Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 14
Clinical Characteristics, Treatment Outcome and Associated Factors of Epilepsy Among Children at Hospitals of North-West Ethiopia
Authors Nasir M , Abebaw E, Ahmed M , Ketema DB
Received 13 September 2023
Accepted for publication 26 October 2023
Published 31 October 2023 Volume 2023:14 Pages 385—404
DOI https://doi.org/10.2147/PHMT.S436022
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Roosy Aulakh
Mohammed Nasir,1 Ermias Abebaw,2 Muluken Ahmed,3 Daniel Bekele Ketema4
1Pediatrics Department, Hawassa University, Hawassa, Ethiopia; 2Pediatrics Department, ALERT Comprehensive Specialized Hospital, Addis Ababa, Ethiopia; 3Pediatrics Department, Arba Minch University, Arba Minch, Ethiopia; 4Public Health Department, Debre Markos University, Debre Markos, Ethiopia
Correspondence: Mohammed Nasir, Pediatrics Department, Hawassa University, Hawassa, Ethiopia, Tel +251-911739081, Email [email protected]
Background: Epilepsy is an important cause of neurological morbidity in children and adolescents. Clinical parameters are the main diagnostic tools, especially in developing countries. Although cost-effective treatments for epilepsy are available, studies have shown that uncontrolled seizures can occur in many patients.
Objective: To assess clinical characteristics, treatment outcomes, and associated factors for controlled epilepsy among children with epilepsy who underwent follow-up at the Debre Markos Comprehensive Specialized Hospital, North-west Ethiopia from October 28, 2020, to April 28, 2021.
Methods: An institutional-based retrospective cohort study was conducted from October 28, 2020, to April 28, 2021. A total of 385 participants who fulfilled the inclusion criteria were included in the study. A pretested, structured, interviewer-administered questionnaire with a chart review was used to collect data. The data were entered into the Epi-data software version 4.4.2.1 and then exported to the Stata version 14 statistical package for analysis. Descriptive statistics were used to describe the sociodemographic and clinical characteristics, treatment profiles, and treatment outcomes of patients with epilepsy. Bivariate and multivariate analyses were used to identify factors associated with treatment outcomes.
Results: The most frequent type of seizure among the 385 respondents was Generalized-tonic-clonic seizures (88.1%). The proximate cause of seizures was identified in 15% of patients, of whom 45 had a perinatal history (8.8%), head injury (3.6%), and CNS infection (2.3%). One-third of patients had poor seizure control. Caregiver relationship (father AOR=0.58; 95th CI:0.35,0.97) and poor adherence (AOR=2.97; 95th CI:1.82, 4.86) were significantly associated with treatment outcome.
Conclusion: One-third of children with epilepsy have poor seizure control. Poor adherence to treatment is implicated in poor control. Counseling caregivers on proper treatment and adherence to anti-epileptic medication is recommended to improve treatment outcome in children.
Keywords: children, epilepsy, seizure, treatment outcome, Ethiopia
Introduction
Epilepsy is a brain disorder caused by the chronic propensity to develop epileptic seizures. A clinical diagnosis of epilepsy is made if there have been at least two unprovoked seizures that occur more than 24 h apart, one unprovoked seizure with a recurrence probability of more than 60% over the subsequent 10 years.1,2 However, when epilepsy is clinically difficult to diagnose, as in childhood absence epilepsy, EEG may help support the diagnosis.3 However, EEG is not available in many centers in developing countries.
An estimated 10 million children under the age of 15 are estimated to have active epilepsy worldwide, constituting approximately 25% of the epilepsy population. Over 80% of the 3.5 million people with epilepsy who obtain diagnoses each year are under the age of 15, and 40% of them are in developing nations.4 There are wide variations in the incidence of epilepsy in Africa, from 5.2 per 1000 people in Ethiopia to 58 per 1000 people in Cameroon.5
Seizure type, epilepsy type, aetiology, and epileptic syndrome are the four categories used by the International League Against Epilepsy (ILAE) to classify epilepsy.6 Prior febrile seizures, birth trauma, head injuries, infections of the central nervous system such as neurocysticercosis, and a family history of epilepsy were discovered in sub-Saharan Africa as risk factors for epilepsy.7 Classification of epilepsy based on whether the seizure type is focal or generalized is of primary importance in localization in the brain focus. Since we are classifying epilepsy, terminology may change, using up-to-date terminology may be useful for communication and research purposes.8–10
There are four distinct phases of an epileptic seizure: prodromal, aura, ictal, and postictal.5 The existence of these phases varies among patients with epilepsy; for example, in contrast to other studies, one study found that many patients with generalized epilepsy also had aura.11 Automatism is another manifestation of epilepsy observed mainly in patients with focal epilepsy.12
The mortality rate of people with epilepsy is reported to be 2 to 3 times higher than that of the general population, necessitating timely intervention.7 In addition, childhood epilepsy has a high psychosocial impact on both parents and children.13–15 Finally, the presence of suicidal ideation and comorbidities related to the disease must be addressed.16
Antiepileptic drugs are the main treatment options.17,18 Their primary treatment goals are to prevent seizures, reduce adverse reactions or drug interactions, improve quality of life, provide effective care, and ensure patient satisfaction.4,19
Around 20–30% of patients with epilepsy develop drug resistance epilepsy.16 Only 50% of children with childhood-onset epilepsy may make a satisfactory transition to adulthood, with seizure remission, the withdrawal of anti-epileptic medications, and good quality of life.20 EEG results, patient history of seizure frequency and density, and etiology of syndromes affect the outcome.21 Ethiopia has been implementing community-based health insurance since 2011. By contributing ETB 240 (less than $5 USD) annually, households will get free health services at public health facilities. However, public health facilities have shortages of medicines, medical supplies, reagents, and laboratory and diagnostic services. Although Ethiopia has a high prevalence of epilepsy, only a few studies have specifically focused on the clinical profile, etiology, and treatment outcomes of epilepsy in children and adolescents. The aim of this study is to evaluate the clinical characteristics, etiology, and therapeutic outcomes of epilepsy in children and adolescents in a specialized tertiary hospital in North-west Ethiopia. In our study, we used a period of 1 year without seizures for controlled seizures.
