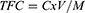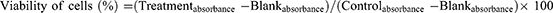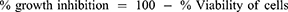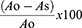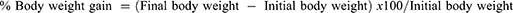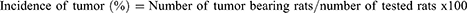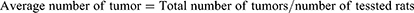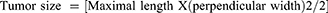Back to Journals » Journal of Experimental Pharmacology » Volume 15
Chemopreventive Activity of 80% Methanol Leaf Extract of Vernonia auriculifera Hiern (Asteraceae) Against Dimethylhydrazine-Induced Colorectal Carcinogenesis in Rats
Authors Wondmkun YT, Engidawork E , Labisso WL , Belete A, Tesfaye S, Girma Shumiye Y
Received 11 June 2023
Accepted for publication 30 August 2023
Published 4 September 2023 Volume 2023:15 Pages 333—347
DOI https://doi.org/10.2147/JEP.S421338
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Yohannes Tsegyie Wondmkun,1 Ephrem Engidawork,1 Wajana Lako Labisso,2 Anteneh Belete,3 Solomon Tesfaye,1 Yonas Girma Shumiye2
1Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, Addis Ababa University, Addis Ababa, Ethiopia; 2Department of Pathology, School of Medicine, Addis Ababa University, Addis Ababa, Ethiopia; 3Department of Pharmaceutics and Social Pharmacy, School of Pharmacy, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Ephrem Engidawork; Yohannes Tsegyie, Email [email protected]; [email protected]; [email protected]
Background: Vernonia auriculifera Hiern (Asteraceae) is among Ethiopian herbal medicines that are traditionally used to treat skin and gastrointestinal cancers. In this study, the chemopreventive potential of Vernonia auriculifera leaf extract in dimethylhydrazine (DMH)-induced colorectal carcinogenesis in rats was investigated.
Methods: Rats were assigned to nine groups (normal, positive, and negative control groups, and three pre- and three post-initiation groups). Except for the normal control group (administered with 1 mL/100 g distilled water), the remaining eight groups were given DMH (20 mg/kg) intraperitoneally (ip) for 15 consecutive weeks to induce colorectal tumours. The extract was given orally to the pre-initiation and post-initiation groups at doses of 100, 200, and 400 mg/kg before and after the induction of cancer, respectively. The positive control group was treated with aspirin (60 mg/kg/day) orally for the whole experimental period. Parameters including body weight, average tumour number, size, progression, incidence, total cholesterol, serum total protein, and triglyceride levels were determined. The cytotoxic activity of the extract in Caco-2 cells was evaluated using the MTT assay, and the antioxidant activity of the extract was also assessed using 2.2-diphenyl-1-picrylhydrazine (DPPH) and reducing power methods. Moreover, total phenol and flavonoid contents were determined using appropriate methods.
Results: Rats treated with the extract showed a lower incidence of up to 50% in the pre-initiation higher dose, average number (p< 0.05),and size (p< 0.05) of tumours compared to untreated rats. It also inhibited colorectal cancer-associated increases in serum total cholesterol and triglycerides. The extract’s IC50 value in the MTT assay was found to be higher than 200 μg/mL. The extract had an IC50 of 74.88 ± 0.86 μg/mL and 84.69 ± 2.02 μg/mL in the reducing power and DPPH assays, respectively. Total flavonoid and phenol contents were 14.51 ± 0.41 mg quercetin acid equivalent/gm and 47.37 ± 0.72 mg gallic acid equivalent/gm of the crude extract, respectively.
Conclusion: The findings collectively indicated that the leaves of V. auriculifera possess chemopreventive activity, probably mediated through antioxidant mechanisms, which supports the traditional claim.
Keywords: antioxidant, cytotoxicity, colorectal cancer, pre-initiation, post-initiation, Vernonia auriculifera
Introduction
Colorectal cancer (CRC) is a kind of malignancy that originates from the epithelial cells of the colon and rectum.1 CRC is the second most frequently identified cancer in both men and women across the globe. Men are more likely than females to get CRC, and regional variation in incidence is nearly ten times greater.2 South-Central Asia and Africa have the lowermost incidence rates, while western countries have the highest rates.3 This might be because of differences in environmental and dietary exposures imposed on a genetically determined susceptibility inheritance.4 In Ethiopia, CRC develops at a younger age than in other nations, and the majority of the cases involve the rectum. However, patients from Ethiopia did not exhibit the transfer of CRC to the right colon, a phenomenon that is frequently reported in other areas of the world.5
Over the years, different approaches (a combination of chemotherapy, surgery, targeted therapy, and radiation therapy) have been employed and are still in use for the management of cancer.6 However, these modalities have their own limitations (serious adverse effects like vomiting, alopecia, and myelosuppression for chemotherapeutic drugs).7 Furthermore, efficacy is often limited by insolubility, a lower rate of absorption and the tumor’s drug resistance. Although numerous studies have shown that many targeted medication classes, notably anti-inflammatory drugs like aspirin, have the capacity to reduce carcinogenesis, these agents’ drawbacks have prevented their indication in high-risk people and individuals with early-stage CRC.8
The use of herbal medicines in the inhibition of malignant growth has a long history, and there is growing attention to plants as a source of possible anticancer drugs.9 Through advancements in drug discovery methods, studies focusing on the development of new molecules from herbs have been reinvigorated.10 Vernonia auriculifera Hiern (Asteraceae) is documented to be used for treatment of headache,11 dysentery, stomachache, malaria, and wounds.12 The leaves are also claimed to be used for treatment of gastrointestinal disorders and cancer13,14 and are one of the most frequently cited plant species for cancer treatment by traditional healers in eleven districts of Ethiopia.15 Screening of secondary metabolites of V. auriculifera indicated the presence of pentacyclic triterpenoids, including lupenyl acetate, β-amyrin acetate, β-sitosterol, oleanolic acid, friedelanone, α-amyrin, β-amyrin, and friedelin acetate together with a sesquiterpene amine, farnesylamine, which has not been identified antecedently in the test plant.16,17
The genus Vernonia has been known to exhibit chemopreventive properties.17 For instance, Vernonia amygdalina Del. mitigated cycasin-induced oxidative damage in rat colonic tissue.17 Vernolide-A and -B, two isolated sesquiterpene lactones from V. cinerea, have also produced a cytotoxic effect against many tumor cell lines.18 Moreover, triterpenes from V. auriculifera are reported to be endowed with cytotoxic and apoptotic properties in several cancer cell lines.18–21 The aforementioned folkloric use and in vitro activity thus formed the rationale to investigate the in vitro activity as well as the chemopreventive effects of the leaf extract of V. auriculifera against CRC in rats.
