Back to Journals » Infection and Drug Resistance » Volume 14
Comparison of Bleeding Risk Between Colistin–Tigecycline and Colistin–Carbapenem Treatment Regimens: A Retrospective Cohort Study
Authors Huang YT, Yu CI, Chen PY, Wang CC , Wu CC
Received 15 September 2021
Accepted for publication 11 November 2021
Published 25 November 2021 Volume 2021:14 Pages 4949—4955
DOI https://doi.org/10.2147/IDR.S339188
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Yu-Ting Huang,1 Chia-I Yu,2 Pao-Yu Chen,3,4 Chi-Chuan Wang,1,2,5 Chien-Chih Wu1,2
1Department of Pharmacy, National Taiwan University Hospital, College of Medicine, National Taiwan University, Taipei, Taiwan; 2School of Pharmacy, College of Medicine, National Taiwan University, Taipei, Taiwan; 3Department of Internal Medicine, National Taiwan University Hospital, College of Medicine, National Taiwan University, Taipei, Taiwan; 4Graduate Institute of Clinical Medicine, College of Medicine, National Taiwan University, Taipei, Taiwan; 5Graduate Institute of Clinical Pharmacy, College of Medicine, National Taiwan University, Taipei, Taiwan
Correspondence: Chien-Chih Wu
Department of Pharmacy, National Taiwan University Hospital, College of Medicine, National Taiwan University, 7 Chung Shan S. Road, Taipei, Taiwan
Tel/Fax +886-2-23123456 ext. 63702/ +886-2-23310930
Email [email protected]
Background: Antibiotic combination is commonly used to treat multidrug-resistant pathogens. Reports have indicated that tigecycline use is associated with hypofibrinogenemia. However, whether the bleeding risk of tigecycline is higher than that of other antibiotics remains unknown. The aim of this study was to compare the bleeding risk between colistin–tigecycline and colistin–carbapenem treatment.
Methods: This retrospective cohort study enrolled adult patients treated with colistin along with tigecycline or carbapenems (doripenem, imipenem–cilastatin, or meropenem) for ˃72 hours during hospitalization. The primary outcome was major bleeding events, which were determined by a hemoglobin drop of ≥ 2 g/d and receipt of blood transfusions with whole blood or packed red blood cells. Multivariate logistic regression was applied to determine risk factors for bleeding events.
Results: In total, 106 and 268 patients in the colistin–tigecycline and colistin–carbapenem groups met the criteria for analysis, respectively. The two groups did not differ significantly in demographic data, except for alanine aminotransferase (ALT), serum creatinine (SCr) and ulcer disease. The colistin–tigecycline group had a higher ALT, SCr and a lower proportion of ulcer disease. Major bleeding events did not differ significantly between the colistin–tigecycline and colistin–carbapenem groups (12.26% vs 9.33%, P = 0.40). Antibiotic duration [OR = 1.06 (1.02– 1.11), P=0.007)] and anticoagulant use [OR = 2.16 (1.05– 4.42), P=0.04] were associated with major bleeding events.
Conclusion: Colistin–tigecycline treatment was not associated with a higher bleeding risk. Antibiotic duration and concurrent use of anticoagulant were the risk factors of bleeding events.
Keywords: tigecycline, carbapenems, bleeding events
Introduction
Multidrug-resistant organisms (MDROs) such as multidrug-resistant Acinetobacter baumannii (MDRAB), Extended-Spectrum β-lactamase Producing Enterobacterales, carbapenem-resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with difficult to treat resistance pose a major threat in hospitals worldwide.1–5 Although new antibiotic have been developed for these MDROs, it is still not available in many countries.1 Concurrent use of antibiotics with different target site could enhance the bacterial killing effect and is a common strategy for MDRO treatment.1,6 For example, colistin–carbapenem and colistin–tigecycline has been demonstrated to have synergic effect on MDRAB and CRE,7–9 and meta-analysis study showed antibiotic combination therapy was significantly associated with better survival.10
Tigecycline is a glycylcycline antibiotic that binds to the 30S ribosomal subunit and inhibits protein synthesis in bacteria.11 It has a broad spectrum of activity against aerobic and anaerobic pathogens including multidrug-resistant bacteria such as MDRAB, methicillin-resistant Staphylococcus aureus,vancomycin-resistant Enterococcus faecium, and Mycobacterium species.12 However, it is not active against Proteus mirabilis and Pseudomonas aeruginosa.13 Because of its broad antibacterial spectrum, it is commonly used to treat community-acquired pneumonia, complicated skin infections, and intra-abdominal infections.14 The most common adverse drug reactions (ADRs) of tigecycline are nausea, vomiting, and diarrhea.12 Tigecycline-associated hypofibrinogenemia was overlooked by clinicians until the first case report in 2010.15 Tigecycline use could reduce fibrinogen levels significantly, although the mechanism underlying this reduction is unknown.16–19 The occurrence rate of tigecycline-associated hypofibrinogenemia is approximately 50%, and the risk factors are age, baseline fibrinogen level, intra-abdominal infection, tigecycline dose, treatment duration, and renal insufficiency.17–20 Only one randomized controlled study was conducted to compare the influence on fibrinogen level between tigecycline and imipenem-cilastatin, and it reported that tigecycline was associated with a significant decrease in fibrinogen levels since day 3 of treatment.21 Hypofibrinogenemia usually resolve within 1 week after the discontinuation of tigecycline use.17,22 Fibrinogen, the precursor of fibrin, is an essential component of the coagulation system for stopping bleeding.23 Although hypofibrinogenemia may increase the risk of bleeding,19 whether tigecycline use increases bleeding events compared with other antibiotics such as carbapenem, which is also a broad-spectrum antibiotic commonly used for treating MDRO-induced infections, remains unknown. The aim of this study was to explore the bleeding risk between colistin–tigecycline and colistin–carbapenem combination therapy.