Methods and Materials
Study Area and Period
The study was conducted at the Debre Markos Comprehensive Specialized Hospital, which is located in the Amhara Region, North-west Ethiopia. Debre Markos is an administrative seat in the East Gojjam Zone in Amhara Region, Ethiopia, located 300 km north-west of Addis Ababa, the capital of Ethiopia. The pediatric department serves both patient care and academic activities at Debre Markos University College of Medicine and Health Sciences. This department provides both inpatient and outpatient services. The follow-up clinic is an activity of the department handled by consultant pediatricians and general practitioners on a twice-weekly basis. An average of 150–200 pediatric epileptic patients up to the age of 17 years are being followed up every 3 months at this clinic. The study was conducted from October 28, 2020, to April 28, 2021.
Study Design
We conducted a hospital-based retrospective cohort study at Debre Markos Comprehensive Specialized Hospital.
Source and Study Population
All pediatric epileptic patients who were attending the Debre Markos Comprehensive specialized hospital and who fulfilled the inclusion criteria were included in the study. Epileptic patients aged 17 years or younger, with a minimum follow-up of 12 months, were included in the study. Pediatric epileptic patients with incomplete medical records or charts or those with uncertain clinical profiles and final seizure control status due to insufficient follow-up duration or loss to follow-up were excluded.
Sample Size Determination
The minimum sample size for this study was calculated using a single population proportion with the following assumptions: 95% confidence level, 5% margin of error, 6% proportion of uncontrolled seizures.8 When the assumed prevalence is too low (going to be below 10%), a precision of 5% appears to be inappropriate. As a result, a conservative choice would be half the prevalence, as the amount of precision in our case was small (P=6%) the calculated sample size will be 347. After considering 10% for incompleteness of cards, the calculated sample size for the first objective was 385.
For the second objective, we calculated the sample size using the double-proportion formula and the Stata Version 14 statistical software. For sample size estimation, the following variables were considered major predictors of uncontrolled seizures: These variables are sex and adherence to anti-epileptic drugs.8 For sex, we used CI=95%, POWER=80%, % of outcomes among unexposed=29%, OR=2.12, and calculated sample size will be 234.8 For second factor, adherence to medication, we took CI=95%, power=80%, % of outcome among unexposed=16.7, OR=4.69, and calculated sample size will be 86.8 Accordingly, the maximum sample size based on the above two methods, after considering 10% incompleteness, was 385. Therefore, it is advisable to use the maximum sample size to obtain a precise parameter estimate. The final sample size was 385. Simple random sampling was used to select the samples.
Variables
The dependent variables were clinical characteristics of the patients and treatment outcomes (uncontrolled vs controlled).
The independent variables were sociodemographic characteristics (age, sex, area of residence, ethnicity, parental marital status, occupation, education, and income), type of epilepsy, type of medication (polytherapy vs monotherapy), age at seizure onset, duration of treatment, type of seizure at onset, adherence to treatment, frequency of seizure before initiation of therapy, and comorbidity.
The Following Terms and Operational Definitions are Used
Epilepsy is considered to be present when >1 unprovoked seizure occurs in a time frame of >24 h or one unprovoked seizure and a probability of further seizures to the general recurrence risk after two unprovoked seizures, occurring over the next 10 years, or diagnosis of epileptic syndrome.
Controlled epilepsy refers to being seizure-free for at least 12 consecutive months following anti-epileptic therapy.
Drug resistant epilepsy is patients whose seizures do not successfully respond to antiseizure medication (ASM) (two tolerated, appropriately chosen, and used ASMs) therapy is considered to have drug-resistant epilepsy (DRE).
Status epilepticus was defined as a seizure lasting greater than 5 min with no interruption or more than one seizure with no regaining of consciousness in between.
Polytherapy uses more than one ASMs for controlling of seizure.
Adherence is defined as the extent to which individuals take their medications as prescribed, with respect to dosage and dosage intervals. Excellent adherence is if the patients took more than 90% of their monthly medications. Good adherence is due to the use of more than 85% of his/her monthly medication. Poor adherence occurs if the patient took less than 85% of their monthly medications.
Data Collection Tool and Procedure
Data were collected using a structured questionnaire and data abstraction sheet. The tool was designed based on a literature review. Face-to-face interviews and record reviews were employed to collect data from the study participants and parents or caregivers. The data were collected and supervised by trained and experienced health-care providers and principals/co-investigators. Two training days were provided to both the data collectors and supervisors. The training focused on the purpose of the study, the content of the tool, the data collection technique, ethical issues, and the roles and responsibilities of the data collectors and supervisors.
Data Quality Control
To ensure the quality of the data, emphasis was placed on properly designed data-collection instruments. Training was provided to both data collectors and supervisors. To ensure the validity and consistency of the tool, the tool was pre-tested in 5% of the samples out of the study area. The principal investigator supervised and coordinated data collection activities.
Data Processing and Analysis
Collected data were manually checked for completeness. Data were coded and entered into the Epi-data software version 4.4.2.1 and then exported to the Stata version 14 statistical package for analysis. For continuous variables, normality is checked using the Shapiro–Wilk test. Descriptive statistics were performed and summarized using tables, graphs, medians, and IQR. The proportion of clinical characteristics was coded as yes or no. Controlled seizures were defined as seizure-free for the last 12 months, and yes and no were the codes for classification. Bivariate logistic regression analysis was performed between dependent and independent variables. Variables with a p-value of <0.25 in ordinal logistic regression, were potential candidates for multivariate logistic regression analysis to control for confounders in the regression models. Variables with a p-value of less than 0.05, in the multivariable ordinal logistic regression model, were considered statistically significant. Model fitness was checked using the Hosmer–Lemeshow goodness-of-fit test. The strength of association between the outcome variable and independent variables was reported by using the adjusted odds ratio with 95% CI).
Ethical Consideration
Prior to data collection, ethical clearance was obtained from the Debre Markos University School of Medicine's Institutional Research Ethics Review Committee (IRERC) (ethical approval NO: IRERC 076/21). Ethical approval was granted on Feb 1/2021. A letter of permission was obtained from the respective hospital body. Written consent was obtained from the parents/guardians, and their rights were respected if they did not want to be involved in the study. The anonymity of the study subjects was maintained confidential and was intended to be the only research purpose.
