Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 15
Complex Case of Tuberculosis Lymphadenitis with Concurrent Takayasu Arteritis in a 14-Year-Old Girl from Ethiopia
Authors Tadesse YG , Mulisa MD , Beyene ET, Adugna BA
Received 28 September 2023
Accepted for publication 2 December 2023
Published 8 December 2023 Volume 2023:15 Pages 231—236
DOI https://doi.org/10.2147/OARRR.S438427
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Chuan-Ju Liu
Yared Getachew Tadesse,1 Merga Daba Mulisa,1 Eden Tesfaye Beyene,1 Becky Abdissa Adugna2
1Department of Internal Medicine, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia; 2Department of Internal Medicine, Rheumatology Unit, College of Health Sciences Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Yared Getachew Tadesse, King George VI Street, Arada, Addis Ababa, 33638, Ethiopia, Tel +251929436214, Email [email protected]
Abstract: Takayasu arteritis (TA) is a large vessel arteritis that predominantly affects the aorta and its major branches. Its association with tuberculosis (TB) has been described in the literature. This association poses a diagnostic and therapeutic challenge, especially in TB-endemic areas. We report a case of a young Ethiopian female patient who was diagnosed with TA associated with TB. We discuss the diagnostic and therapeutic challenges of this association.
Keywords: takayasu arteritis, tuberculosis, tuberculosis lymphadenitis
Introduction
Takayasu arteritis (TA) is a large vessel arteritis of unknown etiology. The aorta and its major branches are the primary affected vessels. It mainly affects young females with a peak age of onset between 20 and 30 years.1–3 Although it has a worldwide distribution, it is much more common in the Asian population, with the highest prevalence in Japan.3 The early systemic phase of TA is characterized by nonspecific symptoms, which include fever, weight loss, and fatigue, and in the late occlusive phase, inflammatory and obliterative changes of the great vessels happen, leading to absent peripheral pulses, limb claudication, hypertension with blood pressure discrepancies between extremities and angina.4
Tuberculosis (TB) is a treatable disease caused by Mycobacterium tuberculosis and is transmitted via inhalation of droplets.5 According to the World Health Organization (WHO), more than 1.7 billion people are estimated to be infected with TB; most have latent infection, which can be reactivated with immunosuppression.5 Even though TB primarily affects the lungs, it can affect any organ system in the body.
TB is one of the challenging differential diagnoses in patients with TA, especially in TB-endemic areas. This is due to the fact that early-phase TA and TB tend to share similar constitutional and other clinical manifestations. A recent study also suggested that more patients with a history of TB were in the active stage of TA further suggesting that TB infection may have an impact on the course and efficacy of TA treatment.6 Furthermore, TA and tuberculous arteritis exhibit similar pathologic and radiologic characteristics, where granulomas and caseous necrosis are hallmarks of both,7 and likewise, imaging techniques are not reliable tools for differentiation.
Clinical management decisions are challenging in circumstances of uncertainty because erroneous immunosuppressive drug administration may negatively impact the course of tuberculosis,8 while a recent literature review suggested that patients with TA had no greater risk of developing TB than individuals with other rheumatic diseases when they are exposed to TNF inhibitors9 indicating a management challenge commonly faced in these areas.
Here, we report a 14-year-old female patient diagnosed with Takayasu arteritis associated with tuberculosis.
Case Presentation
A 14-year-old female presented with worsening easy fatigability and shortness of breath at rest of 01-week duration. She also noted progressive exertional dyspnea 08 months prior to presentation associated with orthopnea of 2 pillows and bilateral lower leg swelling.
Furthermore, she reported difficulty in combing her hair and intermittent claudication of her arms while doing daily activities for the last 01 year. In the same period, she has been experiencing cough productive of whitish sputum, drenching night sweats, and a significant unquantified amount of weight loss.
Otherwise, she had no history of visual disturbances, headaches, or body or facial weaknesses. There was no chest pain, nausea, vomiting, or medication use. She also denies of any rash, photosensitivity ulcers, history of hypertension, previous Tuberculosis treatment history, self or family history of similar illness.
Physical Examination
On general examination, she was chronically sick-looking. There was a solitary, non-tender, firm lymph node in the left axillary fossa measuring 2×3 centimeters. The carotid, brachial, and radial pulses were symmetrically palpated with full volume without bruit. The popliteal, tibialis posterior and dorsal pedis pulses were absent on palpation bilaterally. Average blood pressure recordings in the right brachial/left brachial/right popliteal and left popliteal were 100/75, 105/68, 130/70 and 132/73, respectively. Cardiac examination demonstrated an active precordium, a point of maximal impulse displaced to the sixth intercostal space, and a systolic murmur at the left sternal border. Abdominal examination revealed no abnormal findings.
