Back to Journals » Clinical Ophthalmology » Volume 13
Combined pars plana vitrectomy with phacoemulsification for rhegmatogenous retinal detachment repair
Authors Guber J, Bentivoglio M, Sturm V, Scholl HPN, Valmaggia C
Received 11 May 2019
Accepted for publication 4 July 2019
Published 21 August 2019 Volume 2019:13 Pages 1587—1591
DOI https://doi.org/10.2147/OPTH.S215352
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Josef Guber,1,2 Maico Bentivoglio,1 Veit Sturm,1 Hendrik PN Scholl,2–4 Christophe Valmaggia1
1Eye Clinic, Cantonal Hospital Sankt Gallen, Sankt Gallen, Switzerland; 2Department of Ophthalmology, University of Basel, Basel, Switzerland; 3Institute of Molecular and Clinical Ophthalmology Basel (IOB), Basel, Switzerland; 4Wilmer Eye Institute, Johns Hopkins University, Baltimore, MD, USA
Correspondence: Josef Guber
Eye Clinic, Cantonal Hospital Sankt Gallen, Rorschacherstrasse 95, CH-9007 Sankt Gallen, Switzerland
Email [email protected]
Purpose: To investigate the outcome after combined phaco-vitrectomy in rhegmatogenous retinal detachment (RRD) repair.
Patients and methods: In this retrospective study, we included all patients who underwent pars plana vitrectomy (PPV) for RRD between January 2013 and December 2017. The main outcome measure was the retinal re-detachment rate after combined phaco-vitrectomy.
Results: Overall, 1017 eyes with RRD were included. All eyes received PPV, while in 516 eyes additional phacoemulsification was performed. A retinal re-detachment occurred in 103 patients (10.1%). No significant difference in the rate of re-detachment was found if additional phacoemulsification was performed (p=0.641). Subgroup calculations showed a significant higher rate of re-detachment in patients with a PVR (p=0.0003) and in patients where silicone oil was used as primary tamponade (p=0.0001) as well as in macula off RRDs (p=0.034).
Conclusion: Additional phacoemulsification during vitrectomy for RRD is not associated with higher rate of retinal re-detachment.
Keywords: retinal detachment, cataract, phacoemulsification, vitrectomy
Introduction
Pars plana vitrectomy (PPV) was first introduced by Machemer in 1972. Since then, PPV has undergone a major technical development, becoming a standard procedure for rhegmatogenous retinal detachment (RRD) that produces favorable outcomes.1–3
Despite all advantages, the formation of cataracts in almost every patient after vitrectomy is an inevitable consequence, with up to 80% of PPV patients developing significant cataracts within the first year.4,5 Most of these patients will then undergo cataract surgery at one point. Often intraocular gas or silicone oil is used as tamponade during PPV. That fact further contributes to cataract formation.6,7
Advanced instrumentation and modern surgical techniques have led to increasing popularity for combined cataract and vitreoretinal surgery in the last few years, not only in Europe.8–12 However, despite the increasing acceptance, some vitreoretinal surgeons are still reserved in performing combined procedure in patients with RRD because of concerns regarding higher intra-or postoperative complications rates.13–15 In addition, in many places, combined procedures are avoided for other reasons including the degree of specialization required to implant premium intraocular lenses as well as general practice patterns.
To our knowledge, this is the first and largest study analyzing the surgical outcome in terms of re-detachment rate after combined phaco-vitrectomy in RRD repair.
Patients and methods
The study was approved by the local ethic committee (Ethikkommission Ostschweiz, BASEC Nr. 2018-00104). All data were anonymized, therefore patient consent was waived by the ethics committee. This research adhered to the tenets of the Declaration of Helsinki.
In this retrospective study, we included all patients who underwent PPV for RRD at the Eye Clinic of Cantonal Hospital Sankt Gallen between January 2013 and December 2017. Patients with other types of retinal detachment, such as tractional or exsudative types, were excluded.
The case notes of these patients were reviewed and following data were included in the statistical analysis: patients characteristics, macula involvement, PVR present at the first visit, lens status, phacoemulsification performed during PPV, type of tamponade (gas or silicone oil), occurrence of re-detachment and reason for re-detachment (proliferative vitreoretinopathy (PVR), insufficiently treated break, new location of RRD).
