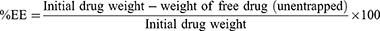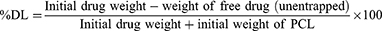Back to Journals » Nanotechnology, Science and Applications » Volume 16
Chitosan-Coated Azithromycin/Ciprofloxacin-Loaded Polycaprolactone Nanoparticles: A Characterization and Potency Study
Authors Yassin AE, Albekairy AM, Omer ME , Almutairi A, Alotaibi Y, Althuwaini S, Alaql OA, Almozaai SS, Almutiri NM, Alluhaim W , Alzahrani RR, Alterawi AM, Halwani MA
Received 5 October 2023
Accepted for publication 9 December 2023
Published 21 December 2023 Volume 2023:16 Pages 59—72
DOI https://doi.org/10.2147/NSA.S438484
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Kattesh Katti
Alaa Eldeen Yassin,1,2 Abdulkareem M Albekairy,1,3 Mustafa E Omer,4 Arwa Almutairi,1 Yousef Alotaibi,1 Salem Althuwaini,1 Osama Aql Alaql,1 Shahad S Almozaai,1 Nouf Mohammed Almutiri,1 Wed Alluhaim,2,5 Raghad R Alzahrani,2,5 Asma M Alterawi,6,7 Majed A Halwani1,2
1College of Pharmacy, King Saud bin Abdulaziz University for Health Sciences, Ministry of National Guard Health Affairs, Riyadh, Saudi Arabia; 2King Abdullah International Medical Research Center, Ministry of National Guard Health Affairs, Riyadh, Saudi Arabia; 3Pharmaceutical Care Services, King Abdulaziz Medical City, Ministry of National Guard Health Affairs, Riyadh, Saudi Arabia; 4Pharmacy Program, College of Health and Sport Sciences, University of Bahrain, Zallaq, Bahrain; 5Department of Botany and Microbiology, College of Science, King Saud University, Riyadh, 11451, Saudi Arabia; 6Department of Pharmaceutics and Center for Pharmaceutical Engineering and Sciences, School of Pharmacy, Virginia Commonwealth University, Richmond, VA, 23298, USA; 7Faculty of Pharmacy, Jordan University of Science and Technology, Irbid, Jordan
Correspondence: Alaa Eldeen Yassin; Majed A Halwani, King Abdullah International Medical Research Center, PO Box: 3660, Riyadh, 11481, Saudi Arabia, Tel +966509426323 ; +966540396771, Fax +966114299999#95058, Email [email protected]; [email protected]
Purpose: Antimicrobial resistance is a major health hazard worldwide. Combining azithromycin (AZ) and ciprofloxacin (CIP) in one drug delivery system was proposed to boost their antibacterial activity and overcome resistance. This study aims to improve azithromycin and ciprofloxacin activity by co-encapsulating them inside chitosan-coated polymeric nanoparticles and evaluating their antibacterial activity.
Methods: The double emulsion method was employed to co-encapsulate AZ/CIP inside chitosan-coated polymeric nanoparticles. The formulations were evaluated for their nanoparticle size, size distribution, and zeta potential. Differential scanning calorimetry (DSC) analysis characterized the formula’s thermal sustainability. Encapsulation efficiency was measured by HPLC and spectrophotometric analysis. Morphological studies used the Transmission Electron Microscopy (TEM). The in vitro release profiles of both AZ and CIP were monitored utilizing the dialysis membrane bag method. The micro-dilution assay assessed the antimicrobial activity against a clinical isolate of Klebsiella pneumoniae.
Results: The prepared AZ/CIP-poly-caprolactone nanoparticles were spherical; their size range was 184.0 ± 3.3– 190.4 ± 5.6 nm and had high size uniformity (poly-dispersity index below 0.2). The zeta potential ranged from − 21.2 ± 2.4 to − 27.0 ± 2.5 mV, while chitosan-coated nanoparticles showed a positive zeta potential value ranging from 8 to 11 mV. The thermal study confirmed the amorphous state of both antibiotics inside the nanoparticles. The results of the in vitro release study indicated a slow and uniform rate of release for both drugs extended over 4-days, with a faster rate in the case of AZ. The MIC values reported for both chitosan-coated NP have been tremendously reduced by at least 15 folds of pure CIP and more than 60 folds of pure AZ.
Conclusion: The co-encapsulation of AZ/CIP into chitosan-coated polymeric nanoparticles has been successfully achieved. The produced particles showed many beneficial attributes of uniform particle sizes below 200 nm and high zeta potential values. Chitosan-coated polymeric nanoparticles extensively enhanced the antibacterial activity of both AZ/CIP against bacteria.
