Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 15
Clinical Course and Outcomes of COVID-19 Infection in Patients Treated with Rituximab: A Tertiary Care Center Experience
Authors Alhowaish TS , Alhamadh MS , Mathkour A, Alamoudi M, Alqahtani HA, Alrashid A
Received 25 June 2023
Accepted for publication 18 August 2023
Published 28 August 2023 Volume 2023:15 Pages 145—159
DOI https://doi.org/10.2147/OARRR.S424316
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Chuan-Ju Liu
Thamer S Alhowaish,1,2 Moustafa S Alhamadh,2,3 Alaa Mathkour,4 Marwan Alamoudi,2,5 Hossam Ali Alqahtani,1,2 Abdulrahman Alrashid2,3,5
1Division of Neurology, King Abdulaziz Medical City, Ministry of the National Guard Health Affairs, Riyadh, Kingdom of Saudi Arabia; 2King Abdullah International Medical Research Center, Ministry of the National Guard Health Affairs, Riyadh, 11481, Kingdom of Saudi Arabia; 3College of Medicine, King Saud Bin Abdulaziz University for Health Sciences (KSAUHS), Ministry of the National Guard-Health Affairs, Riyadh, 14611, Kingdom of Saudi Arabia; 4Ministry of Health, Riyadh, 12613, Kingdom of Saudi Arabia; 5Department of Medicine, Division of Rheumatology, King Abdulaziz Medical City, Ministry of the National Guard-Health Affairs, Riyadh, Kingdom of Saudi Arabia
Correspondence: Thamer S Alhowaish, Division of Neurology, Department of Medicine, King Abdulaziz Medical City, Ministry of the National Guard Health Affairs, P.O. Box 22490, Riyadh, 11426, Kingdom of Saudi Arabia, Email [email protected]
Introduction: Patients receiving rituximab (RTX) may be at increased risk for severe Coronavirus infections and worse outcomes compared with the general population. Because of the conflicting results concerning the effect of RTX on the clinical course and outcomes of COVID-19 infection, we aimed to share our experience with 35 patients infected with COVID-19 while treated with RTX for a variety of clinical indications.
Methods: This was a single-centre retrospective cohort study that included 35 patients. All patients aged ≥ 14 years who were treated with RTX for various conditions and were found to have COVID-19 infection were included. Patients with poor outcomes or patients with suspected COVID-19 infection were excluded.
Results: The patients’ mean age was 42.8 ± 16.3 years with an average BMI of 29.9 ± 11.4 kg/m2. Over half (51.4%, n = 18) of the patients received RTX at a dose of 375 mg/m2 with a median frequency of 4 doses. More than a third (37.1%, n = 13) of the patients had hypogammaglobulinemia and 25.7% had low CD19. Over a third (42.9%, n= 15) of the patients required hospitalization and almost a third (25.7%, n = 9) required treatment in the intensive care unit. There was a statistically significant association between intensive care unit admission and age, steroid use, and low CD19. The mortality rate was 25.7%, and it was significantly higher in elderly, diabetics, corticosteroid users, patients who were hospitalized, treated in the intensive care unit, and had low immunoglobin or CD19.
Conclusion: Treatment with RTX seems to be a potential risk factor for unfavorable outcomes in COVID-19 patients. RTX should be used with caution or avoided unless the benefit clearly outweighs the risk.