Laboratory Tests
Laboratory findings showed normal white blood cells with predominant lymphocytosis, increased erythrocyte sedimentation rate (100mm), C-reactive protein (14mg/dl), and anemia. Liver and renal function tests were within the normal ranges, while HIV and Hepatitis C antibody tests were negative.
Imaging Tests
Chest CT showed a small calcific nodule in the left lower lobe, which suggests a calcific granuloma of a latent tuberculosis. Echocardiography showed a dilated cardiomyopathy with biventricular systolic dysfunction (LV ejection fraction ~15–20%) with mild pulmonary hypertension and moderate secondary mitral regurgitation. Renal Doppler ultrasound demonstrated an echogenic filling defect in the suprarenal abdominal aorta segment with marked luminal expansion and >70% luminal occlusion.
Computed angiography of the thoracic and abdominal aorta (Figures 1 and 2) showed diffuse and irregular narrowing of the distal descending thoracic and proximal abdominal aorta with partially thrombosed aneurysm of the proximal abdominal aorta caudal to the superior mesenteric artery. Also, there was a severe stenosis of the left renal artery, and the findings suggest Takayasu arteritis.
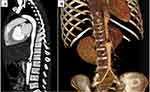 |
Figure 1 Sagittal (A) and 3D volume rendered (B) chest and abdominal CT angiography showing diffuse and irregular abdominal aortic wall thickening and luminal narrowing. |
Histopathology
Fine needle aspiration of the enlarged lymph node showed mainly caseous necrosis and vague granuloma in a reactive lymphoid background. The specimen was stained with Ziehl-Neelsen, and acid-fast bacteria was detected confirming tuberculos lymphadenitis. Soon after, active (ITAS score 8 points) Numano subtype 3 Takayasu arteritis associated with Tuberculosis lymphadenitis was diagnosed. Prednisolone 35 mg PO daily (1mg/kg/day) was used as an induction therapy without the use of other immunosuppressants (azathioprine or anti-TNF therapies). Concomitantly, anti-tuberculous drugs (Isoniazid, Rifampicin, Pyrazinamide, and Ethambutol) were initiated per local protocol. She was also treated with guideline-directed medical therapy for HFrEF (Heart failure with reduced ejection fraction). With these management, she significantly improved her constitutional and heart failure symptoms. After one month, she was seen at our Rheumatology clinic for follow-up and had an inactive TA (ITAS score 1) with improvements in constitutional symptoms and a 3 kg weight gain.
Discussion
TA is an uncommon large vessel granulomatous vasculitis.1 Through time, it brings about changes and narrowing of the vascular wall. Early TA mimics TB, and patients present with nonspecific symptoms. TB is a significant public health concern in Ethiopia, with an incidence of 119 per 100,000 population in 2021, making Ethiopia among the top 30 countries with high TB burden countries in the world.6
A study done on 1105 Chinese patients with TA showed that 9.9% had TB infection, the majority (48.6%) were diagnosed with TB before having symptoms of TA, and 21.1% had TA and TB concurrently.10 Our patient was diagnosed with both TA and TB at the same time.
Most patients having TA and TB concurrently have fever, dyspnea, and blood pressure discrepancies, as was the case in our patient. However, initial occlusive symptoms are uncommon,11,12 unlike our patient, who reported symptoms of upper limb claudication. Acute phase reactants were much higher in patients with both TA and TB at presentation than those with isolated TA,10–12 which was also the case in our patient with an Erythrocyte sedimentation rate of 100mm/hr.
A study of 80 patients with TA concluded that patients with concurrent TA and TB tend to be older, have longer disease duration, and do not have occlusive symptoms with TA being diagnosed incidentally,13 which was not the case in our patient who is young, had occlusive symptoms and relatively shorter disease duration.