Keywords: azithromycin, ciprofloxacin, poly-caprolactone, chitosan-coated nanoparticles, differential scanning calorimetry
Introduction
Antimicrobial resistance (AMR) is currently a worldwide threat that jeopardizes the efficacy of critical antimicrobial treatment.1–3 The rising prevalence of drug-resistant pathogens with new mechanisms, resulting in antibiotic resistance, continues to jeopardize our capacity to cure communicable diseases. The alarming global spread of multi- and pan-resistant bacteria is especially concerning. It renders routine medical procedures such as surgery, organ transplants, chemotherapy, and diabetes management extremely risky.1,4
Azithromycin (AZ) is a semi-synthetic macrolide derived from erythromycin. AZ binds to the 50s subunit of the 70s ribosome of susceptible microorganisms, inhibiting protein synthesis, which is crucial for bacterial survival and growth.5,6 AZ spectrum of antimicrobial activity encompasses many gram-positive and gram-negative bacteria but is more effective against gram-negative bacteria.7–9 Compared to older-generation macrolides, AZ has a distinct pharmacokinetic property. It has a high volume of distribution (Vd) of about 23L/kg.10 AZ rapidly accumulates in many areas of the body, including the lung mucosa, gastrointestinal tract (GIT), and genital tract, making it more useful for pulmonary, sexually transmitted diseases (STDs), and infections of the ear, sinuses, and throat.10–12 AZ bioavailability after oral administration is only 37%—due to poor absorption.13 AZ has a long elimination half-life, allowing for a once-daily dosing regimen.14
Ciprofloxacin (CIP) is a fluoroquinolone antimicrobial synthetic antibiotic. It inhibits bacterial cell reproduction by interfering with DNA gyrase and topoisomerase IV, which are required to separate and replicate bacterial DNA.15 CIP has a broad spectrum of activity against gram-positive and gram-negative bacteria and has demonstrated clinical efficacy in treating complicated urinary tract infections (UTI), STDs, GIT infections, skin and bone infections, and other infections.9,16 CIP bioavailability after oral administration is approximately 70–80%.17 After absorption, it is widely distributed to body tissues, bones, and fluids, and the apparent volume of distribution is 1.7–5L/kg.16,18 Only 33% of the drug is eliminated non-renally as an unchanged drug in feces and metabolites, while the majority is eliminated renally.18 According to the World Health Organization (WHO), AZ and CIP are essential medicines for health systems.1
Many reports in the literature concluded a synergistic effect for CIP and AZ combinations against different bacterial strains.19–23 For example, Martin et al tested the synergism between AZ/CIP against the intracellular non-pneumonic Legionella. They confirmed that this combination was more synergistic against Legionella micdadei and Legionella bozemanii than other macrolide-ciprofloxacin combinations.21 The more exciting and significant impact of the AZ/CIP combination is their efficacy against resistant biofilms such as Pseudomonas aeruginosa’s biofilm-associated urinary tract infections20 and chronic rhinosinusitis.19 Saini et al reported a synergy with a fractional inhibitory concentration index equal to 0.37 against P. aeruginosa PAO1 planktonic cells. They attributed this effect to the ability of AZ to disrupt the biofilm reverting it to a planktonic state and allowing CIP to induce its known powerful antipseudomonal effect.20 Also, Lim et al found that although the biofilm-forming P. aeruginosa lipopolysaccharide layer stimulates the production of interleukin 8 (IL-8), causing increasing rhinosinusitis severity, it was reduced with AZ alone and further decreased when using AZ/CIP combination.19 Interestingly, their tested cell line, human sinonasal epithelial cells (HSNECs), preserved its cellular function and rigidity and showed no toxicity to the drug combination.19 Furthermore, a randomized prospective study conducted by Magri et al revealed that combining these two antibiotics may result in a therapeutic synergistic effect in treating chronic prostatitis syndromes.24
In recent years, polymeric nanoparticles (NPs) have emerged as potential new antimicrobials to combat pathogenic microorganism resistance by controlling therapeutic levels, reducing toxicity, and protecting against premature degradation.25–27 PCL has unique and tailorable physico-chemical properties that make it an ideal nano-drug delivery carrier for a wide range of applications among all aliphatic polyester polymers.28,29 PCL demonstrated excellent drug loading ability and uniform controlled release properties due to its low glass transition and melting points, semi-crystallinity, and high mechanical flexibility.30,31 Chitosan, the N-deacetylated form of chitin, is the world’s second most abundant polysaccharide. It has been widely used for drug delivery because of its warranted safety, biodegradability, biocompatibility, and stability.25,32,33 Coating NPs with chitosan has demonstrated beneficial properties, significantly improving drug penetration, tissue distribution, and overall therapeutic efficacy.34,35 Furthermore, Alzahrani et al36 reviewed the antibacterial activity of chitosan nanoparticles and concluded a significant enhancement of the antibacterial activity of silver and metallic nanoparticles when coupled with chitosan. Mu et al investigated the synergistic effect of several antibiotics combined with chitosan on eradicating Listeria monocytogenes biofilms. The results showed that chitosan combined with other antibiotics, such as amikacin, vancomycin, or erythromycin, was more effective than the antibiotic alone in inhibiting or disrupting L. monocytogenes biofilms.37 This study aims to develop chitosan-coated polymeric nanoparticles loaded with AZ and CIP to enhance the antibacterial activity against resistant bacterial strains, biological isolates, and biofilms.
Materials and Methods
Materials
Azithromycin (AZ), poly-caprolactone (PCL, M.W 42KD), dichloromethane, Tween 80, Mueller Hinton agar, Mueller Hinton broth (cation adjusted), and sodium deoxycholate were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA) through a local vendor. Ciprofloxacin was obtained as a kind gift from Tabuk Pharmaceuticals (Tabuk, Saudi Arabia). All other reagents and chemicals were of analytical grade.
Methods
Preparation of Azithromycin/Ciprofloxacin Co-Encapsulated Polymeric Nanoparticles
A simple O/W emulsion-solvent evaporation method was modified to co-encapsulate AZ/CIP into poly-caprolactone matrix NPs.38 Briefly, specific AZ, CIP, and PCL weights were dissolved in 10 mL dichloromethane and mixed with 20 mL aqueous solution containing 10mg Tween 80 and 5 mg sodium deoxycholate. The mixture was subjected to probe-sonication under ice at 40% intensity for 1 min to form the primary emulsion. Then, the formed emulsion was dispersed in another 40 mL of the surfactant solution and subjected to another 2 min cycle of probe sonication under an ice bath. The formed emulsion was stirred overnight at room temperature inside a fume hood to allow for complete evaporation of dichloromethane and separation of NPs. Then, the dispersion was centrifuged at 15,000 RPM for 30 min at 4°C (Heraeus Megafuge 16R-Thermo Fisher Scientific, Germany) in Eppendorf tubes. The supernatant was removed, and a sample was kept at −30°C to determine entrapment efficiency and drug loading. The particles were washed twice by re-centrifugation of the residue with a small volume of double distilled water. The collected particles were stabilized by freeze-drying (BETA 2–8 LDplus–CHRIST, Germany).