Result
Socio-Demographic Characteristics
Among 385 children and adolescents with epilepsy on follow-up, 61.6% were male. Median age of patients was 12 years with an interquartile range of 8–15 years, more than half (60.3%) of patients are in the age range of 11 to 17 years. Median age of onset of seizure is 7 years (IQR of 4–11 years), and most of patients had onset of seizures above infancy (82.5%). Predominantly (70.9%), patients lived in rural areas, and most of (57.9%) their caretakers are illiterate. The majority of caregivers (50.1%) were fathers of patients (Table 1).
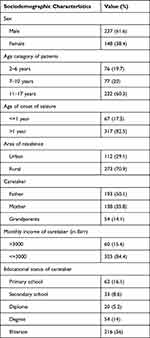 |
Table 1 Socio-Demographic Characteristics of Patients with Epilepsy at Comprehensive Specialized Hospital of North-West Ethiopia, 2021 (n = 385) |
Clinical Characteristic
Many (47.3%) of patients had at least one form of aura. Fear is the most common aura symptom followed by blurred vision. Manifestations of automatism were observed in only a few patients (2.5%). Chewing (1.8%) was the most common automatic manifestation. More than three-third of patients had generalized tonic-clonic seizures. Focal seizures were reported in 4.7% of patients. The majority of patients had a seizure with a dual peak, both during the day and night. More than two-thirds of the patients had recurrent seizures (more than 4 times per week) before starting ASMs. A significant number (67.5%) of patients had no precipitating factors. Anxiety was the most common precipitating factor followed by sleep deprivation. Sleep is the most common type of postictal phenomenon. History of status epilepticus and family history of epilepsy were identified in 7.3% and 5.4% of patients, respectively. In majority of patients, the cause was unidentified; in patients with identified causes, HIE was the common cause. 78.4% had no comorbid condition, and CP with global developmental delay was the most common comorbid condition. Only a few patients underwent EEG and MRI/CT (3.9% and 2.9%, respectively). 54(14%) sustained a seizure-related injury, these include soft tissue injury (abrasions and lacerations) 17(4.42%), dental or nasal injury 10(2.6%), burns 7(1.8%), dislocations 7(1.8%), fractures 7(1.8%), and head injury 6(1.6%) (Table 2).
 |
Table 2 Clinical Characteristics of Patients with Epilepsy at Comprehensive Specialized Hospital of North-West Ethiopia, 2021 (n = 385) |
Treatment Characteristics
Approximately 90% of caregivers had the awareness that epilepsy was treatable. The median follow-up duration was 45 months with IQR 16–98 months. The median time to seizure control was 36 months (IQR=15–48 months). Herbal medicine and prayer were used before ASMs in 49.1% of patients. The median time from onset of epilepsy to treatment was 18 months (IQR = 12–21.6) months and a range of 8.4 −25.2 months in both the seizure-controlled and uncontrolled groups. Most patients were on monotherapy, with phenobarbitone (55.8%) being the most common monotherapy used followed by phenytoin (28.1%). Sixteen (4%) of patients transferred to third ASMs. Phenobarbitone and phenytoin are the most commonly used combinations. 33% of patients had reported adverse drug reactions. Hyperactivity was a common adverse drug reaction (22.3%). Majority of patients had excellent adherence (57.1%). The majority (52%) of patients were in the maintenance phase of therapy. More than half of patients experienced controlled seizures (64.2%). Drug-resistant epilepsy occurred in 16(4.16%) of patients (Table 3).
 |
Table 3 Treatment Characteristics of Patients with Epilepsy at Comprehensive Specialized Hospital of North-West Ethiopia, 2021 (n = 385) |
Associated Factors with Controlled Seizure
Multivariate analysis showed that caregivers’ illiteracy, timing of seizure, frequency of seizure before ASMs, central nervous system infection as the etiology of epilepsy, cerebral palsy with developmental delay as a comorbid condition, age of seizure onset, and drug adherence were associated with controlled seizures. Patients with educated caregivers were more likely to have controlled seizures than those with illiterate caregivers. However, only caregivers with primary education had a statistically significant association with controlled seizures. Patients with caregivers with primary education were 3.2 times more likely to have controlled seizures than those with an illiterate caregiver (95% CI=1.52–6.80; P=0.002). Seizures with dual peaks, both day and night, were 54% less likely to have controlled seizures than those occurring in the afternoon only (95% CI=0.25–0.86, P=0.014). Similarly, patients with recurrent seizures were 62% less likely to develop controlled seizures compared to patients with single seizure (95% CI=0.20–0.71, P=0.002), and meningitis as an etiology of epilepsy was 61% less likely to develop controlled seizures compared to patients with unknown causes (AOR=4.03, 95% CI=0.16–0.90, P=0.028). Patients with an age of onset of seizure > 1 year were 2.2 times more likely to develop controlled seizures than those with an onset age of < 1 year (95% CI=1.18–4.20, P=0.013). Similarly, patients with excellent adherence to ASMs were 2.5 times more likely to develop controlled seizures than those with poor adherence (95% CI=1.23–5.03, P=0.011). Comorbid conditions such as the presence of cerebral palsy associated with developmental delay associated with 72% less likely to have controlled seizure than patients with no comorbidity (95% CI=0.94–0.84, P=0.024). Adverse drug reactions and perinatal asphyxia as the etiology of epilepsy were associated with controlled seizures in bivariate analysis; however, in multivariate analysis, they were not associated with controlled seizures (Table 4).
 |
Table 4 Factors Associated with Treatment Outcome in Epileptic Patients at Comprehensive Specialized Hospital of North-West Ethiopia, 2021 (n = 385) |
Discussion
This study was done in a resource limited country where imaging to reach diagnosis (MRI, CT, and EEG) is not easily available. Newer anti-epileptic drugs, which are very expensive, are also either unaffordable or not available. This study may be used as a representative to most African countries. Our study found that a significant percentage of patients (47.3%) reported having an aura, corroborating the findings that generalized tonic-clonic seizures were the most frequent seizure type (67.5%), and that unknown etiology was the most prevalent etiology (84.9%) in epileptic patients. It also revealed that most patients experience postictal symptoms, as shown in other studies, although fewer triggering situations and automatism were observed. Additionally, it showed a significantly lower percentage of drug-resistant epilepsy (4.2%) and a lower proportion of patients (64.2%) with controlled seizures. Primary education of the caregiver and excellent adherence were factors that increased the rate of controlled seizures. In contrast, frequent seizure episodes before ASMs treatment, seizures with dual peak time, the presence of cerebral palsy with developmental delay such as comorbidity, the presence of autism spectrum disorder as comorbidity, and meningitis as an epilepsy etiology decreased the rate of controlled seizures.