Materials and Methods
Plant Material
The leaves of V. auriculifera were collected in April 2018 in the Western Oromia Region’s Gilgel Gibe River area, around 450 kilometers south of Addis Ababa, Ethiopia. A taxonomist identified and verified the collected plant material, and a voucher sample, designated Bel-025, was placed at the National Herbarium, Addis Ababa University.
Experimental Animals
All animal procedures and tests were performed in compliance with the US National Research Council’s Guides for the Use and Care of laboratory animals22 and also approved by the Ethical Review Board of School of Pharmacy, College of Health Sciences, Addis Ababa University (ERB/SOP/151/12/2017). We used male Wistar Albino rats (6–7 weeks old, weighing 115–200 g) that were obtained from the Addis Ababa University School of Pharmacy’s animal house. Fifty four rats were assigned to nine groups (n = 6) in clean cages with wire mesh tops and sanitary sawdust beds. The rats were familiarized to the working area for a week before the commencement of the study.
Plant Extraction
The collected V. auriculifera leaves were dried and powdered with a mortar and pestle to make a coarse powder. A sample of powder weighing 1.5 kg was macerated in 80% methanol for three days at room temperature. By adding a new solvent to the marcs, the extraction procedure was repeated twice. The mixture was concentrated using Rotavapor at 40°C under vacuum after being filtered via Whatman filter paper. The extract was tested and found to be 9.75% after the concentrate was freeze-dried using a lyophilizer. Before the experiment started, the raw extract was put into a vial and placed in the refrigerator.
Total Flavonoid Content (TFC) Determination
An aluminium chloride colorimetric assay was used to measure the level of total flavonoids.23 In each test tube, 1 mL of the quercetin solution (62.5, 125, 250, 500, and 1000 μg/mL) was incorporated, and a solution of sodium nitrite was added. A 10% aluminium chloride solution was incorporated after waiting for 5 minutes. 2 mL of 1 M NaOH were also applied exactly at minute six. The solution was then extensively vortexed after being diluted with 10 mL of distilled water. The absorbance of the respective quercetin solution was measured at 510 nm exactly after 30 minutes of incubation, and a calibration curve was constructed (Supplementary Figure 1). The TFC of the extract was determined using Eq. (1). The content was reported as milligrams equivalents of quercetin (mg QE/gm) for every gram of the extract’s dried weight. Methanol was the control substance, and the phytochemical analysis was performed three times, with the mean value recorded.
Where C = concentration of sample (µg/mL), V= solvent volume (mL) used, and M= dried extract weight (g).
Total Phenol Content Determination
In this study, to measure the content of total phenol in V. auriculifera leaf extract, Folin-Ciocalteu assay was employed.24 The assay is an electron transfer-based method that provides reducing capacity expressed as phenolic content. Using gallic acid as a standard, a calibration curve was drawn (Supplementary Figure 2). In terms of gallic acid equivalent weight (GAE), the TPC of the sample was reported as mg/gm of the extract. The phytochemical quantification was done in triplicate, with the mean value recorded.
HPLC Analysis
The crude 80% methanol leaf extract of Vernonia auriculifera and its chloroform fraction were prepared. The HPLC fingerprints were obtained by using an Ultimate 3000 HPLC (Thermo Fisher Scientific, USA) fitted with a C-18 column (Nucleodur 100–5 EC-C18, 4.6 mm-250 mm) (Macherey-Negas, Germany). A Diode array detector (DAD) was used. Elution was effected using a ternary gradient of the solvent mixture methanol (A): acetonitrile (B): 1% acetic acid (C) (20: 40: 40, v/v/v), and then followed by A: B: C (60: 20: 20) in 30 min at a flow rate of 1 mL/min. The column temperature was set to 30 0.8 °C, and the sample injection volume was 10 µL. The chromatograms were recorded at a wavelength of 360 nm for both the extract and fraction.
Acute Toxicity Study
According to the OECD/OCDE 425 guideline standard procedures, the acute toxicity of V. auriculifera leaf extract of was evaluated.25 For the toxicity investigation, female rats aged 6 to 8 weeks were fasted overnight. The starting dose was initially established through a sighting study. To do this, 2000 mg/kg of the test sample was given orally by gavage to a single female rat. Following the administration of the extract, the rat was then constantly monitored for the first 30 minutes, and then every 4 h for the following 24 h. In the first 24 hours, neither toxicity nor death were noted. In order to observe the onset, severity, and persistence of toxicity signs, such as changes in behavior and physical appearance, lacrimation, hair erection, appetite loss, salivation, diarrhea, motor and feeding activities, as well as mortality within 14 days, another four female rats were administered a dose that was similar.