Two formulations were prepared using the above preparation method with the composition listed in Table 1.
 |
Table 1 The Composition of Prepared AZ/CIP-Polymeric Nanoparticle Formulations |
Chitosan Coating of Nanoparticles
The method reported by Badran et al39 was employed. Briefly, 100 mg of freeze-dried NPs were dispersed in 5 mL of 2% chitosan solution for 2 hours. Afterward, the NPs were separated by centrifugation at 15,000 rpm for 15 minutes at 4°C, washed with chilled deionized water, and re-centrifuged.
Evaluation of the Prepared Nanoparticles
Measurement of Particle Size
A Brookhaven ZetaPALS (Brookhaven Instruments Corporation, Holtsville, NY, USA) was utilized to determine the particle size and polydispersity for each prepared formulation before and after chitosan coating. Each sample was diluted to a concentration of 0.1% of NPs dispersion using double distilled water. The size measurements were determined at a 90° angle.
Determination of Zeta Potential
The laser Doppler velocimetry (LDV) mode was utilized to determine zeta potential values for each nanoparticle formulation using the same instrument for particle size determination. Diluted samples (0.1%) were also used for the measurement.
Thermal Profile Monitoring of the Prepared Nanoparticles
From each freeze-dried NP formulation, a 3–5 mg sample was placed inside the aluminum crucible pan and spread uniformly before compression. Samples were scanned under nitrogen in a 25–300°C temperature range at a heating rate of 10°C/min using a Differential Scanning Calorimetry (DSC 214 polyma-NETZSCH, Germany). Nitrogen was used as a purging gas at a 40 mL/min flow rate. Thermograms of NP formulations were compared with those of the pure AZ and CIP samples.
Measurement of Drug Entrapment Efficiency and Drug Loading
After the centrifugation processes (as mentioned previously in preparation for AZ/CIP-polymeric NPs), the collected supernatants of each formulation were filtered using a syringe filter (WhatmanTM 0.45 µm PTFE). Then, the concentrations of CIP were determined using a previously described simple spectrophotometric method,40 while another sensitive RP-HPLC method was employed for measuring AZ concentrations.41 Finally, the percentage of drug entrapment efficiency (%EE) was calculated using the following equation.
Spectrophotometric Determination of Ciprofloxacin
Using a previously constructed standard calibration curve, the CIP concentration was determined by measuring UV absorbance at λ= 277 nm with a Thermo Scientific Evolution 60S UV/Visible Spectrophotometer. With R2 = 0.9998, the standard curve demonstrated high linearity in the concentration range of 0.5 to 10 µg of CIP in methanol.
HPLC Determination of Azithromycin
The reversed-phase HPLC method described by Ghari et al41 was utilized to quantify entrapped AZ in poly-caprolactone (PCL) NPs using an Agilent 1200 series HPLC system equipped with a 1260 series Infinity Diode Array Detector (DAD; Agilent, Santa Clara, CA). A C18, 150 mm 4.6 mm ZORBAX Eclipse XDB column (Cat. no. 993967, Agilent) with a particle size of 5 m was used for the separation. The mobile phase was isocratically eluted at a 1.2 mL/min flow rate using an 80:20 (v/v) methanol: 0.02M phosphate buffer pH 8 solution. The measurement was carried out at λ= 210 nm.
Particle Morphology
The morphological properties of the prepared NPs were examined using a transmitted electron microscope (TEM). The measurements were taken with a (JEOL, Tokyo, Japan) JEM-1400 electron microscope set to 120 kV acceleration voltage. A sample of one drop (1 mg/mL) was placed on a 400-mesh copper grid coated with carbon. Before imaging, the samples were stained and allowed to dry at room temperature. Samples were stained with UranyLess, a negative staining reagent introduced by EMS (Hatfield, PA, USA).
In vitro Release
In vitro release profiles of AZ and CIP from NPs were investigated in a phosphate buffer saline (PBS) medium using the dialysis membrane bag method, which had been modified slightly.42 Simply 10 mg of each nanoparticle formulation were dispersed in 2 mL PBS and placed in a dialysis bag (molecular weight cut off: 12 kDa, Livingstone, NSW, Australia), which had been pre-soaked in distilled water for 12 hours and was firmly tied from one end. Each bag was immersed in a container containing 20 mL of PBS after the other end was securely tied. Triplicate samples represented each NP formulation. All the containers were incubated at 37 ± 1°C in a shaking water bath adjusted to 80 rpm shaking speed. To maintain sink conditions, 3 mL samples were removed from the medium at regular intervals and replaced with the same volume of fresh PBS medium incubated at the same conditions. The samples were analyzed for AZ and CIP using the methods described above. The cumulative percentage of each drug released was plotted against time.