Keywords: rituximab, SARS-CoV-2, COVID-19, autoimmunity, hypogammaglobulinemia, rheumatology
Introduction
Coronavirus is an enveloped, single-stranded RNA virus. Infection with this virus was first reported in Wuhan, China, in December 2019, and it was declared a pandemic in March 2020. Coronavirus disease 2019 (COVID-19) may present as mild to moderate, self-limiting respiratory symptoms such as fever, loss of taste and smell, cough, and shortness of breath, but in some patients, it may be severe enough to require hospitalization and intubation with mechanical ventilation.1
Rituximab (RTX) is a monoclonal antibody that is used for the treatment of numerous diseases, including rheumatologic, hematologic, nephrologic, neurologic, and oncologic conditions.2 RTX targets the B-cell transmembrane protein CD20, leading to B-cell destruction. As a result, RTX suppresses the humoral immunity via B-cell depletion. Although generally well-tolerated with only mild adverse effects such as infusion reactions, headache, and chills, RTX can induce severe immunosuppression, resulting in hypogammaglobulinemia, neutropenia, and reactivation of viral infections.3
There is no consensus on whether immunosuppressive and immunomodulatory agents negatively affect the clinical course and outcome of COVID-19 infection.4 To emphasize, some studies found that patients who were already taking immunosuppressive agents are more susceptible to COVID-19 infection and worse outcomes.1,5 More specifically, multiple studies suggested that patients treated with RTX are at increased risk for severe COVID-19 infection and worse outcomes compared to the general population. Also, several reports revealed higher rates of COVID-19-related hospitalization, respiratory failure requiring intubation and mechanical ventilation, and mortality in patients treated with RTX.3,6,7
A descriptive study by Martos et al of SARS-CoV-2 infections in patients with rheumatic diseases treated with RTX showed a high rate of severe illness requiring hospitalization (61.5%) and a high mortality rate (23.1%). Similarly, a cohort study by Singh et al showed that the use of RTX in patients with rheumatoid arthritis was associated with worse COVID-19 outcomes such as hospitalization (26%) and ICU admission (6.6%) than in patients receiving conventional synthetic disease-modifying anti-rheumatic drugs.3
Because of the variable results of different studies on the effect of RTX on the course and outcome of COVID-19 infection and because of the limited literature in Saudi Arabia, we aimed to investigate the outcome of COVID-19 infection in patients who received RTX during the period of the COVID-19 pandemic in Saudi Arabia.
Materials and Methods
Aim
To evaluate the clinical course and outcomes of COVID-19 infection among patients treated with RTX for a variety of clinical indications.
Study Design/Setting
This was a retrospective cohort study conducted at King Abdulaziz Medical City (KAMC), Ministry of National Guard-Health Affairs (MNG-HA), Riyadh, Kingdom of Saudi Arabia. KAMC is an academic, government-funded tertiary hospital that combines clinical care, training, and academics with research and state of the art medical technologies.
Inclusion and Exclusion Criteria
Patients aged ≥ 14 years who were treated with RTX for autoimmune connective tissue diseases, hematologic malignancies, benign hematologic diseases, neurologic diseases, or glomerular diseases and had PCR-proven COVID-19 infection were included. Patients who had a poor outcome due to severe underlying disease before infection with COVID-19 or patients with suspected infection with COVID-19 were excluded. After applying the aforementioned criteria, only 35 patients were eligible for inclusion.
Data Collection
Electronic medical records (via the KAMC electronic system - BestCare; Seoul, South Korea: ezCaretech Co) were used to obtain data of all patients who met our inclusion criteria, from 2019 to 2021. The data collected included the following: demographic data such as age, gender, and body mass index, comorbidities including diabetes mellitus (DM), hypertension (HTN), and chronic kidney disease (CKD), the primary diagnosis for which RTX was used, RTX dose, number of RTX doses, other immunosuppressants used, immunoglobulin (IgG, IgM, and IgA) levels, CD19 levels, presenting signs and symptoms of COVID −19, chest x-ray findings on admission, ferritin levels, site of treatment (outpatient, inpatient, or intensive care unit (ICU)), and outcomes (survival or death).
Statistical Analysis
Statistical analysis was performed with SPSS version 28 (IBM Co., Armonk, NY, USA).
Quantitative nonparametric data were presented as median and interquartile range (IQR) and analyzed with the Mann Whitney test. Categorical variables were presented as frequency and percentage (%) and analyzed with the Chi-square test or Fisher’s exact test, as appropriate. A two tailed P value < 0.05 was considered statistically significant.
Ethical Considerations
This study was approved by the Institutional Review Board of the King Abdullah International Medical Research Center, Ministry of National Guard-Health Affairs, Riyadh, Kingdom of Saudi Arabia (RC20/693/R). Because this was a retrospective study, informed consent was waived. Access to the data was restricted to the investigators. Patient confidentiality and privacy were maintained, and no names or medical record numbers were used, and all data were stored in a secure location at National Guard-Health Affairs. This study complies with the Declaration of Helsinki.