Cells and Culture Conditions
Caco-2 (colorectal adenocarcinoma) cells that were taken from the ATCC® were placed in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 1% sodium pyruvate at 37 °C in a humidified atmosphere of 95% air, 15% (v/v) heat-inactivated foetal bovine serum (FBS), 1% NEAA, and 5% CO2. Once achieving 80% confluence, the cells were passaged.
MTT Assay
The cultured Caco-2 cells were taken from a T-175 TC flask and suspended at a density of 106 cells/mL. Then, a cell suspension of 50 µL was added to a serially diluted test compound (eight two-fold dilutions starting at 200 µg/mL) in the same medium (50 µL) containing a 96-well microtiter plate.26 The final cell density in each well was 50,000. All chemicals were dissolved in 100% DMSO, with the highest concentration wells getting a final DMSO concentration of 1%. Controls were given DMSO alone at the same concentration as the extract-treated cells. After 48 h of incubation with the extract, a 25 µL solution of MTT was incorporated into each well, followed by incubation of the cells at 37°C for 3 h. Then, the incubated cells were lysed by incorporating 100 µL of extraction buffer to each well and plates were kept in the incubator overnight. The MTT assay results were read at 570 nm using a Labsystem multi-scan plate reader. The following formula was applied to determine cell viability (Eq. 2). The measurement was done three times, and the average was taken. The same procedures were also employed to evaluate the cytotoxic activity of the different fractions (aqueous, ethyl acetate, butanol, and chloroform) of the extract.
Antioxidant Assay Using the Reducing Power Method
As per the processes described elsewhere,27 the potassium ferricyanide-ferric chloride method was used to determine the extract’s ferric reducing potential. The UV absorbance was measured at 700 nm against a blank, and a dose-response curve was used to obtain the IC50 (Supplementary Figure 3).
Antioxidant Assay Using the 2, 2-Diphenyl-1-Picrylhydrazine Method
Using the 2.2-diphenyl-1-picrylhydrazine (DPPH) assay (Sigma Chemicals Co, St. Louis, MO, USA), the extract’s capacity to scavenge free radicals was also investigated according to a protocol described elsewhere.28 The IC50 value of each sample was determined from the dosage vs inhibition curve (Supplementary Figure 4), and the percent inhibition was computed using Eq. 4.
Where Ao represents negative control (a 0.004% DPPH solution methanol without the test sample) absorbance, whereas As denotes the absorbance of extract or ascorbic acid-containing solution.
Experimental Groups and Doses
Fifty four male rats were initially acclimated for a week and then randomly assigned into nine groups (n = 6). Apart from the normal control rats, all the others were administered with dimethyl hydrazine (DMH) (20 mg/kg) (TCI Chemicals, India) intraperitoneally (i.p.) for fifteen weeks.29 Different doses of the extract were given either before (pre-initiation group) or after (post-initiation group) the initiation of DMH administration. Group I (the normal control) rats received distilled water only for the whole period. For fifteen weeks straight, Group II (the negative control) was given DMH 20 mg/kg (i.p) (DMH20) once a week, and they were observed until the end of the study. Beginning two weeks after the start of DMH administration and continuing through the cessation of the study period, Groups VI, VII, and VIII (post-initiation groups) received the extract daily orally at doses (POV100, POV200, and POV400) similar to those used during the pre-initiation phase. From the initiation of DMH administration through the end of the study, Group IX (the positive control) was given aspirin at a dose of 60 mg/kg/day (ASA60) orally, similar to the other groups mentioned above. Twenty-five weeks made up the entire experimental period. The extract doses were selected according to the acute oral toxicity study, and the maximum volume given was 10 mL/kg.
Preparation of Carcinogen and Induction of CRC
In order to ensure the stability and suitability of the carcinogen, pH of the solution was adjusted to 7.0 with 1 mM NaOH after DMH (2 mg/mL) was dissolved in normal saline solution (0.9% NaCl). The final solution of the DMH was prepared and administered instantly. The dose of DMH injected was 20 mg/kg/week (i.p.).30
Biochemical Analysis and Body Weight Measurement
At the termination of the experiment, the experimental animals were fasted for the night before being scarified under the proper anesthetic by cervical dislocation. Cardiac puncture was employed to obtain blood samples, which were subsequently centrifuged at 4000 rpm for 10 minutes after clotting for 30 minutes at room temperature. Using a micropipette, serum was collected, and an aliquot was kept in vials pending the completion of the required analyses. Using the colorimetric assay method, the total protein level (mg/dL) was calculated.31 The enzymatic colorimetric method was also used to assess total cholesterol and triglyceride (mg/dL) concentrations.32,33 The Ethiopian Public Health Institute performed all biochemical analyses. On the other hand, the body weight of each rat was weighed at the start of the study, once per week, and just before they were sacrificed.34 The individual growth rate and percent weight gain were calculated using Eq. (5) and (6), respectively:
Colon Tumor and Histopathological Analysis
After the collection of blood samples, the colons were taken out, cut open longitudinally from the cecum to the end of the rectum, and flushed with isotonic saline. The tumor incidence, number, and size were determined (Eq. (7–9)) and recorded. The colons were then embedded in paraffin and preserved in 10% neutral buffered formalin.35 Each polyp (tumor) was afterwards removed and stained for histological investigation using hematoxylin and eosin.36 A blinded pathologist from the Addis Ababa University School of Medicine’s Department of Pathology read and interpreted the slides. Lastly, based on shape, degree of invasion, and differentiation, the colon tumors were divided into invasive adenocarcinoma, tubulovillous, tubular, and villous adenomas.