Antimicrobial Activity of Nanoparticles Formulations
The micro-broth dilution assay was performed to determine the MIC as previously reported43 with some modifications. A clinical isolate of Klebsiella pneumoniae (RKP-R010) was used to test the activity of CIP and AZ loaded inside NPs of NP-1, NP-2, Cs-NP-1, and Cs-NP-2. RKP-R010 inoculum was adjusted to 0.5 McFarland standard and then exposed to different 2-fold serial dilutions of each tested formula/antibiotic. The highest tested concentration (128 µg/mL) of each free antibiotic (CIP) and (AZ) was prepared in Muller-Hinton broth (MHB) and loaded into the 96-well round-bottom plate. In another 96-well plate, a serial dilution of all NPs (NP-1, NP-2, Cs-NP-1, and Cs-NP-2) was also added to the tested strain. Drug-free bacterial suspension and MHB medium alone were used as positive and negative controls, respectively. The plates were then incubated at 37°C for 24 hours. After the incubation period, the (MIC) was determined as the concentration that inhibited bacterial growth visibly. To determine the minimum bactericidal concentration (MBC), 1 µL of the clear wells (without growth) were inoculated on MH agar plates and incubated for 24 hours at 37°C. The clinical isolate was obtained anonymously after routine diagnostic procedures from King Abdulaziz Medical City Microbiology Laboratory and the Infectious Diseases Research Department at King Abdullah International Research Center (KAIMRC). The study was conducted after receiving the IRB approval (SP19/235/R). Escherichia coli ATCC 25922 was used as the quality control bacteria in antibiotic susceptibility testing to verify that the susceptibility results were accurate. The results of the antibiotic susceptibility tests were validated using the CLSI guidelines.44
Results
The Characterization of Nanoparticles for Formulations
The results of the nanoparticle size measurements are summarized in Figure 1. Nanoparticle sizes of the two formulations and the drug-free batch (NP-B) were clearly in a close range between (187.7 to 216.8 nm). This size range was considered very low, considering the encapsulation of two drugs within the nanoparticles. They all exhibited a low coefficient of variation below 3%, indicating the uniformity of nanoparticle sizes. The uniformity of the nanoparticle sizes was also indicated by the polydispersity index values in Figure 1. The polydispersity index values were within the range of (0.152 to 0.189), indicating the size uniformity of formed nanoparticles.
NP-1 and NP-2 formulations had drug-to-polymer ratios of 1:10 and 1:7, respectively. The main nanoparticle population was a bit wider in the range from (70 to 600 nm) with a minor population within the range of 25–50 nm (Figure 1). However, the drug-to-polymer ratio did not affect the particle size. It was empirical that the nanoparticle coating with chitosan would cause an increase in particle sizes by almost 30%. Figure 2 compares the zeta-potential values for both coated and uncoated nanoparticles. The zeta potential of the uncoated polymeric nanoparticles was −21.2, −22.2, and −27.0 mV for the blank NP-1 and NP-2, respectively. After coating with chitosan, the surface charges have turned to +8 and +11.4 for Cs-NP1 and Cs-NP2. This generally indicates reasonable in vitro and in vivo stability of the nanoparticle.
Encapsulation Efficiency of Azithromycin and Ciprofloxacin Inside Chitosan-Coated Polymeric Nanoparticles Formulations
The entrapment efficiency for CIP and AZ into the PCL-nanoparticles was generally higher than 80%, indicating the robustness of the optimized double emulsion preparation method (data is tabulated in Table 2). CIP had %EE of about 90%, showing a significantly higher %EE than those reported with AZ (~83%). This can be attributed to the hydrophilic nature of AZ compared to the CIP base.
 |
Table 2 Mean Percent Entrapment Efficiency (EE) and Percent Drug Loading (DL) Measurements for All Formulations |
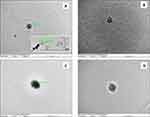
The Morphology Study of the Formulation’s Nanoparticles
All the images showed variation in the texture under high magnification (30,000X-60,000X), indicating the encapsulation of drugs inside the polymer matrices of the nanoparticles. Figure 3A and B depicts the TEM images of different nanoparticles from NP-2 and Cs-NP-2 batches. In Figure 3C and D, the presence of shadows encircling the Cs-NP-2 particles demonstrates the gel layer made by the chitosan coat. The size of all the nanoparticles was smaller than the reported mean nanoparticle sizes obtained by the dynamic light scattering (DLS), and this is common due to the difference in the nature of samples and the principle of measurement in both techniques.45
The Thermal Analysis of AZ and CIP Inside Chitosan-Coated Polymeric Nanoparticles Formulations
The DSC thermograms for NP-1 and NP-2 were compared with those of the pure CIP, pure AZ form, and the blank NP formula (NP-B), as presented in Figure 4. CIP thermogram showed a sharp endothermic peak at 274.2 °C corresponding to the melting point of CIP, which appears to occur close to what was reported before,40 while at a slightly higher temperature than the one reported by Tehler et al at 266°C.46 The thermogram of pure AZ showed a melting endotherm at 124°C, similar to what was reported in the literature.47 A sharp endothermic peak was observed at 59°C with an NP-B thermogram corresponding to the melting of PCL polymer. The DSC thermograms of both NP-1 and NP-2 formulations appeared similar to that of the NP-B with the same melting endotherm of PCL.
AZ and CIP Retention Inside Chitosan-Coated Polymeric Nanoparticles Formulations
The CIP release profile from the prepared nanoparticles was demonstrated within 96 hours in Figure 5. Generally, CIP exhibited a slow uniform release pattern from all nanoparticle formulations with no burst release. Complete drug release was only achieved with Cs-NP-2 after 72 hours; for the other formulations, the maximum amount released after 96 hours was 60–75%. It is clear that the higher the ratio of the drug to PCL, the slower the rate of release. The chitosan-coated formulations exhibited a significantly faster rate of CIP release. This is probably due to the hydrogel nature of chitosan, which allows for more wetting CIP, resulting in faster rate solubility in the particles’ hydrogel coat. Figure 6 depicts the release of AZ from the four formulations. AZ followed a faster release rate than almost complete drug release within the 96-hour study period. The profile was more likely to follow a bi-phasic pattern with a faster drug release in the first 10 hours, followed by a much slower rate after that. The impact of the drug-to-PCL ratio and the chitosan coating were similar to that observed with CIP release. Figure 7 compares the uncoated nanoparticle formulation’s CIP and AZ release profiles. The release of AZ is significantly faster than CIP, with more profound in the initial stage, which can be attributed to the higher water solubility of AZ. This faster rate of AZ was also observed with chitosan-coated formulations; however, the biphasic pattern of AZ has been abolished and turned more uniform, as demonstrated in Figure 8. This can be attributed to chitosan’s hydrogel characteristics, which can provide an additional release control factor to the rapidly dissolved AZ.