Surgical technique
All surgical procedures were performed under general anesthesia with the Stellaris System (Bausch and Lomb, New York, USA) using three 23 gauge-valved ports. A standard core and peripheral was performed in all patients. Retina reattachment was achieved either by direct fluid-air exchange with drainage of subretinal fluid through main break or by using perfluorocarbon liquid followed by fluid-air exchange. Retinopexy was performed either with transconjunctival cryocoagulation or by endolaser. At the end of surgery, 12% octafluoropropane, 20% sulfur hexafluoride, or silicone oil was applied as intraocular tamponade according to the surgeon’s choice.
In phaco-vitrectomy group, three ports were placed at 3.5 mm from the limbus before performing cataract surgery. Phacoemulsification was performed through 2.6 mm clear corneal tunnel incision. Residual cortex was removed using irrigation/aspiration canulas. Subsequently, the anterior chamber was formed with viscoelasticum and vitrectomy was performed as described before. A foldable one-piece hydrophobic acrylic intraocular lens was implanted in the capsular bag using a preloaded injector system before performing fluid-air exchange.
Biometry was obtained using a Carl Zeiss Meditec IOL-Master. In macula off retinal detachment axial length was measured by ultrasound A-scan.
Statistical analysis
The main outcome measure was the rate of retinal re-detachments in patients where a combined phaco-vitrectomy was performed. Subgroup analyses were carried out for patients with primary PVR and/or patients, where silicone oil was used as tamponade to ensure that the outcome is not confounded by these two factors. Furthermore, adjusted calculations were carried out to account for patient characteristics.
The significance between the phacoemulsification and rate of re-detachment was calculated using chi-squared tests with continuity correction, either for all patients or for high-risk subgroups (PVR and silicone oil). Generalized linear models with binomial error distribution were used to test whether outcome depends on PVR or type of tamponade as well as patient characteristics.
A power calculation was performed to demonstrate that the study is adequately powered to detect a meaningful and expected difference in re-detachment rates between the two groups (the number of patients per group needed to show a significant difference with 90% probability was n=342).
All analyses were carried out with R, version 3.3.3.
Results
Overall, 1017 consecutive eyes with retinal detachment were included in this study. The age ranged between 15.2 and 94.5 years with a median age of 63.2 years. Macula was detached in 587 patients (57.7%). In 516 (50.7%) eyes, phacoemulsification was performed simultaneously. Primary PVR was found in 93 (9.3%) patients and silicone oil was used as primary tamponade in 138 (13.6%) patients. Patients’ characteristics at baseline are summarized in Table 1.
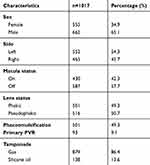 |
Table 1 Patients’ characteristics |
A retinal re-detachment occurred in 103 patients (10.1%). Main reason for re-detachment was an insufficiently treated break which occurred in 53.6% followed by postoperative PVR-reaction in 37.3%. In 9.1%, another break at a different location re-detached the retina again.
No significant difference in the rate of re-detachment was found if phacoemulsification was performed (10.7% (95% CI 8.2–13.7) versus 9.6% (95% CI 7.2–12.6), p=0.641).
Significant higher rates of re-detachments were found in patients with a primary PVR (p=0.0003) and/or patients where silicone oil was used as primary tamponade (p=0.0001). These two factors were clearly associated with each other (X2=290, p<0.0001). Furthermore, patients with a macular involvement had a significant higher re-detachment rate (Table 2). However, if associations between phacoemulsification and retinal re-detachment were calculated individually for the high-risk subgroups, results did not differ from those for all patients (Table 3).
 |
Table 2 Associations between the occurrence of re-detachment and phacoemulsification, high-risk factors and patients’ characteristics |
 |
Table 3 Association between the occurrence of re-detachment and phacoemulsification for high-risk groups |
Discussion
PPV has brought a lot of advantages for the treatment of retinal detachment. However, in phakic patients, postoperative formation of cataract is an inevitable consequence reported in several studies.4–6 We therefore began to perform all our vitrectomies combined with phacoemulsification regardless of degree of lens opacity or age threshold. As mentioned before, despite the increasing acceptance within Europe where combined procedures are often performed routinely, some vitreoretinal surgeons are still reserved, in particular in the US, where combined are rarely performed.