Statistical Analysis
The results of this study were entered and analyzed using the statistical software for social science (SPSS), version 25 for Windows (SPSS Inc, Chicago, Illinois, USA). The findings for each group were expressed as mean ± standard error of the mean (SEM). A One-way analysis of variance (ANOVA) followed by Tukey’s post hoc was used to compare statistical differences between groups. Finally, a p value of < 0.05 was used as the cutoff point for the presence of statistical significance.
Results
Acute Toxicity Test
No mortality was recorded by the extract when it was given at a single dose of 2000 mg/kg orally during the first 24 hours or the next 14 days of the follow-up period. Moreover, no observable signs of acute toxicity such as salivation, hair erection, loss of appetite, lacrimation, or diarrhea were revealed during cage side observation for gross behavioral and physical changes. Hence, the LD50 of the plant extract was obtained to be above 2000 mg/kg. It is of note that all experimental groups tolerated not only oral administration of the plant extract but also oral administration of aspirin as well as i.p. injections of DMH.
Total Flavonoid and Total Phenol Contents
The standards (quercetin and gallic acid) calibration curves and regression equations were used for calculation of TFC and TPC of the extract. Accordingly, TFC was found to be 14.51±0.41 mg QE/gm of dried extract and TPC was 47.37±0.72 mg GAE/gm of dried extract.
HPLC Fingerprinting
The HPLC fingerprints for Vernonia auriculifera crude extract and its chloroform fraction were obtained and shown in Supplementary Figure 5. As can be seen from the figure, with the exception of peak 12, the HPLC chromatograms obtained for the methanolic crude extract and its chloroform fraction had relatively similar retention times. Likewise, both fractions produced fifteen distinctive peaks at 360 nm; however, the relative peak areas varied slightly for peaks observed at a relatively similar retention time (Supplementary Table 1). This difference can be the result of differences in the chemical compositions and corresponding contents of V. auriculifera leaves in these two different solvents. Nevertheless, the peak with a relative retention time of 10.53 min is a reference peak, as it is the maximum peak in both chromatograms.
Cytotoxic Activity
MTT assays revealed that an 80% methanol leaf extract of V. auriculifera did not affect the viability of Caco-2 cells up to a concentration of 200 µg/mL. The maximum percentage cell growth inhibition elicited by the extract was 32.66%, suggesting that the IC50 for the extract is above 200 µg/mL. The same assay was employed to see whether the different fractions (aqueous, ethyl acetate, butanol, and chloroform) could have a different cytotoxicity profile. However, the assay once again showed an IC50 of above 200 µg/mL for all the fractions (Supplementary Table 2). According to specifications from the National Cancer Institute plant screening program, all V. auriculifera extracts are considered to be not active in terms of cytotoxicity.37
Antioxidant Activity of the Extract
As the extract did not exhibit appreciable cytotoxicity activity, we set out to evaluate the antioxidant activity of the plant extract using two types of assays. The IC50 values for the extract were found to be 74.88±0.86 µg/mL and 84.69±2.02 µg/mL in the ferric reducing and DPPH assay, respectively. Quercetin, the standard for the former assay, and ascorbic acid, the standard for the latter assay, had an IC50 of 11.62±0.78 µg/mL and 64.17±0.63 µg/mL respectively.
Induction of Colorectal Cancer
The effect of DMH on incidence, number, and size of tumor as well as biochemical parameters is presented in Table 1. DMH produced on average four tumors per animal (p<0.001) and the incidence of tumor was found to be 100%. The macroscopic view of representative tumors induced is presented in Figure 1. The carcinogenic chemical also resulted in biochemical changes in the DMH20 group in comparison with the normal controls, as shown by a substantial decrease in serum total protein concentration (p<0.05), as well as an increase in serum triglyceride concentration (p<0.01) and total cholesterol concentration (p<0.05) (Table 1).38
 |
Table 1 Parameters Showing Induction of Colorectal Cancer by Dimethylhydrazine After 25 Weeks |
Effect of the Extract on Tumor Number, Size, and Incidence
At the end of the study period, the tumors examined were 26 from DMH20, 2 from ASA60, and an average of 30 and 41 from various doses of PIV and POV groups, respectively (Table 2). Treatment initiated with 200 mg/kg (middle dose) and (400 mg/kg (high dose) of PIV and POV as well as aspirin produced a significant decrease in average tumor size (p<0.05) in comparison with DMH20. In the meantime, there was a significantly lower average number of tumor (p<0.05) in both PIV400 and POV400 groups compared to DMH20. Although ASA60 elicited a significant decrease (p<0.05) in both average size and number of tumor compared to the DMH20 group, no detectable differences were noted between ASA60 and various doses of the plant extract in both phases (Table 2)).38
 |
Table 2 Effect of Vernonia Auriculifera on Number and Size of Tumors in Colon and Rectum of Dimethylhydrazine-Treated Rats |
From the dose response curve (Figure 2), we found that the effective doses of the extract that reduced tumor number by 50% (ED50) were obtained to be 131.83 mg and 178.18 mg in the pre-initiation and post-initiation phases, respectively.
 |
Figure 2 Dose-response curve of the extract in tumor number reduction for the pre-initiation (A) and post-initiation (B) phases. |
Administration of the extract elicited a decrease in tumor incidence (Table 3). Whilst PIV400 reduced incidence by half, all the other doses of the extract, regardless of the time for initiation of treatment, were able to bring the incidence down by a third. ASA60, however, displayed maximum reduction by two-third.