It is evidenced that nano-scaled chitosan, which has a higher surface-to-volume ratio and higher surface charge density, leads to increased affinity to bacteria and higher antimicrobial activity.34
The Antibacterial Activity of Encapsulated AZ and CIP Inside Chitosan-Coated Polymeric Nanoparticles Formulations
The MIC and MBC values of free antibiotics and nanoparticles are illustrated in (Table 3). The assay was performed on the clinical bacterial isolate RKPR010 to examine the antibacterial activity of the CIP/AZ-loaded nanoparticle formulations. The NP-1 and NP-2 formulations exhibited moderated antibacterial activity (18.75 and 37.5 µg/mL) compared to the free CIP and AZ (16 and 64 µg/mL). On the other hand, the other nanoparticle formulations, Cs-NP-1 and Cs-NP-2, significantly inhibited bacterial growth at low concentrations (0.94 µg/mL) compared to the free form of both CIP and AZ (Table 1). In addition, the MBC values of Cs-NP-1 and NP-2 nanoparticles eradicate bacterial growth entirely at a lower concentration of 1.88 µg/mL compared to free forms of CIP and AZ (32 and 128 µg/mL), respectively.
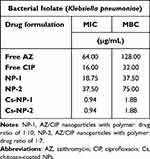 |
Table 3 The MIC and MBC Values for All Uncoated and Chitosan-Coated Polymeric Nanoparticle Formulations |
Discussion
The double-emulsion preparation method successfully overcame many challenges, including differences in solubility, pKa, and molecular mass. The method was optimized to produce nanoparticles with low particle size, high uniformity, reasonable stability, and, more importantly, high entrapment efficiency. The particle sizes reported in this study are comparable to those reported in the literature for chitosan-coated polymeric nanoparticles. Wang et al48 created chitosan-coated 5-fluorouracil-loaded PLGA nanoparticles with particle sizes ranging from 233 to 280 nm. Many studies have found that coating polymeric nanoparticles with chitosan increases particle size by 20 to 30%.39,48 Many biological benefits of drugs incorporated into nanoparticulate systems necessitate boosted cellular uptake. Aside from the chemical nature of the nanoparticles, it has been reported that sizes ranging from 100 to 350 nm can trigger cellular uptake via the clathrin-mediated endocytosis pathway.49
In polymer-based nanoparticle drug delivery, PDI values below 0.2 are considered highly acceptable,50,51 while PDI below 0.3 is considered reliable for lipid-based systems. The FDA’s “Guidance for Industry” for liposome systems did not set a golden number for PDI values. However, it emphasized the importance of both Particle size and PDI as crucial quality parameters.52 The size distribution plot for NP-B (Figure 3) showed a single-particle population ranging from (70 to 500 nm). The obtained zeta potential values below 30mV are not considered a critical issue for the physical stability because chitosan-coated nanoparticle dispersions are lyophilic colloids (solvent liking) stabilized primarily by forming a protective layer of solvent (solvent effect).53,54 Remarkably, the TEM images (Figure 3) of the dispersed particles in the liquid phase exhibited swelling in addition to the presence of a solvent-stagnant layer that is usually measured as part of the particle’s hydrodynamic radius in DLS, which is consistent with what has been shown in the literature.47,53 Fissan et al explained that unlike the geometric size—measuring the size accurately—the hydrodynamic size of the particles will include ligands, stabilizers, hydration coating, or polymer shells, which will result in a larger particle size in DLS compared to TEM.54
The absence of the melting peaks of both CIP and AZ in the DSC of both formulations indicates the existence of both drugs in the amorphous states inside the polymeric matrix of the nanoparticles, confirming the solubility of both drugs in the polymer.55
The observed CIP release profile from the prepared formulations was more uniform and slower than Günday and coauthors reported.56 They showed that CIP release from PLGA nanoparticles followed a biphasic pattern with a maximum of 75% released after 24 hours.57 In another study, the CIP release from nanoparticles made entirely from chitosan was significantly slower, with only 10% of the drug released after 96 hours.58
The AZ release profiles from our nanoparticles are more uniform than the particles reported in another study in which the PLGA nanoparticles were utilized as a matrix for AZ.59 They reported a biphasic release pattern with almost 50% burst release and a slow-release rate that completes within 24 hours.59
The simplest and the most widely used techniques for the qualitative and quantitative assessment of antimicrobial activity are diffusion and dilution (phenotypic) methods.60 In this study, we used the broth microdilution method, which is considered the gold standard bioassay to determine antimicrobial’s MIC against microorganisms.60 Our AZ/CIP formulations (NP-1 and 2) had moderate antibacterial activity. On the other hand, the chitosan-coated AZ/CIP formula significantly inhibited Klebsiella pneumonia growth at a lower concentration (MIC = 1.88 µg/mL) compared to the free AZ and CIP (MIC = 128 and 32 µg/mL). According to previous studies, chitosan encourages negatively charged microbial cell walls to interact with cytoplasmic membranes, resulting in decreased osmotic stability, membrane breakdown, and eventual leakage of intracellular elements. It can also enter bacterial nuclei and bind to microbial DNA, preventing mRNA and protein synthesis.33 Costa et al35 found that chitosan-coated daptomycin-loaded alginate NPs effectively treated methicillin-resistant Staphylococcus aureus causing bacterial endophthalmitis.35 This was attributed to chitosan’s cationic nature, which allows for opening the blood-retinal barrier tight junction, enhancing permeation across ocular epithelia.35 Our findings are consistent with those of Raouf et al.61 They used an ion gelation method to encapsulate AZ and CIP in chitosan nanoparticles and tested their ability to eliminate planktonic and biofilm formed by Pseudomonas aeruginosa. They observed a significant decrease in both the MIC and the minimum biofilm eradication concentration (MBEC) when compared to the combination of free Az and CIP. Also, Jamil et al62 sought to investigate and develop cefazolin-loaded chitosan nanoparticles against multidrug-resistant (MDR) pathogens in vitro. The encapsulation efficiency increased proportionally to the concentration of antibiotics (28–62%).62 Furthermore, growth kinetics using inhibition zone (agar-diffusion) and spectrophotometer (broth dilution) revealed the potency of cefazolin-loaded chitosan-covered NPs against MDR K. pneumoniae, P. aeruginosa, and Extended Spectrum Beta Lactamase (ESBL)-producing Escherichia coli.62 Lastly, we expect that the improved activity of azithromycin and ciprofloxacin is due to coating the particles with chitosan—demonstrated by the lowered effective dose (MIC) of both antibiotics.