Lungs are the commonly involved sites of TB infection in TA,6 and TB is diagnosed with Mantoux or Quantiferon tb gold tests in such patients. A case series of 18 TA patients showed that 57.9% had TB lymphadenitis, with biopsy showing caseating granuloma.14
Since the discovery of Langhans giant cells in the arterial samples from TA patients, which shared morphological similarities with TB granulomas, by Shimizu and Sano in 1948, there has long been a suggestion of an association between latent or active TB and TA.15 In the aftermath, there have been multiple case reports of active TB in patients with TA where majority had pulmonary involvement with variable extrapulmonary involvement of TB.16
Proposed evidences of linkage between TA and TB includes the consistent demonstration of humoral and cellular immune responses against cell stress proteins like the mycobacterial heat shock protein (HSP) in TA patients,17 larger frequency of delayed hypersensitivity to tuberculin in TA patients compared to their counterparts without TA18 and the detection of mycobacterial genetic sequences like IS1610 and HupB in 70% of aortic tissue specimens from autopsies of patients with TA.19
However, recent studies begun challenging physiopathogenic relationship between TA and TB, arguing that the relationship is merely epiphenomenal. It has been discussed that routine BCG vaccination early in life could explain the delayed hypersensitivity to tuberculin seen in TA patients while research employing molecular methods and even directly demonstrating M. tuberculosis in the aortic tissues of TA patients indicate to a lack of pathogenic association between both entities.20,21
While tumor necrosis factor (TNF) inhibition and disruption of formed granulomas with subsequent release of viable mycobacteria partly explain the increased risk of TB in TA patients, a review that encompassed 214 patients reported only 2 cases of active TB that were treated with infliximab.9,22,23
This finding, along with similar results seen in patients with other rheumatic diseases exposed to TNF inhibitors further affirms the absence of TB reactivation with TNF inhibitors as possibly being the proof-of-concept to confirm that the association between TA and TB as being a pure epiphenomenal matter and not a causal one.9,24
Nonetheless, a novel pathogenic model predicated on the loss of self-tolerance to stress-induced cell molecules and an inflammatory response perpetuated by the innate immune system have been proposed to unify laboratory evidence with epidemiological and clinical information, while providing rationality for a judicious use of TNF inhibitors in TA.9
Our patient did not have lung involvement, but fine needle aspiration cytology from the cervical Lymph node showed caseous necrosis and granuloma. Favorable outcomes are seen when patients with concurrent TA and TB are treated with Anti TB first, followed by immunosuppressive. [17] Our patient was started on Anti TB and prednisolone simultaneously, and she had significant improvement.
Conclusion
In conclusion, this case illustrates the difficulty in differentiating between coincidental diagnosis of TB and TA versus causal relationship. Furthermore, concomitant diagnoses of TA and tuberculosis have both diagnostic and therapeutic implications. Although our patient did not experience worsening of tuberculosis symptoms with high dose glucocorticoid therapy, there exists a risk of reactivation of latent TB and worsening of active TB cases with the use of such therapies. Hence, treating physicians should be weary of underlying/associated tuberculosis while caring for TA patients with constitutional symptoms and increased levels of markers of inflammation, especially in tuberculosis-endemic areas.
Limitation of the Paper
Our paper lacks to conclusively discern between causal or causal link of Tuberculosis and Takayasu arteritis in this patient.
Data Sharing Statement
The laboratory investigations, patient physical findings, and imaging results used to support the findings of this study are included in the article.
Ethical approval and consent to participate
The authors’ institution does not require ethical approval for the publication of a single case report.
Consent for Publication
Written informed consent was obtained from the patient’s parent for publication of this case report and any accompanying diagnostic images.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lupi-Herrera E, Sánchez-Torres G, Marcushamer J, Mispireta J, Horwitz S, Vela JE Takayasu’s arteritis. Clinical study of 107 cases. Am Heart J. 1977;93(1):94–103. doi:10.1016/S0002-8703(77)80178-6
2. Arend WP, Michel BA, Bloch DA, et al. The American college of rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum. 1990;33(8):1129–1134. doi:10.1002/art.1780330811
3. Epidemiology of Takayasu arteritis – pubMed [Internet]; [cited November 9, 2023]. Available from: https://pubmed.ncbi.nlm.nih.gov/28756072/.
4. Updates in pathophysiology, diagnosis and management of Takayasu arteritis - pubmed[internet]; [cited November 9, 2023]. Available from: https://pubmed.ncbi.nlm.nih.gov/27238990/.