Methods
This retrospective cohort study was conducted at the National Taiwan University Hospital (NTUH), a 2600-bed medical center in Taiwan. All data were retrieved from electronic health records from January 1, 2006, to December 31, 2018, from the NTUH Integrated Medical Database (NTUH-iMD). Adult patients (>20 years) treated with colistin along with tigecycline or carbapenems (doripenem, imipenem–cilastatin, or meropenem) for ˃72 hours during hospitalization were screened for inclusion. Only the first combination therapy during hospitalization was assessed. Index date was defined as the first day of antibiotic combination. Patients who had coagulopathy (INR > 1.5, aPTT > 60 s, or fibrinogen < 200 mg/L), preexisting bleeding [hemoglobin (Hb) drop of ≧ 2 g/dL or receipt of whole blood or packed red blood cell (pRBC) transfusions of ≧ 2 units within 7 days before the index date], and re-admission within 30 days were excluded. This study was registered and approved by the Research Ethics Committee of NTUH (202005025RINB). Informed consent was waived for this study by the Research Ethics Committee of NTUH because patient identification in the database was removed. This study was conducted in accordance with the Declaration of Helsinki.
The primary outcome was major bleeding events between the colistin–tigecycline and colistin–carbapenem groups. Major bleeding events were determined by Hb drop of ≥ 2 g/dL and receipt of blood transfusions with whole blood or pRBCs, which referred to the criteria made by International Society of Thrombosis and Hemostasis (ISTH).24 Minor bleeding events were also explored. Minor bleeding events were defined as receipt of a transfusion [platelet (PLT), cryoprecipitate, or fresh frozen plasma (FFP)] or new prescription of tranexamic acid for ˃3 days.25 Because tigecycline-associated hypofibrinogenemia usually resolves within 1 week after the discontinuation of tigecycline, bleeding events were followed up for 1 week after the discontinuation of the antibiotic combination.17,26 Demographic data and associated clinical characteristics such as age, sex, total bilirubin (T-bil), alanine aminotransaminase (ALT), serum creatinine (SCr), Hb, PLT, albumin, concomitant medications (anticoagulation, antiplatelet, nonsteroidal anti-inflammatory drugs, and glucocorticosteroids), shock, and comorbidities were extracted from electronic medical records. Shock was defined as the presence of vasopressors or inotropic agents. Comorbidities, particularly those associated with increased bleeding risk, were selected. Clinical Classifications Software (Healthcare Cost and Utilization Project, https://www.hcup-us.ahrq.gov/toolssoftware/ccs) was used to identify International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification (ICD-9-CM, ICD-10-CM) codes for comorbidities of interest. These covariates were collected during the period of combination treatment.
Pearson’s chi-square test or Fisher’s exact test was employed to analyze categorical variables, and Student’s t-test or the Wilcoxon rank sum test was used for continuous variables. A multivariate logistic regression model was constructed to identify the predictors of major bleeding events by using variables for which the P value was <0.1 in the univariate analysis. Sensitivity analysis was conducted by shortening the follow-up period for bleeding events to the date of combination therapy discontinuation. Statistical results were considered significant using a two-tailed P value of <0.05 and 95% CI. All statistical analyses were performed using SAS (version 9.4) software (SAS Institute, Cary, NC, USA).