Conclusion
A simple, reliable, and reproducible method was used to successfully encapsulate AZ/CIP into chitosan-coated polymeric nanoparticles. The nano-particles produced had uniform spherical particles with sizes less than 200 nm, high size uniformity indicated by PDI values less than 0.2, and reasonably high zeta potential values. The method successfully encapsulated a high percentage of both drugs within the polymer matrix of the nanoparticles. The physical adsorption of chitosan on the surface of particles was confirmed by increasing particle size and changing the particle surface charge from negative to positive. Both drugs released slowly and uniformly over a long period of time. The antibacterial activity of chitosan-coated polymeric nanoparticles against a clinical isolate of Klebsiella pneumoniae was greatly enhanced.
Acknowledgments
This work was funded by two grants from King Abdullah International Medical Research Center (KAIMRC), Ministry of National Guard Health Affairs (MNG-HA), Riyadh, Saudi Arabia (Grants No. SP19/235/R) and (SP19/237/R). The funding agency had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Antimicrobial Resistance. World Health Organization. Available from: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance;.
2. Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. 2015;109(7):309–318. doi:10.1179/2047773215Y.0000000030
3. CDC’sAntibiotic Resistance Threats in the United States, 2019. U.S. Department of Health and Human Services, CDC; Available from: www.cdc.gov/DrugResistance/Biggest-Threats.html.
4. Akash MSH, Rehman K, Fiayyaz F, Sabir S, Khurshid M. Diabetes-associated infections: development of antimicrobial resistance and possible treatment strategies. Arch Microbiol. 2020;202(5):953. doi:10.1007/s00203-020-01818-x
5. Parnham MJ, Haber VE, Giamarellos-Bourboulis EJ, Perletti G, Verleden GM, Vos R. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol Ther. 2014;143(2):225–245. doi:10.1016/j.pharmthera.2014.03.003
6. Drew RH, Gallis HA. Azithromycin--spectrum of activity, pharmacokinetics, and clinical applications. Pharmacotherapy. 1992;12(3):161–173. doi:10.1002/j.1875-9114.1992.tb04504.x
7. Peters DH, Friedel HA, McTavish D. Azithromycin. Drugs. 1992;44(5):750–799. doi:10.2165/00003495-199244050-00007
8. Lalak NJ, Morris DL. Azithromycin Clinical Pharmacokinetics. Clin Pharmacokinet. 1993;25(5):370–374. doi:10.2165/00003088-199325050-00003
9. Annex W World Health Organization Model List of Essential Medicines – 23rd List. In:
10. Foulds G, Shepard RM, Johnson RB. The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother. 1990;25(Suppl A):73–82. doi:10.1093/jac/25.suppl_A.73
11. Singlas E. Clinical pharmacokinetics of azithromycin. Pathol Biol. 1995;43(6):505–511.
12. Bakheit AHH, Al-Hadiya BMH, Abd-Elgalil AA. Azithromycin. Profiles Drug Subst Excip Relat Methodol. 2014;39:1–40.
13. Heidary M, Ebrahimi Samangani A, Kargari A, et al. Mechanism of action, resistance, synergism, and clinical implications of azithromycin. J Clin Lab Anal. 2022;36(6):e24427. doi:10.1002/jcla.24427
14. Sandman Z, Iqbal OA. Azithromycin. StatPearls Publishing; 2023.
15. Spencer AC, Panda SS. DNA Gyrase as a Target for Quinolones. Biomedicines. 2023;11(2):371. doi:10.3390/biomedicines11020371
16. Campoli-Richards DM, Monk JP, Price A, Benfield P, Todd PA, Ward A. Ciprofloxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1988;35(4):373. doi:10.2165/00003495-198835040-00003
17. Thai T, Salisbury BH, Zito PM. Ciprofloxacin. StatPearls Publishing; 2023.
18. Ungphakorn W. Pharmacometric Models of Oral Ciprofloxacin for Children with Malnutrition. University of Strathclyde; 2012.