The majority of the epileptic patients in our study were male (61.6%), as they were in many other studies involving epileptic patients, including those conducted in China (50.9%), the United States (53%), Nigeria (52.3%), Uganda (56.7%), Northern Ethiopia’s Finote Selam and Debre Markos regions (58.7%), and Southern Ethiopia, Mizan Tepi region (51.7%).22–27 In our study, the majority of them resided in rural areas (70.9%), similar to studies conducted in Southwest Ethiopia (Jima study, 57.6%), Mizan Tepi (51.7%), Finote Selam and Debre Markos (75.1%).26–28 A study in Gondar found that the patient’s father (44.8%) provided the majority of the patient’s care, which was also the case in our study (50.1%).29 However, studies in southwest Ethiopia and Malaysia found that both the patient’s mother and father provided care equally, with 44.1% each in the former and 40% each in the latter.28,30
According to one study involving multiple African countries, proximate causes of epilepsy in Africa are prenatal events (11%), acute encephalopathy (10%), and head injuries before seizures started (3%), are the most common proximate causes of epilepsy in Africa.31 In a previous study conducted in Nigeria, similar to our study, meningitis and perinatal asphyxia were the two most frequently identified causes of epilepsy.32 This can be explained by the high incidence of infectious disorders, such as meningitis with inadequate treatment and poor perinatal care for women in poorer nations, where the majority of mothers delivered at home, predisposing the infant to sepsis with meningitis and perinatal asphyxia. Most of the time the cause of epilepsy cannot be determined. Unknown causes were reported in 50.6, 50.5%, and 64% in Turkey, Canada, and Finland studies, respectively.33–35 The cause was also unknown in 84.9% of patients in our study. The presence of a notably high unknown etiology in this study can be explained by the fact that the majority of our patients did not undergo a thorough investigation because genetic testing, MRI and CT scans were unavailable, there were financial limitations to conducting these investigations, and there were not enough experts to interpret these investigations.
It is crucial to identify any precipitating causes that are crucial for characterizing the clinical features. Even if the presence of at least one precipitating factor varies among studies—53%, 27.5%, 86.9%, and 60% in combined samples in USA, Denmark, and Norway, (Polish, Indian, and Ethiopian studies, respectively) stress is one of the precipitating factors that is present in all of these studies. In these studies, additional triggering factors included skipping meals, skipping medications, sleep deprivation, fever or infection, smoking, fatigue, and flashing lights.36–39 In our study, precipitating factors were present in 32.5% of the patients. Correspondingly, anxiety, lack of sleep, and photic stimulation were the three most frequently recognized precipitating causes.
In our study, GTCS was the most common type of seizure, identified in 87.8% of patients. This result is comparable to those of Rwanda (80.4%) and Jima study (78.7%). Two Nigerian (61.1 and 59.3%) and Turkish studies also showed generalized seizures were the predominant seizure type.24,32,40,41 In contrast to our study, complex partial seizures with secondary generalization and focal seizures were the most common types reported in Canadian and Qatar studies.34,42 Misclassification may have occurred in our study because patients arrived late, mostly to determine whether the generalized seizure was primary or secondary, and the use of EEG, which is important for the classification of seizures, was minimal. The sociodemographic characteristics of the studies can also be used to explain the differences in seizure types across studies.
Epilepsy causes not only neuronal injury but also seizure-related physical injury to the patient. Studies have shown that patients with epilepsy are more likely to require hospitalization after suffering an injury, which causes epilepsy-related injuries to both the victim and their caregiver. Injuries included fractures, burns, concussions, and dislocations.43 In a study conducted in Gondar, seizures injured 27.9% of patients with epilepsy. Abrasions made up 12.5% of these injuries, followed by burns (5.9%), dental wounds (4.4%), fractures (2.2%), and head injury and dislocations (1.5%, respectively).44 Our study’s injury rate was much lower at 54 (14%), but abrasions were still the most common type of injury, just like in the Gondar study. The presence of a significant percentage of patients who experienced seizures while receiving ASMs in the Gondar study caused a discrepancy in the proportion of seizure-related damage between our study and the previous study.
Regarding the type of treatment used, monotherapy was the most common regimen utilized in our study (86%), which has similar findings to those reported from China, Nigeria, Debre Markos and Finote Selam.26,45,46 Various initial ASMs were used as monotherapy throughout the study because, as mentioned previously, the choice of ASMs is affected by many factors. In our study, phenobarbitone (55.8%) was used as a monotherapy commonly followed by phenytoin (28.1%) Contrary to our study, two African studies and one European study conducted in Rwanda, Nigeria, and Holland frequently utilize sodium valproate as a monotherapy, whilst two Ethiopian studies conducted in Ambo and Gondar and study done in five sub-Saharan sites revealed that phenobarbital is frequently used as a monotherapy.31,39,40,46–48 Since managing epilepsy is costly in our situation, choosing an ASM is primarily based on cost, because the majority of patients have a poor socioeconomic status.
Regarding adverse drug reactions is the target of ASMs, a study conducted in southern Nigeria and Turkey revealed that side effects occur in 38.3% and 3.61% of patients, respectively.33,46 However, studies conducted in our nation’s Gondar region revealed 47.6% and 17.6% of adverse medication reactions, whereas, in our study, only 25% occurred.29,48 In our study, the most typical medication side effect was hyperactivity (22.3%). Because the majority of our patients were from low socioeconomic backgrounds and could not undergo laboratory testing to check for certain consequences that require testing, such as hepatotoxicity, the frequency of drug reactions may have been higher in our study. In our study, the occurrence of hyperactivity as a common adverse drug reaction was explained by the frequent use of phenobarbitone, either as a monotherapy or in combination with other drugs.