Results
During the study period, 1390 patients treated with colistin–tigecycline and colistin–carbapenem combination therapy were screened for inclusion, and 106 and 268 patients in the colistin–tigecycline and colistin–carbapenem groups met the criteria for analysis, respectively (Figure 1). The two groups did not differ significantly in demographic data, except for ALT, SCr and ulcer disease. The colistin–tigecycline group had a higher ALT (51 ± 85 vs 34 ± 29 U/L, P =0.01), worse Scr (1.69 ± 1.62 vs 1.41 ±1.44 mg/dL, P =0.03) and a lower proportion of ulcer disease (1.89% vs 8.96%, P = 0.02; Table 1). The rates of major bleeding events did not differ significantly between the colistin–tigecycline and colistin–carbapenem groups (12.26% vs 9.33%, P = 0.40). Receiving FFP, cryoprecipitate, or PLT transfusion and intravenous tranexamic acid also did not exist significant difference (Table 2). Hypertension, antibiotic duration, anticoagulant use, and gastrointestinal bleeding met the enrollment criteria according to the multivariate logistical regression model. Antibiotic duration [OR = 1.06 (1.02–1.11), P=0.007)] and anticoagulant use [OR = 2.16 (1.05–4.42), P=0.04] were associated with increasing risk of major bleeding events (Table 3). In the sensitivity analysis, we observed no difference in major bleeding events between the colistin–tigecycline and colistin–carbapenem groups (8.49% vs 5.97%, P = 0.38).
 |
Table 1 Patient Demographic and Clinical Characteristics |
 |
Table 2 Major and Minor Bleeding Events of Colistin–Tigecycline and Colistin–Carbapenem Treatment |
 |
Table 3 Multivariate Logistic Regression Analysis with Major Bleeding Events as Dependent Variable |
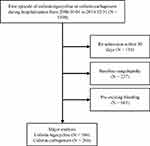 |
Figure 1 Flowchart of patient selection. |
Discussion
According to our review of the literature, this study is the first to compare bleeding risks between colistin-tigecycline and colistin-carbapenem use. The results indicate that patients who received colistin–tigecycline treatment did not have a significantly higher bleeding risk than did those who received colistin–carbapenem treatment. Tigecycline is a broad-spectrum antibiotic that is active against multidrug-resistant pathogens. The most common adverse effects of tigecycline use are nausea, vomiting, and diarrhea.13 Hepatotoxicity and coagulopathy are also mentioned in the drug’s package insert. However, tigecycline-associated hypofibrinogenemia was not established until 2010. Pieringer et al reported the first case of severe coagulation disorder with hypofibrinogenemia from tigecycline use, and more cases have since been reported.15,26 All of the case reports have indicated that the fibrinogen level recovered within 7 days after the discontinuation of tigecycline use.26 A few retrospective studies have since explored the risk factors for tigecycline-associated hypofibrinogenemia.16–20 These risk factors include age, treatment dose, treatment duration, baseline fibrinogen level, intra-abdominal infection, and renal failure.16–20 Hakeam et al conducted a randomized controlled trial to compare the impact of tigecycline on fibrinogen level with imipenem-cilastatin, and demonstrated that tigecycline was associated with a significant decrease in fibrinogen since day 3 of treatment. Therefore, tigecycline use may increase bleeding risk compared to carbapenems during MDRO treatment due to its effect on fibrinogen.
The mechanism underlying tigecycline-associated hypofibrinogenemia remains unknown. Fibrinogen is produced by hepatocytes, and it could be converted to insoluble fibrin to form blood clots if the patient has encountered trauma or a diagnosis of sepsis.27 An in vitro study demonstrated a rapid loss of mitochondrial activity in hepatic cells with supra-therapeutic tigecycline doses; this loss could decrease fibrinogen production.28 Fibrinogen is also considered an acute phase protein, and its production is regulated by cytokines. For example, interleukin (IL)-6 enhances the biosynthesis of fibrinogen, and tigecycline has been demonstrated to reduce the levels of IL-6, which may interfere with the production of fibrinogen.29 Fibrinogen is an essential component of the coagulation system for stopping bleeding,23 and lower level of fibrinogen may increase the risk of bleeding.19,30 Campany‑Herrero et al found that 5 of 12 patients with tigecycline-associated hypofibrinogenemia had bleeding events and that 4 of them received blood transfusions.20 In Zhang et al’s study, patients with hypofibrinogenemia had more bleeding events compared to those with normal fibrinogen level although it did not achieve clinical significance (12.09% vs 8.06%, P = 0.17).18 Similar finding was also reported by Hu et al that severe bleeding was more common in patients with tigecycline associated hypofibrinogenemia (11.3% vs 3.6%, P = 0.13).17 Liu et al reported that bleeding risk was higher in patients with hypofibrinogenemia (13.3% vs 1.7%, P = 0.01), and hypofibrinogenemia was independently associated with bleeding [OR 8.96 (1.132–70.946, p = 0.038)].19 However, the definition of bleeding events was not mentioned in these studies, and this lack created a non-robust conclusion. In this study, we clearly defined the major bleeding events by ISTH criteria, and did not demonstrate a significantly higher bleeding risk in the tigecycline-based regimen. However, major bleeding events were numerically higher in the colistin–tigecycline group. This discrepancy could be attributed to the relatively small sample size of our study that prevented the necessary power to detect the difference. Based on the result of our study, we calculated that 3644 patients would need to be enrolled to provide 80% power to reject the null hypothesis of no difference between the groups in bleeding risk, at a two-sided alpha level of 0.05. Additional studies are warranted to confirm our results.