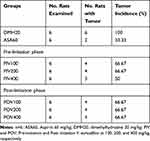 |
Table 3 Effect of Vernonia Auriculifera on Tumor Incidence in Dimethylhydrazine-Treated Rats |
Effects of the Extract on Body Weight Change and Histopathology
In all groups of rats, body weight was increased gradually during the 25th week of the experimental period (Table 4). However, treatment with the extract generally resulted in decreased weight gain when compared to the normal control (p<0.05), with the exception of PIV400, regardless of dose. ASA60 group, the bodyweight of rats was increased by 120% (p<0.05). A noticeable difference was not observed in terms of weight gain between the pre-initiation groups and ASA60. Similarly, except for the higher dose (p<0.05), the percentage weight gain of rats among the pre-initiation groups was not significantly different from the DMH20 group. Moreover, there was no statistically substantial difference in body weight gain between the post-initiation groups and the DMH20 and ASA60 groups.
 |
Table 4 Effect of Vernonia Auriculifera on Body Weight Gain and Growth Rate of Experimental Rats |
Also, treatment with low (100 mg/kg) and medium (200 mg/kg) doses of the extract resulted in a substantial slowing of the growth rate in both the pre-initiation (p<0.05) and post-initiation (p< 0.01) treatment groups when compared to the normal control. However, neither phases of treatment with the extract nor ASA60 resulted in a noticeably different growth rate (Table 4). Up on pathological examination, a total of 99 tumors were histologically analyzed. Among them, 41 were found to be tubular adenomas, 6 villous adenomas, 16 tubulovillous adenomas, and 36 invasive adenocarcinomas (Table 5).
 |
Table 5 Histopathological Classification of Tumors Induced by Dimethylhydrazine in the Various Groups |
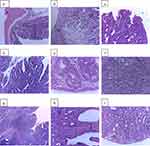
In DMH20 rats, 21 invasive adenocarcinomas and 5 tubular adenomas were observed. After treatment, incidence of invasive adenocarcinomas was reduced. Both the dose and the time of administration had an impact on the reduction. Earlier initiation of treatment, as shown in the pre-initiation phases, produced significant reduction of tumor compared to post-initiation phases (4 vs 11). Moreover, only one post-initiation group had villous adenomas, whereas three pre-initiation groups had none. There were no invasive adenocarcinomas or tubulovillous adenomas detected in the higher dose extract groups, indicating that the medium and higher (400 mg/kg) doses were more effective in both stages than the lower dose. Under a high power (40X) light microscope, histopathological differences in the colon and rectum of several treatment groups were analyzed. The photomicrographs of colorectal tumor sections showed different histological architectures among the experimental groups (Figure 3).
The colorectal region of control rats displayed conventional colonic architecture, normal mucosal and sub-mucosal layers, and normal glands without any obvious abnormalities (Figure 3a). Moreover, the proportion of glands bordered by regular cells with sporadic goblet cells was normal. Although colon tumors were present in DMH20 rats, histopathological investigation also indicated invasive adenocarcinoma with invasive single cells and glands formed of highly malignant epithelial cells infiltrating muscle proper (Figure 3b). Representative colon tumors from PIV100 rats, on the other hand, were discovered to be tubulovillous, with villous and tubular components clearly visible and lined by normal epithelial cells (Figure 3c). However, a villous adenoma with villous extensions from mucosa bordered by typical epithelial cells was revealed in PIV200 rats. (Figure 3d). Moreover, PIV400’s lamina propria and submucosa contained a tubular adenoma without any signs of invasion (Figure 3e).
An invasive carcinoma was identified in a colorectal tumor removed from POV100 rats, which had a proliferation of highly pleomorphic epithelial cells with dysplastic nuclei that formed glandular structures and invaded the sub-mucosa (Figure 3f). When villous components made up more than 75% of the tumor mass in a tumor slice from POV200 rats, the diagnosis of villous adenoma was made (Figure 3g). Furthermore, a tubular adenoma with rounded features in cut cross section and branching tubules embedded in the lamina propria was identified in a pedunculated polyp removed from POV400 rats (Figure 3h). Last but not least, it was determined that a tumor removed from an ASA60 rat was a tubular adenoma with a greater glandular number and fewer villous components (Figure 3i).
Effect of the Extract on Biochemical Parameters
At the end of the study period, levels of biochemical parameters in the various treatment groups were assessed and are shown in Table 6. Comparing DMH20 to control group, it was found that triglycerides (p<0.01), total cholesterol (p<0.05) and total protein (p<0.05) were all greater. Depending on the parameter measured, treatment with the extract had varying results. Only the medium and higher doses were able to reduce triglycerides in the post-initiation phase (p<0.05) as compared to DMH20 rats. Triglycerides were considerably decreased by ASA60 (p<0.05) and all doses (p<0.05) of the pre-initiation phase. On the other hand, only ASA60 and PIV400 significantly lowered total cholesterol (p<0.01 and p<0.05, respectively). Neither the extract nor aspirin produced a significant change in total protein compared to DMH20 rats, although the treatments tended to increase the protein levels.