Results
Baseline Characteristics
A total of 35 patients (15 men and 20 women) were included. The patients’ mean age was 42.8 ± 16.3 years, and over two-thirds (65.9%, n= 23) of them were ≤ 50 years old. About half of the patients (48.6%, n= 17) were obese with an average BMI of 29.9 ± 11.4 kg/m2. The most prominent comorbid condition was HTN, followed by DM and CKD, accounting for 28.6%, 25.7%, and 25.7%, respectively. The most common primary diagnoses for using RTX were neurologic diseases (22.9%), autoimmune connective tissue diseases (20%), and hematological malignancies (20%) Figure 1. Over half (51.4%, n= 18) of the patients received RTX at a dose of 375 mg/m2, around a third (37.1%, n= 13) at a dose of 1000 mg/m2, and only a few (11.4%, n= 4) at a dose of 500 mg/m2. The median frequency of RTX administration was 4 times with an IQR of 3 to 6 times. More than half (51.4%) of the patients were on corticosteroids, and a few were on azathioprine (14.3%), mycophenolate (14.3%), and hydroxychloroquine (11.4%). Hypogammaglobulinemia, defined as low IgG, IgM, and/or IgA, was diagnosed in 37.1% of the patients and 25.7% had low CD19. The mortality rate was 25.7% as shown in Table 1
 |
Table 1 Baseline Characteristics of the Studied Patients (n=35) |
 |
Figure 1 The Primary Diagnosis for Using Rituximab. |
COVID-19 Data
As summarized in Table 2, 22.9% of the patients had symptomatic COVID-19 infection. The most common presenting symptoms were lower respiratory symptoms, which were reported by more than half (60%, n= 21) of the patients, followed by gastrointestinal and neurological symptoms, each reported by 17.1%. Chest radiography revealed pneumonia in 22.9% and bilateral airspace opacities in 17.1%. More than half (57.1%, n= 20) of the patients were treated as outpatients, and 25.7% were admitted to ICU.
 |
Table 2 COVID-19 Data Among the Studied Patients (n=35) |
Mortality
Comparison between survivors and nonsurvivors revealed a statistically significant difference in age, as nonsurvivors were older than those who recovered (P=0.016). Compared with the recovered patients, the nonsurvivors had higher rates of DM (55.6% vs 15.4%, P=0.03), steroid administration (88.9% vs 38.5%, P=0.018), inpatient care (77.8% vs 30.8%, P=0.022), and ICU admission (66.7% vs 11.5%, P=0.003). Nonsurvivor patients had significantly lower immunoglobulins (hypogammaglobulinemia) than the survivors (77.8% vs 23.1%, P=0.006), manifested by an abnormal decrease in either IgG (44.4% vs 7.7%), IgM (66.7% vs 23.1%), or IgA (11.1% vs 7.7%, which was similar between the two groups). Moreover, nonsurvivors had lower CD19 levels than survivors (55.6% vs 15.4% respectively had low CD19, P=0.03) as summarized in Table 3, Figures 2–4.
 |
Table 3 Association Between Mortality and Patients’ Characteristics, Outcome |
 |
Figure 2 Association between mortality and age of the studied patients. |
 |
Figure 3 Association between mortality and steroids administration of the studied patients. |
 |
Figure 4 Association between mortality and hypogammaglobulinemia rate of the studied patients. |
Intensive Care Unit Admission
As shown in Table 4, there was a statistically significant association between ICU admission and age, as patients who were admitted to the ICU were older than those who were not admitted (P=0.045). Compared with patients who were not admitted to the ICU, patients admitted to the ICU had higher rates of steroid use (88.9% vs 38.5%, P=0.018) and lower CD19 levels (55.6% vs 15.4%, P=0.03). Patients admitted to the ICU had a higher mortality rate (66.7% vs 11.5%, P=0.003) (Figure 5).
 |
Table 4 Association Between ICU Admission and Patients’ Characteristics, Outcome |
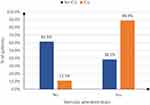 |
Figure 5 Association between ICU admission and steroids administration of the studied patients. |
Discussion
In this study, we sought to evaluate the outcome of COVID-19 infection in patients who received RTX for various underlying diseases in Saudi Arabia. We found that RTX at a dose of 375 mg/m2 was the most commonly used protocol. Corticosteroids were used in more than half of the patients. This study showed that a large number of RTX receivers were found to have hypogammaglobulinemia and low CD19. Our study showed a relatively high mortality rate among patients treated with RTX. In addition, patients treated with RTX who did not survive tended to be older, diabetic, taking steroids, and hospitalized or admitted to ICU. Also, hypogammaglobulinemia and lower CD19 levels were found to be associated with a high mortality rate. Moreover, this study showed that there was a statistically significant association between ICU admission and age, steroid use, and low CD19 levels.