In the present study, approximately 15% had developed ASMs with ASM-related adverse effects. This was lower than that in studies conducted in Ambo (42%), Hawassa (35%), Jima (46%), and Bahr Dar (38%) studies.28,39,49,50 The higher adherence rate in our study may be explained by the lower incidence of drug reactions. Alternatively, since patient self-reporting was used to monitor adherence, self-reporting bias may have occurred because patients may have reported better adherence in need of acceptance by health-care professionals.
Our study’s-controlled seizure rate of 62.4% was lower than studies carried out in China, Canada, Turkey, Spain, which showed controlled seizure rates of 72%, 68.4%, 67.5%, 81%, respectively, but higher than Scotland, Zambia, Ethiopia-Ambo region, Ethiopia-Mizan Tepi, Ethiopia-Jimma, and Ethiopia-Gondar regions, which showed 59.2%, 23.6%, 55.3%, 39.2%, 54.1%, and 94%, respectively22,27–29,33,34,39,51–53 (Figure 1).
 |
Figure 1 Comparison of good treatment response between other studies and this study. |
Contrary to two Scottish studies that revealed that the majority (more than 50%) of seizure control occurred within the first 13 months of ASM treatment, our results demonstrate that seizure control occurs mostly throughout the 13-to 24-month period.51,54 This difference can be attributed to a variety of factors, including the use of various methodologies, models, variations in study adherence levels, exclusion of non-adherent patients in some studies, use of various ASMs as monotherapy or in combination therapy, and variations in sociodemographic characteristics, such as age and ethnicity (Figure 2).
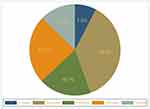 |
Figure 2 Timing of seizure control after initiation of anti-epileptic drugs used of patients with epilepsy at Comprehensive Specialized Hospital of North-west Ethiopia, 2021 (n = 385). |
In our study, children receiving monotherapy had a seizure control rate of 65.3%. This is corroborated by studies from Nepal (57.5%), Turkey (67.5%), Australia (55.3%), and an Ethiopian study from Gondar (73%), which the majority of the seizures were similarly controlled by monotherapy.29,33,55,56 Because the controlled seizure rate is different in various studies, there must be factors that affect treatment outcomes in patients with epilepsy (Figure 3).
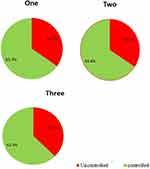 |
Figure 3 Number of anti-epileptic drugs used and treatment outcome of patients with epilepsy Comprehensive Specialized Hospital of North-west Ethiopia, 2021 (n = 385). |
The age at which the patient’s epilepsy first manifested itself during infancy is one of the factors that lead to poor treatment outcomes for epilepsy patients in this study. Research from the US, which demonstrated that while the age of onset > 1 year was related to controlled seizures, the age of onset during infancy was associated with poor seizure control, supported this finding.23 Additionally, the three-year seizure remission rate was low in a Japanese study of patients with seizure onset below 1 year. The age-specific encephalopathies such as Ohtahara syndrome, severe myoclonic epilepsy, and West syndrome, which are difficult to control the seizure begin to manifest in this age group, as well as congenital central nervous system anomalies and cerebral palsy, may explain the association between poor seizure control and the onset of epilepsy during infancy.57
In our study, caregiver illiteracy was associated with poor treatment outcome. A relationship between epilepsy treatment outcomes and caregivers’ educational levels was observed in an Egyptian study. In this study, owing to its correlation with poor ASM adherence, caregiver illiteracy was found to be associated with poor treatment outcomes.58 Additionally, literature suggests that it is feasible to improve patient treatment and outcomes by preventing communication gaps between patients with epilepsy, their caregivers, and health-care professionals through greater two-way communication and goal alignment through good adherence.59 This was supported by a quasi-experimental study conducted in Saudi Arabia, which found that caregivers’ educational level was related to their epilepsy knowledge, attitude, reported practice score level, and self-efficacy score after epilepsy education. In other words, two-way communication becomes easier, and educated caregivers can easily understand counseling.60
In our study, poor treatment outcomes were associated with more than four seizures per week before starting ASM therapy. In line with our study, according to an Australian study, there is a 6% lower chance of being seizure-free during the last clinic visit for every increase in the number of seizures experienced prior to therapy.56 Studies from Mizan Tepi, Ambo, and Jimma found that having more than three and four seizures per week, respectively, were associated with poorly controlled seizures.27,39,41 This may be explained by neuronal damage caused by seizures, which may result in the formation of an epileptic focus in the brain.
Even though a study in Turkey showed no statistically different outcomes in unknown, crying and symptomatic epilepsy, a study conducted in Japan showed etiology of epilepsy significantly affects epilepsy treatment outcomes, with a 3-year remission rate for unknown epilepsy is 62% and 50% for symptomatic seizures.33,61 Another Japanese study showed three-year remission rate for post-encephalitis epilepsy was much lower than unknown etiology 50% and 91%, respectively.57 Our study also demonstrated that symptomatic causes, such as CNS infections, are related to poor treatment outcomes, similar to a Japan study that showed encephalitis and meningitis had remission of 58.3% (84). Unprovoked seizures caused by bacterial meningitis tend to be recurrent and focal mostly, with a reported remission rate of 50%.62 Additionally, a study conducted in India showed that the 6 months seizure outcome in tuberculosis meningitis was poor.63 Viral encephalitis such as herpes simplex encephalitis is associated with drug-resistant epilepsy.64
In our study, the presence of CP in individuals with epilepsy affected treatment outcomes, as these patients were more likely to experience poor seizure control than those without CP. The remission rate in our study (3.6%) was comparable to remission reported in other studies looking at 2-year outcomes in Germany and Hong Kong, which were 12% and 16%, respectively.65,66
Adherence to ASMs was another factor that affected treatment outcomes in our study. Similarly, epilepsy treatment outcomes in studies conducted in Zambia and two Jimma studies, Mizan Tepi, Ayder, Addis Ababa, and Afar, were affected by the degree of adherence to ASMs.27,28,41,53,67,68 Even though the clinician orders the appropriate medication and performs the right dosing titration, it will be challenging to control seizures in patients with epilepsy if they are not taking the medications properly.