 |
Table 6 Effect of Vernonia Auriculifera on Serum Biochemical Parameters in Dimethylhydrazine-Induced Colorectal Cancer |
Discussion
In this study, the hydroalcoholic leaf extract of the experimental plant was evaluated for its chemopreventive activity in the pre- and post-initiation phases against DMH-induced CRC. Average number, size, and incidence of tumors increased (p<0.001) as a result of DMH; this was more effectively reversed by treatment with a larger dose of the extract during the pre-initiation phase. This may be related to the combined impact of early treatment initiation and the presence of more phytochemicals, as demonstrated by the assay used to evaluate TFC and TPC within this dose of the extract. According to preliminary phytochemical investigations, V. auriculifera includes tannins, flavonoids, terpenoids, saponins, and triterpenoids.39 Moreover, flavonoids were found in the majority of the fractions of the methanol extract of this plant, evidenced by a thin layer chromatographic analysis.40
The finding of increased concentrations of phenolic and flavonoids in the current study provides more evidence that the plant is endowed with these secondary metabolites. At the pre-initiation phase, the medium and higher doses of the extract reduced the progression of adenoma to invasive cancer. Moreover, fewer villous and tubulovillous adenomas were found than tubular adenomas, despite having a higher chance of transforming into malignant tumors. This could be attributed to the prolonged length of treatment and the suppressive effect of secondary metabolites on tumor growth when the plant extract was administered during the study period. This idea is supported by the finding that many chemopreventive medicines work best when used earlier in the carcinogenesis process and for a longer period of time.41
As a result of the higher tumor incidence in the colon and rectum, which may in turn cause a decrease in food and fluid intake, body weight loss and growth rate reduction are regarded as diagnostic markers of CRC advancement.42,43 Body weight gain was more pronounced in the pre-initiation than the post-initiation groups in this study, despite the difference not being statistically significant. As a result, the PIV400 group experienced the most body weight gain. This may suggest that early administration of extract with a larger dose had the potential to be chemopreventive against DMH-induced CRC and to considerably decrease the growth of cancer cells. Moreover, the growth rate of DMH20 rats was significantly (p<0.01) lower than that of normal control rats. The growth rate of the PIV400 group had a similar pattern to that of the normal control. This suggests that early treatment with the extract at a higher dose may be more effective than late treatment in maintaining a normal growth rate and inhibiting tumor formation.
Also, the results of biochemical analysis revealed that DMH20 rats had much higher serum levels of cholesterol and triglycerides, indicating that this feature may be linked to cancerous cells in the colon and rectum. In fact, elevated levels of triglycerides and total cholesterol were strongly correlated with colorectal polyps.44,45 The fact that levels of serum cholesterol were lowered by PIV400 and triglyceride by all doses of the pre-initiation phase suggests that administration of V. auriculifera before induction of colon cancer has the potential to inhibit lipid derangements caused by the noxious agent. Loss of protein is commonly related to many cancer types and may explain the decrease in total protein noted in the present study.46 However, serum total protein in the extract received rats in all doses of the pre-initiation and at the higher dose of the post-initiation phases tended to restore the level to normal. In agreement with this study Coffee arabica administration sustained the normal level of serum proteins in DMH-induced CRC in an experimental rat model.47
The anti-oxidative and anti-inflammatory effects, cell cycle arrest, apoptosis, stimulation of Phase II enzymes, changes in gut microbiota, and induction of autophagy by chemopreventive drugs are frequently linked to reductions in tumor number and size.44 Assays performed to link chemoprevention activity to cytotoxic and/or antioxidative activity revealed the latter activity to be more important than the former, as the extract did not affect cell viability significantly at the maximum concentration used (200 µg/mL) in the present study. Previous studies have demonstrated that V. amygdalina, a related species to the plant of interest, mitigated cycasin-induced oxidative damage in rat colonic tissue,17 possibly attributed to scavenging free radicals, inducing detoxification, and inhibiting stress response proteins.48 It is thus plausible to assume that the flavonoid and phenolic compounds present in the extract might inhibit the formation of highly reactive metabolites of DMH, which are azoxymethane, the proximate carcinogen methyl-azoxymethanol, and the ultimate carcinogen, methyl-diazonium ion. Further evidence supporting this notion comes from the observation that phenolic compounds can exert direct preventive effects on the gastrointestinal tract via scavenging and reduction of free radical formation49 and that flavonoids (luteolin 7-O glucuronide, luteolin, and luteolin 7-O glycoside) isolated from V. amygdalina Del. scavenge free radicals and induce detoxification.38
Indeed, the higher effect observed in the pre- than the post-initiation phase and the lack of detectable cytotoxic activity in Caco-2 (the present study) as well as human myeloid leukemia (MV4-11) (IC50 678.1 ±15.8),50 lung adenocarcinoma (A-427) (72.9±37.4), human breast cancer (MCF-7) (105.2±11.9), human urinary bladder tumor (RT-4) (98.9±5.2), and human cervical adenocarcinoma (SISO) (90.2±12.8) cell lines15 strongly argue for the antioxidant activity to be the likely mechanism for the chemopreventive effect.
Conclusions
In conclusion, the extract showed chemopreventive potential, as early treatment significantly reduced tumor number, size and incidence, probably mediated through its antioxidant than cytotoxic activity. Early administration of the extract might have effect on delaying the progression and invasion as well as reduction of the aggressiveness of colon carcinogenesis. Moreover, the potential of V. auriculifera to decrease serum triglyceride and cholesterol level and prevent the decrease in serum protein could be used to suppress the growth of cancerous cells.
Abbreviations
ASA, Acetyl salicylic acid; CRC; Colorectal Cancer, DMH; 1, 2-dimethylhydrazine.
Ethical Approval
The experimental protocol was approved by the school of pharmacy institutional review board with Reference No. ERB/SOP/151/12/2017.
Acknowledgments
The authors acknowledge Addis Ababa University and staff members of School of Pharmacy for funding the research and for technical assistance respectively. This manuscript is a part of the first author’s thesis archived at the Addis Ababa University repository.
Funding
A thematic research grant (TR/35/2015) from Addis Ababa University funded this research.
Disclosure
The authors report that they have no conflicts of interest.
References
1. Rehemtulla A. Dinosaurs and ancient civilizations: reflections on the treatment of cancer. Neoplasia. 2010;12(12):957–968. doi:10.1593/neo.101588
2. Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023. doi:10.3322/caac.21772
3. Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22(04):191–197. doi:10.1055/s-0029-1242458
4. Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87(2):159–170. doi:10.1016/S0092-8674(00)81333-1
5. Ashenafi S. The frequency of large bowel cancer as seen in Addis Ababa University, Pathology Department. Ethiop Med J. 2000;38(4):277–282.