5. Tuberculosis[Internet]; [cited November 9, 2023]. Available from: https://www.who.int/health-topics/tuberculosis.
6. Zhang Y, Fan P, Luo F, et al. Tuberculosis in Takayasu arteritis: a retrospective study in 1105 Chinese patients. J Geriatr Cardiol JGC. 2019;16(8):648–655. doi:10.11909/j.issn.1671-5411.2019.08.003
7. Ishikawa K. Diagnostic approach and proposed criteria for the clinical diagnosis of Takayasu’s arteriopathy. J Am Coll Cardiol. 1988;12(4):964–972. doi:10.1016/0735-1097(88)90462-7
8. Moura C, Aquino MA, Filho JR, Santiago M. Takayasu’s or tuberculous arteritis? Case Rep. 2015;2015:bcr2014208717.
9. Castillo-Martínez D, Amezcua-Castillo LM, Granados J, Pineda C, Amezcua-Guerra LM. Is Takayasu arteritis the result of a Mycobacterium tuberculosis infection? The use of TNF inhibitors may be the proof-of-concept to demonstrate that this association is epiphenomenal. Clin Rheumatol. 2020;39(6):2003–2009. doi:10.1007/s10067-020-05045-z
10. Reshkova V, Kalinova D, Rashkov R. Takayasu’s arteritis associated with tuberculosis infections. J Neurol Neurosci. 2016;7(3):114
11. Tian Y, Chen Y. Tuberculosis and Takayasu arteritis: a case report. J Med Case Rep. 2023;doi:10.1186/s13256-023-04037-2
12. Aggarwal A, Chag M, Sinha N, Naik S. Takayasu’s arteritis: role of mycobacterium tuberculosis and its 65 kDa heat shock protein. Int J Cardiol. 1996;55(1):49–55. doi:10.1016/0167-5273(96)02660-5
13. Zhou J, Ji R, Zhu R, et al. Clinical features and risk factors for active tuberculosis in Takayasu arteritis: a single-center case-control study. Front Immunol. 2021;12: doi:10.3389/fimmu.2021.749317
14. Jansson MK, Geerdes-Fenge HF, Kangowski A, Kneitz C, Reisinger EC. Tuberculosis and Takayasu arteritis: case-based review. Rheumatol Int. 2019;39(2):345–351. doi:10.1007/s00296-018-4231-x
15. Sugiyama K, Ijiri S, Tagawa S, Shimizu K. Takayasu disease on the centenary of its discovery. Jpn J Ophthalmol. 2009;53(2):81–91. doi:10.1007/s10384-009-0650-2
16. Takayasu’s Arteritis and Its Potential Pathogenic Association with Mycobacterium tuberculosis. Intechopen [Internet]; [cited November 6, 2023]. Available from: https://www.intechopen.com/chapters/21616.
17. Raised agalactosyl IgG and antimycobacterial humoral immunity in Takayasu’s arteritis – PubMed [Internet]; [cited November 6, 2023]. Available from: https://pubmed.ncbi.nlm.nih.gov/7837153/.
18. Takayasu’s arteritis in children - PubMed[Internet]; [cited November 6, 2023]. Available from: https://pubmed.ncbi.nlm.nih.gov/1681102/.
19. Detection of IS6110 and HupB gene sequences of Mycobacterium tuberculosis and bovisin the aortic tissue of patients with TAKAYASU’s arteritis. BMC Infectious Diseases. Full Text Internet; November 6, 2023. Available from: https://bmcinfectdis.biomedcentral.com/articles/10.1186/1471-2334-12-194.
20. Lalvani A. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. CHEST. 2007;131(6):1898–1906. doi: 10.1378/chest.06-2471
21. Carvalho ES, de Souza AWS, Leão SC, et al. Absence of mycobacterial DNA in peripheral blood and artery specimens in patients with Takayasu arteritis. Clin Rheumatol. 2017;36(1):205–208. doi:10.1007/s10067-016-3400-0
22. The risk of tuberculosis related to tumour necrosis factor antagonist therapies: a TBNET consensus statement | European Respiratory Society[Internet]; [cited November 6, 2023]. Available from: https://erj.ersjournals.com/content/36/5/1185.long.
23. Handa R, Upadhyaya S, Kapoor S, et.al. Tuberculosis and biologics in rheumatology: a special situation - handa - 2017. Int J Rheum Dis. 2023; doi:10.1111/1756-185X.13129
24. Incidence of tuberculosis in patients receiving anti-TNF therapy for rheumatic diseases: a systematic review. SpringerLink [Internet]; [cited November 6, 2023]. Available from: https://link.springer.com/article/10.1007/s10067-019-04866-x.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