This study has several limitations. First, because of its retrospective nature, selection bias and confounding factors may still exist. Second, we did not include fibrinogen level in our analysis. During our study period, fibrinogen level was not routinely followed up in our institution, and only six and two patients had pretreatment and post-treatment fibrinogen levels followed up, respectively. Although two patients in the colistin–tigecycline group had considerable decreases in fibrinogen levels, further analysis was not possible because of missing data. Our study was an exploratory study comparing the bleeding risk of a tigecycline-based regimen with that of other regimens. Additional studies that analyze fibrinogen levels and have a definitive definition of bleeding events are still required.
In conclusion, colistin–tigecycline treatment was not associated with a higher bleeding risk than that of colistin–carbapenem treatment.
Acknowledgment
The authors acknowledge the staff of the Department of Medical Research at NTUH for facilitating the use of NTUH-iMD.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest.
References
1. Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America Guidance on the treatment of Extended-Spectrum beta-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin Infect Dis. 2021;72(7):e169–e183. doi:10.1093/cid/ciaa1478
2. Ayobami O, Willrich N, Harder T, Okeke IN, Eckmanns T, Markwart R. The incidence and prevalence of hospital-acquired (carbapenem-resistant) Acinetobacter baumannii in Europe, Eastern Mediterranean and Africa: a systematic review and meta-analysis. Emerg Microb Infect. 2019;8(1):1747–1759. doi:10.1080/22221751.2019.1698273
3. Acar A, Karaahmetoğlu G, Akalın H, Altay AF. Pooled prevalence and trends of antimicrobial resistance in Pseudomonas aeruginosa clinical isolates over the past 10 years in Turkey: a meta-analysis. J Glob Antimicrob Res. 2019;18:64–70. doi:10.1016/j.jgar.2019.01.032
4. van Loon K, Voor I, ‘t Holt AF, Vos MC. A systematic review and meta-analyses of the clinical epidemiology of carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother. 2018;62:e01730–17.
5. Teerawattanapong N, Panich P, Kulpokin D, et al. A systematic review of the burden of multidrug-resistant healthcare-associated infections among intensive care unit patients in Southeast Asia: the rise of multidrug-resistant Acinetobacter baumannii. Infect Control Hosp Epidemiol. 2018;39(5):525–533. doi:10.1017/ice.2018.58
6. Scudeller L, Righi E, Chiamenti M, et al. Systematic review and meta-analysis of in vitro efficacy of antibiotic combination therapy against carbapenem-resistant Gram-negative bacilli. Int J Antimicrob Agents. 2021;57(5):106344. doi:10.1016/j.ijantimicag.2021.106344
7. Abdul-Mutakabbir JC, Yim J, Nguyen L, et al. In vitro synergy of colistin in combination with meropenem or tigecycline against carbapenem-resistant Acinetobacter baumannii. Antibiotics. 2021;10(7). doi:10.3390/antibiotics10070880.