Limitation of the Study
The first limitation of this study is its type. This type of study is a retrospective cohort study. It was difficult to remember the basic information. Another limitation of the study is that generalized tonic-clonic seizure is the one who worried about the parents and sought medical help. Therefore, the number of patients with focal seizures is small.
Conclusion and Recommendation
The median age of the participants and age at seizure onset were 12 and 7 years, respectively. Generalized tonic-clonic seizures were the most common seizure type identified, and phenobarbitone was the single most common drug that the participants were treated with. 62.4% participants had good control of seizure. Caregiver illiteracy, timing of seizure, frequency of seizure before ASMs, central nervous system infection such as etiology of epilepsy, cerebral palsy with developmental delay as a comorbid condition, age of seizure onset, and drug adherence are associated with controlled seizures.
Abbreviations
ADR, Adverse Drug Reaction; ASMs, Anti Seizure Medication; AOR, Adjusted Odd Ratio; CBZ, Carbamazepine; CI, Confidence interval; DRE, Drug Resistance Epilepsy; EEG, Electroencephalogram; GTC, Generalized Tonic-Clonic; HIV, Human Immunodeficiency virus; ILAE, International League against Epilepsy; OR; Odd Ratio; PHB, Phenobarbitone; PHT, Phenytoin; SD, Standard Deviation; SNNPR, Southern nations and nationalities people region; SPSS, Statistical Package for Social Science; VPA, Valproic acid; WHO, World Health Organization.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethical Clearance
The study complied with the principles of the Declaration of Helsinki. Ethical approval was obtained from the Debre Markos University School of Medicine's Institutional Research Ethics Review Committee (IRERC) (ethical approval NO: IRERC 076/21). Ethical clearance was granted on Feb 1/2021.
Consent
Written informed consent was obtained from each parent/guardian and child aged > 12 years to participate in the interview and to extract data from their medical charts. Consent was obtained and verified by a research ethics review board. The address of the person mentioned is stated in the ethical clearance letter submitted to the manuscript. Privacy and confidentiality were ensured during the parents/guardians, patient interviews, and medical chart reviews.
Acknowledgment
We would like to acknowledge the participants and data collectors for providing us with consent to share their history and collect the data. We also wish to express our gratitude to Arba Minch University for funding this research and providing ethical clearance.
Disclosure
The authors declare no conflicts of interest regarding the publication of this article.
References
1. Perucca P, Scheffer IE, Kiley M. The management of epilepsy in children and adults. Med J Austra. 2018;208(5):226–233. doi:10.5694/mja17.00951
2. Singh A, Trevick S. The epidemiology of global epilepsy. Neurol Clin. 2016;34(4):837–847. doi:10.1016/j.ncl.2016.06.015
3. Kim HR, Kim GH, Eun SH, Eun BL, Byeon JH. Therapeutic outcomes and prognostic factors in childhood absence epilepsy. J Clin Neurol. 2016;12(2):160–165. doi:10.3988/jcn.2016.12.2.160
4. Guerrini R. Epilepsy in children. Lancet. 2006;367(9509):499–524.
5. Dekker PA; World Health Organization. Epilepsy: A Manual for Medical and Clinical Officers in Africa. World Health Organization; 2002.
6. Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58(4):512–521. doi:10.1111/epi.13709
7. Ba-Diop A, Marin B, Druet-Cabanac M, Ngoungou EB, Newton CR, Preux PM. Epidemiology, causes, and treatment of epilepsy in sub-Saharan Africa. Lancet Neurol. 2014;13(10):1029–1044. doi:10.1016/S1474-4422(14)70114-0
8. Fisher RS, Bonner AM. The revised definition and classification of epilepsy for neurodiagnostic technologists. Neurodiagn J. 2018;58(1):1. doi:10.1080/21646821.2018.1428455
9. Brodie MJ, Zuberi SM, Scheffer IE, Fisher RS. The 2017 ILAE classification of seizure types and the epilepsies: what do people with epilepsy and their caregivers need to know? Epil Dis. 2018;20(2):77–87. doi:10.1684/epd.2018.0957
10. Sarmast ST, Abdullahi AM, Jahan N. Current classification of seizures and epilepsies: scope, limitations and recommendations for future action. Cureus. 2020;12(9):1.
11. Polat H, Aluçlu MU, Özerdem MS. Evaluation of potential auras in generalized epilepsy from EEG signals using deep convolutional neural networks and time-frequency representation. Biomed Eng Biomed Tech. 2020;65(4):379–391. doi:10.1515/bmt-2019-0098
12. Blair RD. Temporal lobe epilepsy semiology. Epilepsy Res Treat. 2012;2012:1–10. doi:10.1155/2012/751510
13. Spangenberg JJ, Lalkhen N. Children with epilepsy and their families: psychosocial issues. South African Family Pract. 2006;48(6):60–63. doi:10.1080/20786204.2006.10873411
14. Kokeb A Lived Experience of Young People with Epilepsy in Bahir Dar City Government Specialized and Referral Hospitals, Ethiopia, (Doctoral dissertation); 2021.
15. Hairrell L. The role of foundations and NGOs in mitigating epilepsy stigma. African Middle East Epil J. 2021;10(2):1.