6. Nakayama G, Tanaka C, Kodera Y. Current options for the diagnosis, staging and therapeutic management of colorectal cancer. Gastrointestinal tumors. 2014;1(1):25–32. doi:10.1159/000354995
7. Wang H, Yu P, Bai J, et al. Ocotillol enhanced the antitumor activity of doxorubicin via p53-dependent apoptosis. Evid Based Complementary Alternative Med. 2013;2013:57.
8. Mohammed A, Yarla NS, Madka V, Rao CV. Clinically relevant anti-inflammatory agents for chemoprevention of colorectal cancer: new perspectives. Int J Mol Sci. 2018;19(8):2332. doi:10.3390/ijms19082332
9. Andrade PHM, Schmidt Rondon E, Carollo CA, et al. Effect of Powdered Shells of the Snail Megalobulimus lopesi on Secondary-Intention Wound Healing in an Animal Model. Evid Based Complementary Alternative Med. 2015;2015:1–9. doi:10.1155/2015/120785
10. Brown BD, Thomas W, Hutchins A, Martini MC, Slavin JL. Types of dietary fat and soy minimally affect hormones and biomarkers associated with breast cancer risk in premenopausal women. Nutr Cancer. 2002;43(1):22–30. doi:10.1207/S15327914NC431_2
11. Chifundera K. Contribution to the inventory of medicinal plants from the Bushi area, South Kivu Province, Democratic Republic of Congo. Fitoterapia. 2001;72(4):351–368. doi:10.1016/S0367-326X(00)00294-X
12. Kiplimo JJ, Koorbanally NA, Chenia H. Triterpenoids from Vernonia auriculifera Hiern exhibit antimicrobial activity. Af J Pharm Pharmacol. 2011;5(8):1150–1156.
13. Esubalew ST, Belete A, Lulekal E, Gabriel T, Engidawork E, Asres K. Review of ethnobotanical and ethnopharmacological evidences of some Ethiopian medicinal plants traditionally used for the treatment of cancer. Ethiopian J Health Dev. 2017;31(3):161–187.
14. Albejo B, Endale M, Kibret B, Anza M. Phytochemical investigation and antimicrobial activity of leaves extract of Vernonia auriculifera Hiern. J Pharm Pharm Res. 2015;3(6):141–147.
15. Tesfaye S, Belete A, Engidawork E, Gedif T, Asres K. Ethnobotanical study of medicinal plants used by traditional healers to treat cancer-like symptoms in eleven districts, Ethiopia. Evid Based Complementary Alternative Med. 2020;2020:1–23. doi:10.1155/2020/7683450
16. Leake I. Chemopreventive action of synthetic triterpenoids in CRC. Nat Rev Gastroenterol Hepatol. 2014;11(7):395. doi:10.1038/nrgastro.2014.87
17. Lolodi O, Eriyamremu G. Effect of methanolic extract of Vernonia amygdalina (common bitter leaf) on lipid peroxidation and antioxidant enzymes in rats exposed to cycasin. Pak J Biol Sci. 2013;16(13):642–646. doi:10.3923/pjbs.2013.642.646
18. Kuo Y-H, Kuo Y-J, A-S Y, et al. Two novel sesquiterpene lactones, cytotoxic vernolide-A and-B, from Vernonia cinerea. Chem Pharm Bull (Tokyo). 2003;51(4):425–426. doi:10.1248/cpb.51.425
19. Maiyoa F, Moodley R, Singh M. Phytochemistry, cytotoxicity and apoptosis studies of Β-sitosterol-3-oglucoside and Β-amyrin from Prunus africana. Af J Traditional Complem Alternative Med. 2016;13(4):105–112. doi:10.21010/ajtcam.v13i4.15
20. Ghante MH, Jamkhande PG. Role of pentacyclic triterpenoids in chemoprevention and anticancer treatment: an overview on targets and underling mechanisms. J Pharmacopuncture. 2019;22(2):55. doi:10.3831/KPI.201.22.007
21. Dinku W, Isaksson J, Rylandsholm FG, et al. Anti-proliferative activity of a novel tricyclic triterpenoid acid from Commiphora africana resin against four human cancer cell lines. App Biol Chem. 2020;63(1):1–11. doi:10.1186/s13765-020-00499-w
22. National Research Council. Guide for the care and use of laboratory animals; 2010.
23. Chantiratikul P, Meechai P, Nakbanpotec W. Antioxidant activities and phenolic contents of extracts from Salvinia molesta and Eichornia crassipes. Res J Biol Sci. 2009;4(10):1113–1117.
24. Kamtekar S, Keer V, Patil V. Estimation of phenolic content, flavonoid content, antioxidant and alpha amylase inhibitory activity of marketed polyherbal formulation. J Appl Pharm Sci. 2014;4(9):61.
25. Merkel D Acute oral toxicity up and down procedure in rats. Unpublished report prepared by the Product Safety Laboratories, Dayton, NJ, USA, for the Flavor and Extract Manufacturers Association, Washington, DC, USA Submitted to WHO by the International Organization of the Flavor Industry, Brussels, Belgium (Study No 16114). 2004;300.
26. Chen X-M, Elisia I, Kitts DD. Defining conditions for the co-culture of Caco-2 and HT29-MTX cells using Taguchi design. J Pharmacol Toxicol Methods. 2010;61(3):334–342. doi:10.1016/j.vascn.2010.02.004
27. Dehpour AA, Ebrahimzadeh MA, Fazel NS, Mohammad NS. Antioxidant activity of the methanol extract of Ferula assafoetida and its essential oil composition. Grasas y aceites. 2009;60(4):405–412. doi:10.3989/gya.010109
28. Tadesse S, Asres K, Veeresham C. Antioxidant activities of three Rubus species growing in Ethiopia. Ethiop Pharm J. 2007;25(2):103–110.