Most of our patients were young females, and the most common cause of RTX use was autoimmune diseases such as multiple sclerosis and myasthenia gravis. This can be explained by the fact that autoimmune diseases tend to affect young individuals and have a female tendency.1,8
Some previous reports found that the use of RTX does not worsen the outcomes of patients with COVID-19 and is not associated with disease progression.9 Our study shows a mortality rate of 25.7% in patients treated with RTX.1 In addition, a large proportion of our cohort were hospitalized (42.9%) and required treatment in the ICU (25.7%). MacKenna et al reported that patients receiving RTX had an increased risk of COVID-19-related hospitalization, ICU admissions, and deaths compared with patients receiving standard systemic therapy.8 Similarly, a cohort study by Singh et al showed that RTX use in patients with rheumatoid arthritis is associated with worse COVID-19 outcomes such as hospitalization (26%) and ICU admission (6.6%) compared to those on conventional synthetic disease modifying anti-rheumatic drugs. Likewise, a descriptive study by Martos et al of SARS-CoV-2 infections in patients with rheumatic diseases treated with RTX showed a high rate of severe illness requiring hospitalization (61.5%) and a high mortality rate (23.1%).3 In agreement with ours, Tepasse et al, Stradner et al, and Alpizar-Rodriguez et al also reported that RTX use was associated with a higher risk of severe disease and/or mortality in patients with COVID-19 infection.9,10
Some immunosuppressants selectively target T lymphocytes and suppress their function, such as calcineurin inhibitors, while largely preserving B cell activity. However, other immunosuppressants, such as RTX, an anti-CD20 monoclonal antibody, result in complete depletion of B cells and suppress B cell functions such as immunoglobulin production. Therefore, the rapid depletion of B cells associated with RTX use can cause secondary hypogammaglobulinemia, which may be persistent or take up to 12 months to recover. Patients with hypogammaglobulinemia develop impaired opsonization and do not form antibodies to exposed antigens. Therefore, these patients develop susceptibility to infection and to the complications that can occur as a result of infection, which may necessitate hospitalization and/or ICU admission.9,11 There are case reports of persistent COVID-19 infection or delayed serological response to COVID-19 vaccination in patients receiving RTX therapy. These data raise concerns that RTX may directly influence adaptive immune response, which is important for the control and clearance of viral infections.12
It is unclear whether the underlying primary diagnosis for which RTX is used alone or in combination with the effect of RTX use and/or additional risk factors, influences mortality. Suppressed immunity and comorbidities are considered the main reasons for the increased infection and mortality rates of COVID-19 in these patients.13,14 Mortality was reported in a quarter (25.7%) of our patients who were treated with RTX. Also, Ekin et al showed that the mortality rate was 27% in patients with rheumatologic diseases who received RTX.11 In a study of the outcomes of COVID-19 in patients with inflammatory rheumatic diseases, the mortality rate was 11.5% in patients on RTX, which is consistent with some other studies.1 For clarification, Montero et al mentioned a mortality rate of 16%, and Sharmeen et al reported a mortality rate of 5.9%.15,16 However, A cohort study by Singh et al and the French national study did not find an association with an increased risk of death in the RTX-exposed vs not-exposed group.12,17 The differences between these studies could be due to different underlying diseases for which RTX was used. This could also explain the high mortality rate in our study because we included different disease groups such as cancer patients and not only inflammatory rheumatic diseases. Moreover, from these studies, it appears that patients receiving RTX have a higher mortality rate than patients receiving other immunosuppressants. As in our study and in previous studies, patients receiving RTX may have other concomitant diseases, such as DM, HTN, and CKD, which also increase the risk of poor outcomes.1,11 This study showed that the mortality rate was higher in RTX receivers if they were older, had diabetes, were taking steroids, were hospitalized, needed ICU, or had lower immunoglobulins or CD19. This is consistent with Ekin et al and the French RMD COVID-19 cohort, which also found a higher mortality rate in older patients. However, unlike our study, Ekin et al showed a significant association between COVID-19-related mortality and CKD.11 Some studies have shown that steroids have no association with mortality. In a meta-analysis of seven trials involving a total of 1703 critically ill COVID-19 patients, steroids reduced 28-day mortality compared with standard treatment or placebo.18 Gianfrancesco et al and the French RMD cohort study, however, found that long-term use of corticosteroids in the treatment of COVID-19 patients increased the risk of severe COVID-19 infection and/or COVID-19 mortality.19,20 To be more specific in patients treated with RTX, unlike our study, Ekin et al showed no significant association between steroid use and COVID-19-related mortality in RTX patients. Therefore, they concluded that the advantages of steroid use in the treatment of rheumatologic diseases and COVID-19 infections seem to outweigh the disadvantages when used in a timely manner.11