19. Lim DJ, Thompson HM, Walz CR, et al. Azithromycin and ciprofloxacin inhibit IL-8 secretion without disrupting human sinonasal epithelial integrity in vitro. Int Forum Allergy Rhinol. 2021;11(2):136. doi:10.1002/alr.22656
20. Saini H, Chhibber S, Harjai K. Azithromycin and ciprofloxacin: a possible synergistic combination against Pseudomonas aeruginosa biofilm-associated urinary tract infections. Int J Antimicrob Agents. 2015;45(4):359–367. doi:10.1016/j.ijantimicag.2014.11.008
21. Martin SJ, Pendland SL, Chen C, Schreckenberger P, Danziger LH. In vitro synergy testing of macrolide-quinolone combinations against 41 clinical isolates of Legionella. Antimicrob Agents Chemother. 1996;40(6):1419–1421. doi:10.1128/AAC.40.6.1419
22. Saini H, Chhibber S, Harjai K. Antimicrobial and antifouling efficacy of urinary catheters impregnated with a combination of macrolide and fluoroquinolone antibiotics against Pseudomonas aeruginosa. Biofouling. 2016;32(5):511–522. doi:10.1080/08927014.2016.1155564
23. Lim DJ, Skinner D, Mclemore J, et al. In-vitro evaluation of a ciprofloxacin and azithromycin sinus stent for Pseudomonas aeruginosa biofilms. Int Forum Allergy Rhinol. 2020;10(1):121–127. doi:10.1002/alr.22475
24. Magri V, Montanari E, Škerk V, et al. Fluoroquinolone-macrolide combination therapy for chronic bacterial prostatitis: retrospective analysis of pathogen eradication rates, inflammatory findings and sexual dysfunction. Asian J Androl. 2011;13(6):819–827. doi:10.1038/aja.2011.36
25. Ahmed TA, Aljaeid BM. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des Devel Ther. 2016;10:483–507. doi:10.2147/DDDT.S99651
26. Pandey R, Khuller G. Polymer based drug delivery systems for mycobacterial infections. Curr Drug Deliv. 2004;1(3):195–201. doi:10.2174/1567201043334669
27. Spirescu VA, Chircov C, Grumezescu AM, Andronescu E. Polymeric Nanoparticles for Antimicrobial Therapies: an Up-To-Date Overview. Polymers (Basel). 2021;13(5):1–27.28. doi:10.3390/polym13050724
28. Washington KE, Kularatne RN, Karmegam V, Biewer MC, Stefan MC. Recent advances in aliphatic polyesters for drug delivery applications. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017;9(4):e1446. doi:10.1002/wnan.1446
29. El Yousfi R, Brahmi M, Dalli M, et al. Recent Advances in Nanoparticle Development for Drug Delivery: a Comprehensive Review of Polycaprolactone-Based Multi-Arm Architectures. Polymers. 2023;15(8):1835. doi:10.3390/polym15081835
30. Sisson AL, Ekinci D, Lendlein A. The contemporary role of ε-caprolactone chemistry to create advanced polymer architectures. Polymer. 2013;54:4333–4350. doi:10.1016/j.polymer.2013.04.045
31. Blazquez-Blazquez E, Perez E, Lorenzo V, Cerrada ML. Crystalline Characteristics and Their Influence in the Mechanical Performance in Poly(epsilon-Caprolactone)/High Density Polyethylene Blends. Polymers. 2019;11(11):1874. doi:10.3390/polym11111874
32. Park JH, Cho YW, Son YJ, et al. Preparation and characterization of self-assembled nanoparticles based on glycol chitosan bearing Adriamycin. Colloid Polym Sci. 2006;284(7):763–770. doi:10.1007/s00396-005-1438-7
33. Zeeshan A, Farhan M, Siddiqui A. Nanomedicine and drug delivery: a mini review. Int Nano Lett. 2014;4(1):1–7.
34. Sharma A, Kumar Arya D, Dua M, Chhatwal GS, Johri AK. Nano-technology for targeted drug delivery to combat antibiotic resistance. Expert Opin Drug Deliv. 2012;9(11):1325–1332. doi:10.1517/17425247.2012.717927
35. Costa JR, Silva NC, Sarmento B, Pintado M. Potential chitosan-coated alginate nanoparticles for ocular delivery of daptomycin. Eur J Clin Microbiol Infect Dis. 2015;34(6):1255–1262. doi:10.1007/s10096-015-2344-7
36. Al-Zahrani SS, Bora RS, Al-Garni SM. Antimicrobial activity of chitosan nanoparticles. Biotech Biotechnol Equipment. 2021;35(1):1874–1880. doi:10.1080/13102818.2022.2027816
37. Mu H, Zhang A, Zhang L, Niu H, Duan J. Inhibitory effects of chitosan in combination with antibiotics on Listeria monocytogenes biofilm. Food Control. 2014;38(1):215–220. doi:10.1016/j.foodcont.2013.10.032
38. Ashour AE, Badran MM, Kumar A, Rishi AK, Yassin AE. Di-Block PLCL and Tri-Block PLCLG Matrix Polymeric Nanoparticles Enhanced the Anticancer Activity of Loaded 5-Fluorouracil. IEEE Trans Nanobiosci. 2016;15(7):739–747. doi:10.1109/TNB.2016.2612340
39. Badran MM, Alomrani AH, Harisa GI, Ashour AE, Kumar A, Yassin AE. Novel docetaxel chitosan-coated PLGA/PCL nanoparticles with magnified cytotoxicity and bioavailability. Biomed Pharmacother. 2018;106:1461–1468. doi:10.1016/j.biopha.2018.07.102
40. Al-Omar M. Ciprofloxacin: analytical Profile. In: Brittain HG, editor. Profiles of Drug Substances, Excipients, and Related Methodology. Vol. 31. Academic Press, Elsevier Inc.; 2005:179–207.