29. Kanna PS, Mahendrakumar C, Chakraborty T, Hemalatha P, Banerjee P, Chatterjee M. Effect of vanadium on colonic aberrant crypt foci induced in rats by 1, 2 dimethyl hydrazine. World J Gastroenterol. 2003;9(5):1020. doi:10.3748/wjg.v9.i5.1020
30. Ravnik-Glavač M, Cerar A, Glavač D. Animal model in the study of colorectal carcinogenesis. Pflügers Archiv Eur J Physiol. 2000;440(1):R055–R7. doi:10.1007/s004240000005
31. Giusti MM, Wrolstad RE. Determination of total phenolics. Curr Protocols Food Analytical Chem. 2001;6:l1.
32. Cole TG. Triglyceride Concentration. Handbook Lipoprotein Testing. 2000;16:207.
33. Sharma A, Artiss JD, Zak B. A method for the sequential colorimetric determination of serum triglycerides and cholesterol. Clin Biochem. 1987;20(3):167–172. doi:10.1016/S0009-9120(87)80115-7
34. Ghadi FE, Ghara AR, Bhattacharyya S, Dhawan DK. Selenium as a chemopreventive agent in experimentally induced colon carcinogenesis. World J Gastrointest Oncol. 2009;1(1):74. doi:10.4251/wjgo.v1.i1.74
35. Aranganathan S, Nalini N. Efficacy of the potential chemopreventive agent, hesperetin (citrus flavanone), on 1, 2-dimethylhydrazine induced colon carcinogenesis. Food Chem Toxicol. 2009;47(10):2594–2600. doi:10.1016/j.fct.2009.07.019
36. Bancroft JD, Gamble M. Theory and practice of histological techniques. Elsevier health sciences; 2008.
37. Cragg GM, Boyd M. Drug discovery and development at the National Cancer Institute: the role of natural products of plant origin. Med Plant Resources Tropical Forest. 1996;101–136.
38. Tsegyie Y, Engidawork E, Chemopreventive Effect of 80% Methanol Leaf Extract of Vernonia auriculifera Hiern (Asteraceae) against Dimethylhydrazine-Induced Colorectal Carcinogenesis in Rat. Available from: http://etd.aau.edu.et/handle/123456789/21153.
39. Tarwish B, Ngeranwa JJ, Matasyoh JC. Phytochemical composition of crude extracts derived from Vernonia spp. and its larvicidal activity against Anopheles gambiae. Int J Bonorowo Wetlands. 2017;7(2):108–116. doi:10.13057/bonorowo/w070207
40. Garcea G, Berry DP, Jones DJ, et al. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Prevention Biomarkers. 2005;14(1):120–125. doi:10.1158/1055-9965.120.14.1
41. Akerlund J, Björkhem I, Angelin B, Liljeqvist L, Einarsson K. Apparent selective bile acid malabsorption as a consequence of ileal exclusion: effects on bile acid, cholesterol, and lipoprotein metabolism. Gut. 1994;35(8):1116–1120. doi:10.1136/gut.35.8.1116
42. Byun TJ, Han DS, Ahn SB, et al. Pseudoinvasion in an adenomatous polyp of the colon mimicking invasive colon cancer. Gut Liver. 2009;3(2):130. doi:10.5009/gnl.2009.3.2.130
43. Yang W, Chang Y, Huang H, Wang Y, Yu X. Association between obesity, serum lipids, and colorectal polyps in old Chinese people. Gastroenterol Res Pract. 2013;2013:1–6. doi:10.1155/2013/931084
44. Laws A, Reaven G. Evidence for an independent relationship between insulin resistance and fasting plasma HDL‐cholesterol, triglyceride and insulin concentrations. J Intern Med. 1992;231(1):25–30. doi:10.1111/j.1365-2796.1992.tb00494.x
45. Thomas E, Gopalakrishnan V, Somasagara RR, Choudhary B, Raghavan SC. Extract of Vernonia condensata, inhibits tumor progression and improves survival of tumor-allograft bearing mouse. Sci Rep. 2016;6(1):1–12. doi:10.1038/srep23255
46. Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335(7630):1134. doi:10.1136/bmj.39367.495995.AE
47. Gedamu S, Labisso W, Yimer G. Coffee arabica complies Chemo-preventive Activity against DMH-induced Colorectal Cancer in Experimental Rat Model. J Med Diagn Meth. 2017;6(239):2. doi:10.4172/2168-9784.1000239
48. Farombi EO, Owoeye O. Antioxidative and chemopreventive properties of Vernonia amygdalina and Garcinia biflavonoid. Int J Environ Res Public Health. 2011;8(6):2533–2555. doi:10.3390/ijerph8062533
49. Ammar RB, Bhouri W, Sghaier MB, et al. Antioxidant and free radical-scavenging properties of three flavonoids isolated from the leaves of Rhamnus alaternus L.(Rhamnaceae): a structure-activity relationship study. Food Chem. 2009;116(1):258–264. doi:10.1016/j.foodchem.2009.02.043
50. Tesfaye S, Tadesse S, Engidawork E, et al. Screening of Twenty-one Ethiopian Medicinal Plants for their Antiproliferative Activity against Human Acute Myeloid Leukemia (MV4-11) Cell Line. Ethiop Pharm J. 2019;35:143–148. doi:10.4314/epj.v35i2.7
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.