Regarding sex and COVID-19 outcomes, there are some conflicting results in the literature. Several studies conducted in the general COVID-19 population reported higher rates of severe COVID-19 infections and COVID-19-related deaths in men compared with women.21,22 In our study, no significant association was found between COVID-19-related mortality and gender, and the same was reported by Ekin et al. However, in the French RMD cohort study which investigated the impact of SARS-CoV-2 on patients with inflammatory rheumatic and musculoskeletal diseases, a significant association was found between female sex and death/serious illness.19 Another cohort study on the outcomes of COVID −19 patients with inflammatory rheumatic diseases showed that women had a more better survival rate compared to men.1 We believe that gender may not be directly related to severe COVID-19 infections. Rather, the underlying primary diagnosis for which RTX is used could play a role. To clarify this point, most of the available literature addressing RTX and the risk of severe COVID-19 infections and COVID-19-related deaths is related to a specific disease such as rheumatoid arthritis, or a group of diseases such as autoimmune diseases which could predominantly affect a specific gender. However, in our study, the fact that our patients had heterogeneous diagnoses could explain the even gender distribution in our study.
Some patients in RTX may develop hypogammaglobulinemia, which can be persistent and clinically significant and may require antibiotic prophylaxis or immunoglobulin replacement therapy to prevent severe infections and infection-related complications.23,24 Several risk factors for hypogammaglobulinemia in patients treated with RTX have been reported in the literature including repeated RTX treatments, advanced age, and concurrent glucocorticoid therapy.25 In our study, 37.1% of patients had hypogammaglobulinemia, and Casulo et al reported almost the same percentage (38.54%).26 However, the incidence was higher in Roberts and Ekin et al, 56% and 68.5%, respectively. Unfortunately, not all patients in our study were tested for immunoglobulin levels, which may explain the difference in percentage between our study and the latter two studies. Both our study and Ekin et al showed that there was a significant association between hypogammaglobulinemia and COVID-19-related mortality.11
In our study, the hospitalization rate was 42.9%, which is higher than the general hospitalization rate in the general population.1 In our study population, 25.71% of patients needed ICU. There was a statistically significant association between ICU admission and older age, steroid use, and lower CD19 . In the study by Ekin et al 28.8% of patients with a history of infection required hospitalization. In the French RMD COVID-19 cohort study of 694 adults, 63% developed mild (not hospitalized), 24% developed moderate (hospitalized out of the ICU), and 13% developed severe (treated/died in the ICU) disease.19 Previously published studies agree with the high rate of admission rate in people with immunotherapy.15,20 COVID-19 patients in the general population, comorbidities such as chronic respiratory disease and DM could be the reason for admission.27,28 In addition, disease-specific factors of the patients could be another explanation for admission as patients with cancer or autoimmune diseases may require more medical attention. To clarify, Sahraian et al showed that in multiple sclerosis patients infected with COVID-19, the hospitalization rate was 25%, Which is higher than the admission rate in the age group of patients with multiple sclerosis in the general population.29 MacKenna et al showed adults with immune-mediated inflammatory diseases are also at increased risk for COVID-19-related ICU admission and death.8 Based on the literature, people with immune-mediated inflammatory diseases have a higher risk of COVID-19-related death, critical care admission or death, and hospital admissions than people without immune-mediated inflammatory diseases. The poor outcomes in these patients could be due to underlying diseases, immunotherapy, and/or other comorbidities.1,8
Study Limitations and Future Directions
This study had some limitations. First, it was a retrospective study, and some defects in the study design cannot be ignored (eg, small sample size, single-center design). Second, some of the patients were not tested for immunoglobulins. Third, data on vaccination were not available, so the effects of vaccination on patient outcomes were ignored in the cohort. We plan to conduct two follow-up studies to assess the effects of vaccination on the outcomes of patients on RTX and the incidence of hypogammaglobulinemia in RTX receivers.