16. Dalic L, Cook MJ. Managing drug-resistant epilepsy: challenges and solutions. Neuropsychiatr Dis Treat. 2016;12:2605. doi:10.2147/NDT.S84852
17. Ramiah R. Newer interventions in epilepsy management. Indian J Pract Pediat. 2020;22(1):14.
18. Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. Lancet. 2006;367(9516):1087–1100. doi:10.1016/S0140-6736(06)68477-8
19. Garnett WR. Antiepileptic drug treatment: outcomes and adherence. Pharmacotherapy. 2000;20(8P2):191S–9S. doi:10.1592/phco.20.12.191S.35250
20. Camfield P, Camfield C, Arts WF, Brouwer OF, Arzimanoglou A. The outcome of childhood epilepsy: what improvements are needed? Epil Dis. 2013;15(2):101–104. doi:10.1684/epd.2013.0583
21. Leach JP. Treatment of epilepsy–towards precision. F1000Research. 2018;7:1932. doi:10.12688/f1000research.16448.1
22. Yao L, Cai M, Chen Y, Shen C, Shi L, Guo Y. Prediction of antiepileptic drug treatment outcomes of patients with newly diagnosed epilepsy by machine learning. Epil Behav. 2019;96:92–97. doi:10.1016/j.yebeh.2019.04.006
23. Berg AT, Wusthoff C, Shellhaas RA, et al. Immediate outcomes in early life epilepsy: a contemporary account. Epil Behav. 2019;97:44–50. doi:10.1016/j.yebeh.2019.05.011
24. Ipingbemi AE. Management, treatment outcome and cost of epilepsy in a tertiary health care facility in northern Nigeria. Int J Med Update. 2015;10(2):25–36. doi:10.4314/ijmu.v10i2.5
25. Kaddumukasa M, Kaddumukasa M, Matovu S, Katabira E. The frequency and precipitating factors for breakthrough seizures among patients with epilepsy in Uganda. BMC Neurol. 2013;13(1):1–7. doi:10.1186/1471-2377-13-182
26. Getnet A, Woldeyohannes SM, Bekana L, et al. Antiepileptic drug nonadherence and its predictors among people with epilepsy. Behav Neurol. 2016;2016:1–6. doi:10.1155/2016/3189108
27. Zewudie A, Mamo Y, Feyissa D, Yimam M, Mekonen G, Abdela A. Epilepsy treatment outcome and its predictors among ambulatory patients with epilepsy at Mizan-Tepi University Teaching Hospital, southwest Ethiopia. Neurol Res Int. 2020;2020:1–8. doi:10.1155/2020/8109858
28. Mohammed H, Lemnuro K, Mekonnen T, Melaku T. Adherence to anti-seizure medications and associated factors among children with epilepsy at tertiary Hospital in Southwest Ethiopia: a cross-sectional study. BMC Neurol. 2022;22(1):1. doi:10.1186/s12883-022-02842-8
29. Beyene A, Ayalew AF, Mulat G, Simachew Kassa A, Birhan T, Russo E. The treatment outcomes of epilepsy and its root causes in children attending at the University of Gondar teaching hospital: a retrospective cohort study, 2018. PLoS One. 2020;15(3):e0230187. doi:10.1371/journal.pone.0230187
30. Lua PL, Khairuzzaman NK, Aziz ZA, Foo JL. The needs and problems in epilepsy caregiving: a qualitative exploration. ASEAN J Psych. 2015;2015:116–126.
31. Kariuki SM, Matuja W, Akpalu A, et al. Clinical features, proximate causes, and consequences of active convulsive epilepsy in A frica. Epilepsia. 2014;55(1):76–85. doi:10.1111/epi.12392
32. Eyong KI, Ekanem EE, Asindi AA, Chimaeze T. Clinical profile of childhood epilepsy in Nigerian children seen in a tertiary hospital. Int J Contemp Pediatr. 2017;4(4):1138–1141. doi:10.18203/2349-3291.ijcp20172658
33. Arhan E, Serdaroglu A, Kurt AN, Aslanyavrusu M. Drug treatment failures and effectivity in children with newly diagnosed epilepsy. Seizure. 2010;19(9):553–557. doi:10.1016/j.seizure.2010.07.017
34. Dudley RW, Penney SJ, Buckley DJ. First-drug treatment failures in children newly diagnosed with epilepsy. Pediatr Neurol. 2009;40(2):71–77. doi:10.1016/j.pediatrneurol.2008.09.021
35. Eriksson KJ, Koivikko MJ. Prevalence, classification, and severity of epilepsy and epileptic syndromes in children. Epilepsia. 1997;38(12):1275–1282. doi:10.1111/j.1528-1157.1997.tb00064.x
36. Nakken KO, Solaas MH, Kjeldsen MJ, Friis ML, Pellock JM, Corey LA. Which seizure-precipitating factors do patients with epilepsy most frequently report? Epil Behav. 2005;6(1):85–89. doi:10.1016/j.yebeh.2004.11.003
37. Stanuszek A, Wnękowicz E, Kuźniar E, Krakowska K, Gergont A, Kaciński M. Seizure-precipitating factors in relation to medical recommendations: especially those limiting physical activity. J Child Neurol. 2015;30(12):1569–1573. doi:10.1177/0883073815574334
38. Balamurugan E, Aggarwal M, Lamba A, Dang N, Tripathi M. Perceived trigger factors of seizures in persons with epilepsy. Seizure. 2013;22(9):743–747. doi:10.1016/j.seizure.2013.05.018
39. MekonenTefera G, Woldehaimanot TE, Tarekegn M. Poor treatment outcomes and associated factors among epileptic patients at Ambo Hospital, Ethiopia. Euro J Ther. 2015;21(1):9–16.
40. Van Steenkiste F, Fidèle S, Nsanzabaganwa W, et al. An ambispective cohort study on treatment outcomes of patients with epilepsy in a tertiary epilepsy center in Rwanda and recommendations for improved epilepsy care. Epilepsia Open. 2019;4(1):123–132. doi:10.1002/epi4.12304
41. Gidey K, Chelkeba L, Gemechu TD, Daba FB. Treatment response and predictors in patients with newly diagnosed epilepsy in Ethiopia: a retrospective cohort study. Sci Rep. 2019;9(1):1–7. doi:10.1038/s41598-019-52574-y
42. Haddad N, Melikyan G, Al Hail H, et al. Epilepsy in Qatar: causes, treatment, and outcome. Epil Behav. 2016;63:98–102. doi:10.1016/j.yebeh.2016.07.043
43. Nguyen R, Tellez-Zenteno J. Injuries in epilepsy: a review of its prevalence, risk factors, type of injuries and prevention. Neurol Int. 2009;1(1):e20. doi:10.4081/ni.2009.e20
44. Bifftu BB, Tadesse Tiruneh B, Mekonnen Kelkay M, et al. Seizure-Related Injuries Among People with Epilepsy at the Outpatient Department of the Cross-Section. North-west Ethiopia: University of Gondar Hospital; 2017.