In the treatment of RRD in particular, the combined procedure offers a number of advantages. Removing the lens before performing vitrectomy improves visualization of the posterior pole as well as to the peripheral retina, especially if the IOL is implanted at the end of vitrectomy. Good access to the vitreous base without risk of lens touch allows a better shaving of the periphery resulting in a more complete vitrectomy. Furthermore, a combined procedure allows faster visual recovery and avoids the need for phacoemulsification in the vitrectomized eye, which is more complicated.16,17 Intraoperative cataract complications such as posterior capsular rupture with nucleus drop are less of a concern because the vitreoretinal surgeon has the ability to address such issues immediately.
However, a few specific concerns are associated with the combined procedure. Combined procedures may provoke a more intense postoperative inflammation and higher rate of posterior synechiae formation.13–15 In our experience, we do not have particularly more intense postoperative inflammation. Nevertheless, in some cases, a more aggressive control with additional steroids may reduce these events.9,18 Furthermore, some studies have reported a higher rate of posterior capsule opacification, especially if a gas tamponade was used.13,14,16 Finally, the use of an intraocular tamponade in phaco-vitrectomy may increase the risk of intraocular-lens-related complications, such as intraocular lens decentration, pupillary capture and anterior displacement of the intraocular lens which may induce a myopic shift.19,20 To prevent these complications, in combined surgeries, the IOLs chosen should have an optic diameter of at least 6 mm and should be rigid when unfolded in order to keep the capsular bag stable. In addition, a slightly smaller performed capsulorhexis during cataract surgery and waiving early postoperative dilatation can prevent anterior displacement.
Conclusion
In conclusion, this study refutes the superstition that combined procedures elicit more PVR reaction and therefore increases the risk for re-detachment. This study proves that there is no significant difference in the rate of re-detachment if an additional phacoemulsification was performed.
Acknowledgment
We thank Dr. sc. nat. Sabine Güsewell for her assistance in statistics.
Disclosure
Professor Hendrik PN Scholl reports support by the Swiss National Science Foundation, National Center of Competence in Research Molecular Systems Engineering & ldquo, Molecular Systems Engineering & rdquo, the Wellcome Trust, and the Foundation Fighting Blindness Clinical Research Institute. Professor Scholl is member of the Scientific Advisory Board of: Astellas Institute for Regenerative Medicine; Gensight Biologics; Ionis Pharmaceuticals, Inc.; Gyroscope Therapeutics Ltd.; ReNeuron Group Plc/Ora Inc.; and Vision Medicines, Inc. Dr. Scholl is paid consultant of: Gerson Lehrman Group; Guidepoint. Professor Scholl is member of the Data Monitoring and Safety Board/Committee of ReNeuron Group Plc/Ora Inc. and member of the Steering Committee of Novo Nordisk (FOCUS trial). Professor Scholl is co-director of the Institute of Molecular and Clinical Ophthalmology Basel (IOB) which is constituted as a non-profit foundation and receives funding from the University of Basel, the University Hospital Basel, Novartis, and the government of Basel-Stadt. These arrangements have been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. Johns Hopkins University and Bayer Pharma AG have an active research collaboration and option agreement. These arrangements have also been reviewed and approved by the University of Basel (Universitatsspital Basel [USB]) in accordance with its conflict of interest policies. Professor Scholl is the principal investigator of grants at the University of Basel (USB) sponsored by the following entity: Kinarus AG; Novartis Pharma AG; Ophthotech Corporation; Pharma Research & Early Development (pRED) of F. Hoffmann-La Roche Ltd; Spark Therapeutics. Grants at USB are negotiated and administered by the USB which receives them on its proper accounts. Individual investigators who participate in the sponsored project(s) are not directly compensated by the sponsor but may receive salary or other support from the institution to support their effort on the project(s). The authors report no other conflicts of interest in this work.
References
1. Heimann H, Bartz-Schmidt KU, Bornfeld N, et al. Scleral buckling versus primary vitrectomy in rhegmatogenous retinal detachment: a prospective randomized multicenter clinical study. Ophthalmology. 2007;114:2142–2154. doi:10.1016/j.ophtha.2006.10.027
2. de la Rua ER, Pastor JC, Fernandez I, et al. Non-complicated retinal detachment management: variations in 4 years. Retina 1 project; report 1. Br J Ophthalmol. 2008;92:523–525. doi:10.1136/bjo.2007.127688
3. Falkner-Radler CI, Myung JS, Moussa S, et al. Trends in primary retinal detachment surgery: results of a Bicenter study. Retina. 2011;31:928–936. doi:10.1097/IAE.0b013e3181f2a2ad
4. Hsuan JD, Brown NA, Bron AJ, Patel CK, Rosen PH. Posterior subcapsular and nuclear cataract after vitrectomy. J Cataract Refract Surg. 2001;27:437–444.