Conclusion
We found that neurological disorders were the most common reason for RTX use. Approximately half of the patients received RTX at a dose of 375 mg/m2. Almost half of the patients received steroids. Hypogammaglobulinemia was seen in 37.1% of patients. ICU admission occurred in 25.7% of cases, and there was a statistically significant association between ICU admission and age, steroid use, and low CD19 levels. The mortality rate was 25.7%, and the mortality rate was higher in patients receiving RTX if they were older, had diabetes, were on steroids, were hospitalized or admitted to the ICU, or had low immunoglobulin or CD19 levels.
Recommendation
Although RTX is an effective and safe medication for the treatment of various autoimmune diseases and hematologic malignancies, still RTX may cause an increased risk of infection and worse outcomes because of its immunosuppressive effects. Based on our findings, we recommend that patients who will start on RTX should be counseled regarding the risk of COVID-19 infection outcomes based on their individual risk factors. RTX should be used with caution or avoided unless the benefits clearly outweigh the risks. Finally, immunoglobulin testing should be performed in all patients started on RTX to know the baseline level of the patients. Multicenter studies with larger samples are needed to validate the results of this study.
Abbreviations
RTX, Rituximab; COVID-19, Coronavirus disease 2019; KAMC, King Abdulaziz Medical City; DM, Diabetes Militias; CKD, Chronic Kidney Disease; ICU, Intensive Care Unit.
Data and Materials Availability
The dataset used and/or analyzed to write the current study is available upon request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
All authors declare no conflicts of interest in this work.
References
1. Alhowaish TS, Alhamadh MS, Alhabeeb AY, et al. Outcomes of COVID-19 in inflammatory rheumatic diseases: a retrospective cohort study. Cureus. 2022;14(6). doi:10.7759/CUREUS.26343
2. Kaegi C, Wuest B, Schreiner J, et al. Systematic review of safety and efficacy of rituximab in treating immune-mediated disorders. Front Immunol. 2019;10:1990. doi:10.3389/FIMMU.2019.01990/BIBTEX
3. Loarce-Martos J, García-Fernández A, López-Gutiérrez F, et al. High rates of severe disease and death due to SARS-CoV-2 infection in rheumatic disease patients treated with rituximab: a descriptive study. Rheumatol Int. 2020;40(12):2015–2021. doi:10.1007/S00296-020-04699-X/TABLES/2
4. Pablos JL, Galindo M, Carmona L, et al. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann Rheum Dis. 2020;79(12):1544–1549. doi:10.1136/ANNRHEUMDIS-2020-218296
5. Hsu CY, Ko CH, Wang JL, Hsu TC, Lin CY. Comparing the burdens of opportunistic infections among patients with systemic rheumatic diseases: a nationally representative cohort study. Arthritis Res Ther. 2019;21(1):1–10. doi:10.1186/S13075-019-1997-5/TABLES/5
6. Nuño L, Novella Navarro M, Bonilla G, et al. Clinical course, severity and mortality in a cohort of patients with COVID-19 with rheumatic diseases. Ann Rheum Dis. 2020;79(12):1659–1661. doi:10.1136/ANNRHEUMDIS-2020-218054
7. Schulze-Koops H, Krueger K, Vallbracht I, Hasseli R, Skapenko A. Increased risk for severe COVID-19 in patients with inflammatory rheumatic diseases treated with rituximab. Ann Rheum Dis. 2021;80(5):e67–e67. doi:10.1136/ANNRHEUMDIS-2020-218075
8. MacKenna B, Kennedy NA, Mehrkar A, et al. Risk of severe COVID-19 outcomes associated with immune-mediated inflammatory diseases and immune-modifying therapies: a nationwide cohort study in the OpenSAFELY platform. Lancet Rheumatol. 2022;4(7):e490–e506. doi:10.1016/S2665-9913(22)00098-4
9. Tepasse PR, Hafezi W, Lutz M, et al. Persisting SARS‐CoV‐2 viremia after rituximab therapy: two cases with fatal outcome and a review of literature. Br J Haematol. 2020;190(2):185–188. doi:10.1111/BJH.16896
10. Alpizar-Rodriguez D, Irazoque-Palazuelos F, Rodriguez-Reyne TS, et al. POS1242 FACTORS ASSOCIATED WITH MORTALITY IN PATIENTS WITH RHEUMATIC DISEASES AND COVID-19 IN MEXICO. Ann Rheum Dis. 2021;80(Suppl 1):904. doi:10.1136/ANNRHEUMDIS-2021-EULAR.3342
11. Ekin A, Coskun BN, Dalkilic E, Pehlivan Y. The effects of COVID-19 infection on the mortality of patients receiving rituximab therapy. Ir J Med Sci. 2022;1. doi:10.1007/S11845-022-03193-6
12. Singh N, Madhira V, Hu C, et al. Rituximab is associated with worse COVID-19 outcomes in patients with rheumatoid arthritis: a retrospective, nationally sampled cohort study from the U.S. National COVID Cohort Collaborative (N3C). Semin Arthritis Rheum. 2023:58. doi:10.1016/J.SEMARTHRIT.2022.152149
13. Sarzi-Puttini P, Giorgi V, Sirotti S, et al. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol. 2020;38(2):337–342.