45. Yu L, Feng J, Yu Z, Dai H. Trends of anti-seizure medication use in pediatric patients in six cities in China from 2013 to 2018. Epilepsy Res. 2020;167:106448. doi:10.1016/j.eplepsyres.2020.106448
46. Eshiet UI, Erah PO, Ekeh BC. Utilisation of antiepileptic drugs in a tertiary referral centre, Southern Nigeria. J Pharm Res Int. 2018;24(6):1.
47. Geerts A, Arts WF, Stroink H, et al. Course and outcome of childhood epilepsy: a 15‐year follow‐up of the Dutch Study of Epilepsy in Childhood. Epilepsia. 2010;51(7):1189–1197. doi:10.1111/j.1528-1167.2010.02546.x
48. Birru EM, Shafi M, Geta M. Drug therapy of epileptic seizures among adult epileptic outpatients of University of Gondar referral and teaching hospital, Gondar, North West Ethiopia. Neuropsychiatr Dis Treat. 2016;12:3213. doi:10.2147/NDT.S119030
49. Dima SA, Shibeshi MS, Papadelis C. Antiepileptic drug adherence in children in southern Ethiopia: a cross sectional study. PLoS One. 2022;17(2):e0263821. doi:10.1371/journal.pone.0263821
50. Gurshaw M, Agalu A, Chanie T. Anti-epileptic drug utilization and treatment outcome among epileptic patients on follow-up in a resource poor setting. J Young Pharma. 2014;6(3):47. doi:10.5530/jyp.2014.3.8
51. Hitiris N, Mohanraj R, Norrie J, Sills GJ, Brodie MJ. Predictors of pharmacoresistant epilepsy. Epilepsy Res. 2007;75(2–3):192–196. doi:10.1016/j.eplepsyres.2007.06.003
52. Ramos-Lizana J, Rodriguez-Lucenilla MI, Aguilera-López P, Aguirre-Rodríguez J, Cassinello-García E. A study of drug-resistant childhood epilepsy testing the new ILAE criteria. Seizure. 2012;21(4):266–272. doi:10.1016/j.seizure.2012.01.009
53. Mwansa NJ, Daka V, Mulenga D, et al. Correlates of seizure control among patients with epilepsy at two referral hospitals in Zambia. Int J Trans Med Res Pub Health. 2020;4(2):130–138. doi:10.21106/ijtmrph.182
54. Brodie MJ, Barry SJ, Bamagous GA, Norrie JD, Kwan P. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78(20):1548–1554. doi:10.1212/WNL.0b013e3182563b19
55. Poudel P, Kafle SP, Pokharel R. Clinical profile and treatment outcome of epilepsy syndromes in children: a hospital‐based study in Eastern Nepal. Epilepsia Open. 2021;6(1):206–215. doi:10.1002/epi4.12470
56. Chen Z, Brodie MJ, Liew D, Kwan P. Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: a 30-year longitudinal cohort study. JAMA Neurol. 2018;75(3):279–286. doi:10.1001/jamaneurol.2017.3949
57. Oka E, Yamatogi Y, Ohtsuka Y, Ohtahara S. Clinical course and prognosis of childhood epilepsy. Pedia Int. 1989;31(3):259–266. doi:10.1111/j.1442-200X.1989.tb01300.x
58. Korkmaz MF, Erdem-Uzun M, Korkmaz M, Ekici A. Adherence to antiepileptic drugs and the health literacy of caregivers in childhood epilepsy. P R Health Sci J. 2020;39(1):45–50.
59. Becker DA, Long L, Santilli N, Babrowicz J, Peck EY. Patient, caregiver, and healthcare professional perspectives on seizure control and treatment goals. Epil Behav. 2021;117:107816. doi:10.1016/j.yebeh.2021.107816
60. Hameed SM, Mohammed HR. Knowledge, attitude, practice, and self-efficacy of caregivers of children with epilepsy: impact of a structured educational intervention program. Epilepsy Seizure. 2021;13(1):1–6. doi:10.3805/eands.13.1
61. Okuma T, Kumashiro H. Natural history and prognosis of epilepsy: report of a multi‐institutional study in Japan: the group for the study of prognosis of epilepsy in Japan. Epilepsia. 1981;22(1):35–53. doi:10.1111/j.1528-1157.1981.tb04331.x
62. Murthy JM, Prabhakar S. Bacterial meningitis and epilepsy. Epilepsia. 2008;49(s6):8–12. doi:10.1111/j.1528-1167.2008.01750.x
63. Misra UK, Kumar M, Kalita J. Seizures in tuberculous meningitis. Epilepsy Res. 2018;148:90–95. doi:10.1016/j.eplepsyres.2018.10.005
64. Misra UK, Tan CT, Kalita J. Viral encephalitis and epilepsy. Epilepsia. 2008;49(s6):13–18. doi:10.1111/j.1528-1167.2008.01751.x
65. Aksu F. Nature and prognosis of seizures in patients with cerebral palsy. Dev Med Child Neurol. 1990;32(8):661–668. doi:10.1111/j.1469-8749.1990.tb08426.x
66. Kwong KL, Wong SN, So KT. Epilepsy in children with cerebral palsy. Pediatr Neurol. 1998;19(1):31–36. doi:10.1016/S0887-8994(98)00011-3
67. Niriayo YL, Mamo A, Kassa TD, et al. Treatment outcome and associated factors among patients with epilepsy. Sci Rep. 2018;8(1):1–9. doi:10.1038/s41598-018-35906-2
68. Nasir BB, Yifru YM, Engidawork E, Gebrewold MA, Woldu MA, Berha AB. Antiepileptic drug treatment outcomes and seizure-related injuries among adult patients with epilepsy in a tertiary care hospital in Ethiopia. Patient Relat Outcome Meas. 2020;11:11. doi:10.2147/PROM.S243867
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