41. Ghari T, Kobarfard F, Mortazavi SA. Development of a Simple RP-HPLC-UV Method for Determination of Azithromycin in Bulk and Pharmaceutical Dosage forms as an Alternative to the USP Method. Iran J Pharm Res. 2013;12(Suppl):57–63.
42. Albekery MA, Alharbi KT, Alarifi S, et al. Optimization of a nanostructured lipid carriers system for enhancing the biopharmaceutical properties of valsartan. Dig J Nanomater Biostructures. 2017;12:381–389.
43. Halwani M, Blomme S, Suntres ZE, et al. Liposomal bismuth-ethanedithiol formulation enhances antimicrobial activity of tobramycin. Int J Pharm. 2008;358(1–2):278–284. doi:10.1016/j.ijpharm.2008.03.008
44. CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically.
45. Souza TGF, Ciminelli VST, Mohallem NDS. A comparison of TEM and DLS methods to characterize size distribution of ceramic nanoparticles. J Phys Conf Ser. 2016;733(1):012039. doi:10.1088/1742-6596/733/1/012039
46. Tehler U, Fagerberg JH, Svensson R, Larhed M, Artursson P, Bergström CAS. Optimizing solubility and permeability of a biopharmaceutics classification system (BCS) class 4 antibiotic drug using lipophilic fragments disturbing the crystal lattice. J Med Chem. 2013;56(6):2690–2694. doi:10.1021/jm301721e
47. Arora SC, Sharma PK, Irchhaiya R, Khatkar A, Singh N, Gagoria J. Development, characterization and solubility study of solid dispersions of Cefuroxime Axetil by the solvent evaporation method. J Adv Pharm Technol Res. 2010;1(3):326–329. doi:10.4103/0110-5558.72427
48. Wang Y, Li P, Kong L. Chitosan-modified PLGA nanoparticles with versatile surface for improved drug delivery. AAPS PharmSciTech. 2013;14(2):585–592. doi:10.1208/s12249-013-9943-3
49. Gustafson HH, Holt-Casper D, Grainger DW, Ghandehari H. Nanoparticle Uptake: the Phagocyte Problem. Nano Today. 2015;10(4):487–510. doi:10.1016/j.nantod.2015.06.006
50. Clarke SP. Development of Hierarchical Magnetic Nanocomposite Materials for Biomedical Applications. Thesis (Phd). Dublin City University.; 2013.
51. Putri DCA, Dwiastuti R, Marchaban M, Nugroho AK. Optimization of Mixing Temperature and Sonication Duration in Liposome Preparation. J Pharm Sci Community. 2017;14(2):79–85. doi:10.24071/jpsc.142728
52. Liposome Drug Products: chemistry, Manufacturing, and Controls; Human Products Pharmacokinetics and Bioavailability; and Labeling Documentation. Guidance for Industry; Center for Drug Evaluation and Research (CDER), US Food and Drug Administration; 2018. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/liposome-drug-products-chemistry-manufacturing-and-controls-human-pharmacokinetics-and.
53. Verwey EJW. Theory of the Stability of Lyophobic Colloids. J Phys Colloid Chem. 1947;51(3):631–636. doi:10.1021/j150453a001
54. Ding B, Ahmadi SH, Babak P, Bryant SL, Kantzas A. On the Stability of Pickering and Classical Nanoemulsions: theory and Experiments. Langmuir. 2023;39(20):6975–6991. doi:10.1021/acs.langmuir.3c00133
55. Karmakar S. Particle size distribution and zeta potential based on dynamic light scattering: techniques to characterize stability and surface charge distribution of charged colloids. In: Recent Trends in Materials Physics and Chemistry. New Delhi: Studium Press India Pvt Ltd; 2019:117–159.
56. Fissan H, Ristig S, Kaminski H, Asbach C, Epple M. Comparison of different characterization methods for nanoparticle dispersions before and after aerosolization. Anal Methods. 2014;6(18):7324–7334. doi:10.1039/C4AY01203H
57. Leyva-Porras C, Cruz-Alcantar P, Espinosa-Solís V, et al. Application of Differential Scanning Calorimetry (DSC) and Modulated Differential Scanning Calorimetry (MDSC) in Food and Drug Industries. Polymers (Basel). 2020;12(1). doi:10.3390/polym12122793
58. Günday Türeli N, Torge A, Juntke J, et al. Ciprofloxacin-loaded PLGA nanoparticles against cystic fibrosis P. aeruginosa lung infections. Eur J Pharm Biopharm. 2017;117:363–371. doi:10.1016/j.ejpb.2017.04.032
59. Sobhani Z, Samani SM, Montaseri H, Khezri E. Nanoparticles of Chitosan Loaded Ciprofloxacin: fabrication and Antimicrobial Activity. Adv Pharm Bull. 2017;7(3):427–432. doi:10.15171/apb.2017.051
60. Abo-zeid Y, Amer A, Bakkar MR, El-Houssieny B, Sakran W. Antimicrobial Activity of Azithromycin Encapsulated into PLGA NPs: a Potential Strategy to Overcome Efflux Resistance. Antibiotics. 2022;11(11):1623. doi:10.3390/antibiotics11111623
61. Raouf M, Essa S, El Achy S, Essawy M, Rafik S, Baddour M. Evaluation of Combined Ciprofloxacin and azithromycin free and nano formulations to control biofilm producing Pseudomonas aeruginosa isolated from burn wounds. Indian J Med Microbiol. 2021;39(1):81–87. doi:10.1016/j.ijmmb.2021.01.004
62. Jamil B, Habib H, Abbasi S, et al. Cefazolin loaded chitosan nanoparticles to cure multi drug resistant Gram-negative pathogens. Carbohydr Polym. 2016;136:682–691. doi:10.1016/j.carbpol.2015.09.078
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.