14. Law HKW, Chung YC, Hoi YN, et al. Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood. 2005;106(7):2366–2374. doi:10.1182/BLOOD-2004-10-4166
15. Montero F, Martínez-Barrio J, Serrano-Benavente B, et al. Coronavirus disease 2019 (COVID-19) in autoimmune and inflammatory conditions: clinical characteristics of poor outcomes. Rheumatol Int. 2020;40(10):1593–1598. doi:10.1007/S00296-020-04676-4
16. Sharmeen S, Elghawy A, Zarlasht F, Yao QP. COVID-19 in rheumatic disease patients on immunosuppressive agents. Semin Arthritis Rheum. 2020;50(4):680. doi:10.1016/J.SEMARTHRIT.2020.05.010
17. Avouac J, Drumez E, Hachulla E, et al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study. Lancet Rheumatol. 2021;3(6):e419–e426. doi:10.1016/S2665-9913(21)00059-X
18. Sterne JAC, Murthy S, J V. D, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1. doi:10.1001/JAMA.2020.17023
19. Florence A, Nassim AA, Jean-David A, et al. Severity of COVID-19 and survival in patients with rheumatic and inflammatory diseases: data from the French RMD COVID-19 cohort of 694 patients. Ann Rheum Dis. 2021;80(4):527. doi:10.1136/ANNRHEUMDIS-2020-218310
20. Gianfrancesco M, Hyrich KL, Hyrich KL, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 global rheumatology alliance physician-reported registry. Ann Rheum Dis. 2020;79(7):859–866. doi:10.1136/ANNRHEUMDIS-2020-217871
21. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi:10.1056/NEJMOA2002032
22. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574. doi:10.1001/JAMA.2020.5394
23. Thiel J, Rizzi M, Engesser M, et al. B cell repopulation kinetics after rituximab treatment in ANCA-associated vasculitides compared to rheumatoid arthritis, and connective tissue diseases: a longitudinal observational study on 120 patients. Arthritis Res Ther. 2017;19(1). doi:10.1186/S13075-017-1306-0
24. Cooper N, Davies EG, Thrasher AJ. Repeated courses of rituximab for autoimmune cytopenias may precipitate profound hypogammaglobulinaemia requiring replacement intravenous immunoglobulin. Br J Haematol. 2009;146(1):120–122. doi:10.1111/J.1365-2141.2009.07715.X
25. Zeevi A, Husain S, Spichty KJ, et al. Recovery of functional memory T cells in lung transplant recipients following induction therapy with alemtuzumab. Am J Transplant. 2007;7(2):471–475. doi:10.1111/J.1600-6143.2006.01641.X
26. Casulo C, Maragulia J, Zelenetz AD. Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin Lymphoma Myeloma Leuk. 2013;13(2):106. doi:10.1016/J.CLML.2012.11.011
27. Pritchard M. COVID-19 symptoms at hospital admission vary with age and sex: ISARIC multinational study. medRxiv Prepr Serv Heal Sci. 2020. doi:10.1101/2020.10.26.20219519
28. Dananché C, Elias C, Hénaff L, et al. Baseline clinical features of COVID-19 patients, delay of hospital admission and clinical outcome: a complex relationship. PLoS One. 2022;17(1). doi:10.1371/JOURNAL.PONE.0261428
29. Sahraian MA, Azimi A, Navardi S, Ala S, Naser Moghadasi A. Evaluation of the rate of COVID-19 infection, hospitalization and death among Iranian patients with multiple sclerosis. Mult Scler Relat Disord. 2020;46. doi:10.1016/J.MSARD.2020.102472
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