8. Zhou YF, Liu P, Zhang CJ, Liao XP, Sun J, Liu YH. Colistin combined with tigecycline: a promising alternative strategy to combat Escherichia coli harboring bla NDM- 5 and mcr-1. Front Microbiol. 2019;10:2957. doi:10.3389/fmicb.2019.02957
9. Yu L, Zhang J, Fu Y, et al. Synergetic effects of combined treatment of colistin with meropenem or amikacin on Carbapenem-Resistant Klebsiella pneumoniae in vitro. Front Cell Infect Microbiol. 2019;9:422. doi:10.3389/fcimb.2019.00422
10. Vardakas KZ, Athanassaki F, Pitiriga V, Falagas ME. Clinical relevance of in vitro synergistic activity of antibiotics for multidrug-resistant Gram-negative infections: a systematic review. J Glob Antimicrob Resist. 2019;17:250–259. doi:10.1016/j.jgar.2019.01.004
11. Greer ND. Tigecycline (Tygacil): the first in the glycylcycline class of antibiotics. Proc (Bayl Univ Med Cent). 2006;19(2):155–161. doi:10.1080/08998280.2006.11928154
12. Peterson LR. A review of tigecycline–the first glycylcycline. Int J Antimicrob Agents. 2008;32(Suppl 4):S215–S222. doi:10.1016/S0924-8579(09)70005-6
13. Wenzel R, Bate G, Kirkpatrick P. Tigecycline. Nat Rev Drug Discov. 2005;4(10):809–810. doi:10.1038/nrd1857
14. Cai Y, Wang R, Liang B, Bai N, Liu Y. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob Agents Chemother. 2011;55(3):1162–1172. doi:10.1128/AAC.01402-10
15. Pieringer H, Schmekal B, Biesenbach G, Pohanka E. Severe coagulation disorder with hypofibrinogenemia associated with the use of tigecycline. Ann Hematol. 2010;89(10):1063–1064. doi:10.1007/s00277-010-0911-7
16. Zhang Q, Zhou S, Zhou J. Tigecycline treatment causes a decrease in fibrinogen levels. Antimicrob Agents Chemother. 2015;59(3):1650–1655. doi:10.1128/AAC.04305-14
17. Hu J, Xiao YH, Zheng Y, Lai YX, Fang XL, Fang Q. Clinical characteristics and risk factors of tigecycline-associated hypofibrinogenaemia in critically ill patients. Eur J Clin Pharmacol. 2020;76(7):913–922. doi:10.1007/s00228-020-02860-w
18. Zhang Q, Wang J, Liu H, Ma W, Zhou S, Zhou J. Risk factors for tigecycline-induced hypofibrinogenaemia. J Clin Pharm Ther. 2020;45(6):1434–1441. doi:10.1111/jcpt.13250
19. Liu J, Yan Y, Zhang F. Risk factors for tigecycline-associated hypofibrinogenemia. Ther Clin Risk Manag. 2021;17:325–332. doi:10.2147/TCRM.S302850
20. Campany-Herrero D, Larrosa-Garcia M, Lalueza-Broto P, et al. Tigecycline-associated hypofibrinogenemia in a real-world setting. Int J Clin Pharm. 2020;42(4):1184–1189. doi:10.1007/s11096-020-01072-7
21. Hakeam HA, Al Duhailib Z, Salahuddin N, Amin T. Impact of tigecycline versus imipenem-cilastatin on fibrinogen levels following cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC): a randomized-controlled study. J Chemother. 2018;30(4):224–232. doi:10.1080/1120009X.2018.1452333
22. Wu PC, Wu CC. Tigecycline-associated hypofibrinogenemia: a case report and review of the literature. IDCases. 2018;11:56–57. doi:10.1016/j.idcr.2018.01.003
23. Kamath S, Lip GY. Fibrinogen: biochemistry, epidemiology and determinants. QJM. 2003;96(10):711–729. doi:10.1093/qjmed/hcg129
24. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3(4):692–694. doi:10.1111/j.1538-7836.2005.01204.x
25. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119–2126. doi:10.1111/jth.13140
26. Fan Q, Huang W, Weng Y, Xie X, Shi Z. Hypofibrinogenemia induced by high-dose tigecycline-case report and review of literature. Medicine. 2020;99(43):e22638. doi:10.1097/MD.0000000000022638
27. Mosesson MW. The roles of fibrinogen and fibrin in hemostasis and thrombosis. Semin Hematol. 1992;29(3):177–188.
28. Brandtner A, Bachler M, Fries D, et al. Tigecycline interferes with fibrinogen polymerization independent of peripheral interactions with the coagulation system. Antibiotics. 2020;9(2). doi:10.3390/antibiotics9020084.
29. Saliba R, Paasch L, El Solh A. Tigecycline attenuates staphylococcal superantigen-induced T-cell proliferation and production of cytokines and chemokines. Immunopharmacol Immunotoxicol. 2009;31(4):583–588. doi:10.3109/08923970902838672
30. Yang L, Vuylsteke A, Gerrard C, Besser M, Baglin T. Postoperative fibrinogen level is associated with postoperative bleeding following cardiothoracic surgery and the effect of fibrinogen replacement therapy remains uncertain. J Thromb Haemost. 2013;11(8):1519–1526. doi:10.1111/jth.12304
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
