;20(5):467–471. doi:10.1111/j.1600-0501.2008.01679.x
31. Consolo U, Travaglini D, Todisco M, Trisi P, Galli S. Histologic and biomechanical evaluation of the effects of implant insertion torque on peri-implant bone healing. J Craniofac Surg. 2013;24(3):860–865. doi:10.1097/SCS.0b013e31827ca3cf
32. Khayat PG, Arnal HM, Tourbah BI, Sennerby L. Clinical outcome of dental implants placed with high insertion torques (up to 176 Ncm). Clin Implant Dent Relat Res. 2013;15(2):227–233. doi:10.1111/j.1708-8208.2011.00351.x
33. Winwood K, Zioupos P, Currey JD, Cotton JR, Taylor M. The importance of the elastic and plastic components of strain in tensile and compressive fatigue of human cortical bone in relation to orthopaedic biomechanics. J Musculoskelet Neuronal Interact. 2006;6(2):134–141.
34. Haider R, Watzek G, Plenk H. Histomorphometric analysis of bone healing after insertion of IMZ-1 implants independent of bone structure and drilling method (in German). Z Stomatol. 1991;88:507–521.
35. Berglundh T, Armitage G, Araujo MG, et al. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Clin Periodontol. 2018;45(Suppl 20):S286–S291. doi:10.1111/jcpe.12957
36. Schwarz F, John G, Schmucker A, Sahm N, Becker J. Combined surgical therapy of advanced peri-implantitis evaluating two methods of surface decontamination: a 7-year follow-up observation. J Clin Periodontol. 2017;44(3):337–342. doi:10.1111/jcpe.12648
37. Bashutski JD, Nj D, Wang HL. Implant compression necrosis: current understanding and case report. J Periodontol. 2009;80(4):700–704. doi:10.1902/jop.2009.080581
38. Staedt H, Rossa M, Lehmann KM, Al-Nawas B, Kämmerer PW, Heimes D. Potential risk factors for early and late dental implant failure: a retrospective clinical study on 9080 implants. Int J Implant Dent. 2020;6(1):81. doi:10.1186/s40729-020-00276-w
39. Raj R, Manju V, Kumar-Gopal V, Eswar M. Analysis of factors determining thermal changes at osteotomy site in dental implant placement - An in-vitro study. J Clin Exp Dent. 2021;13(3):e234–e239. doi:10.4317/jced.57346
40. Dhok K, Adhikari M, Palange A, Dhatrak P. Heat generation during implant site preparation and its effects on osseointegration: a review. Mater Today. 2023;72(3):1035–1040.
41. Duyck J, Corpas L, Vermeiren S, et al. Histological, histomorphometrical, and radiological evaluation of an experimental implant design with a high insertion torque. Clin Oral Implants Res. 2010;21(8):877–884. doi:10.1111/j.1600-0501.2010.01895.x
42. Cha JY, Pereira MD, Smith AA, et al. Multiscale analyses of the bone-implant interface. J Dent Res. 2015;94(3):482–490. doi:10.1177/0022034514566029
43. Toia M, Stocchero M, Cecchinato F, Corrà E, Jimbo R, Cecchinato D. Clinical considerations of adapted drilling protocol by bone quality perception. Int J Oral Maxillofac Implants. 2017;32(6):1288–1295. doi:10.11607/jomi.5881
44. Misch CE. Density of bone: effect on surgical approach and healing. In: Misch CE, editor. Contemporary Implant Dentistry. St Louis: C,V.: Mosby; 1999:372–383.
45. Piatelli A, Scarano A, Balleri P, Favero GA. Clinical and histological evaluation of an active “implant periapical lesion”: a case report. Int J Oral Maxillofac Implants. 1998;13:713–716.
46. Eriksson AR, Albrektsson T, Albrektsson B. Heat caused by drilling cortical bone. Temperature measured in vivo in patients and animals. Acta Orthop Scand. 1984;55(6):629–631. doi:10.3109/17453678408992410
47. Chacon GE, Bower DL, Larsen PE, McGlumphy EA, Beck FM. Heat production by 3 implant drill systems after repeated drilling and sterilization. J Oral Maxillofac Surg. 2006;64(2):265–269. doi:10.1016/j.joms.2005.10.011
48. Sanz-Sanchez I, Sanz-Martin I, Ortiz-Vigon A, Molina A, Sanz M. Complications in bone grafting procedures: classification and management. Periodontol. 2000;88:86–102. doi:10.1111/prd.12413
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