5. Melberg NS, Thomas MA. Nuclear sclerotic cataract after vitrectomy in patients younger than 50 years of age. Ophthalmology. 1995;102:1466–1471. doi:10.1016/s0161-6420(95)30844-5
6. Thompson JT. The role of patient age and intraocular gas use in cataract progression after vitrectomy for macular holes and epiretinal membranes. Am J Ophthalmol. 2004;137:250–257. doi:10.1016/j.ajo.2003.09.020
7. Federman JL, Schubert HD. Complications associated with the use of silicone oil in 150 eyes after retina-vitreous surgery. Ophthalmology. 1988;95:870–876. doi:10.1016/s0161-6420(88)33080-0
8. Soheilian M, Mirdehghan SA, Peyman GA. Sutureless combined 25-gauge vitrectomy, phacoemulsification, and posterior chamber intraocular lens implantation for management of uveitic cataract associated with posterior segment disease. Retina. 2008;28(7):941–946. doi:10.1097/IAE.0b013e31816ed5c7
9. Sisk RA, Murray TG. Combined phacoemulsification and sutureless 23-gauge pars plana vitrectomy for complex vitreoretinal diseases. Br J Ophthalmol. 2010;94(8):1028–1032. doi:10.1136/bjo.2009.175984
10. Heath G, Rahman R. Combined 23-gauge, sutureless transconjunctival vitrectomy with phacoemulsification without face down posturing for the repair of idiopathic macular holes. Eye(Lond). 2010;24(2):214–220. doi:10.1038/eye.2010.7
11. Sood V, Rahman R, Denniston AK. Phacoemulsification and foldable intraocular lens implantation combined with 23-gauge transconjunctival sutureless vitrectomy. J Cataract Refract Surg. 2009;35(8):1380–1384. doi:10.1016/j.jcrs.2009.02.047
12. Lee DY, Jeong HS, Sohn HJ, Nam DH. Combined 23-gauge sutureless vitrectomy and clear corneal phacoemulsification in patients with proliferative diabetic retinopathy. Retina. 2011;31(9):1753–1758. doi:10.1097/IAE.0b013e31820d4057
13. Smith M, Raman SV, Pappas G, Simcock P, Ling R, Shaw S. Phacovitrectomy for primary retinal detachment repair in presbyopes. Retina. 2007;27:462–467. doi:10.1097/01.iae.0000243066.19645.de
14. Treumer F, Bunse A, Rudolf M, Roider J. Pars plana vitrectomy, phacoemulsification and intraocular lens implantation. Comparison of clinical complications in a combined versus two-step surgical approach. Graefes Arch Clin Exp Ophthalmol. 2006;244:808–815. doi:10.1007/s00417-005-0146-9
15. Lahey JM, Francis RR, Kearney JJ. Combining phacoemulsification with pars plana vitrectomy in patients with proliferative diabetic retinopathy: a series of 223 cases. Ophthalmology. 2003;110:1335–1339. doi:10.1016/S0161-6420(03)00454-8
16. Muselier A, Dugas B, Burelle X, et al. Macular hole surgery and cataract extraction: combined vs consecutive surgery. Am J Ophthalmol. 2010;150:387–391. doi:10.1016/j.ajo.2010.04.008
17. Dugas B, Ouled-Moussa R, Lafontaine PO, et al. Idiopathic epiretinal macular membrane and cataract extraction: combined versus consecutive surgery. Am J Ophthalmol. 2010;149:302–306. doi:10.1016/j.ajo.2009.09.011
18. Lee GH, Ahn JK, Park YG. Intravitreal triamcinolone reduces the morphologic changes of ciliary body after pars plana vitrectomy for retinal vascular diseases. Am J Ophthalmol. 2008;145:1037–1044. doi:10.1016/j.ajo.2008.01.029
19. Rahman R, Rosen PH. Pupillary capture after combined management of cataract and vitreoretinal pathology. J Cataract Refract Surg. 2002;28:1607–1612.
20. Shioya M, Ogino N, Shinjo U. Change in postoperative refractive error when vitrectomy is added to intraocular lens implantation. J Cataract Refract Surg. 1997;23:1217–1220.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
