Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 17
Characteristics of Vascular Access Cannulation Complications in End Stage Kidney Disease Patients in West Java from 2018 to 2022: A Retrospective Observational Study
Authors Djajakusumah TM , Hapsari P, Nugraha P, Muhammad A , Lukman K
Received 12 November 2023
Accepted for publication 1 February 2024
Published 12 February 2024 Volume 2024:17 Pages 47—58
DOI https://doi.org/10.2147/IJNRD.S440467
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Pravin Singhal
Teguh Marfen Djajakusumah, Putie Hapsari, Prapanca Nugraha, Arrayyan Muhammad, Kiki Lukman
Department of Surgery, Faculty of Medicine, Universitas Padjadjaran/Dr. Hasan Sadikin General Hospital, Bandung, Indonesia
Correspondence: Kiki Lukman; Teguh Marfen Djajakusumah, Email [email protected]; [email protected]
Background: End-stage kidney disease (ESKD) is associated with a tremendous financial burden. Data in Indonesia shows an increasing number of patients with ESKD taking hemodialysis as a routine procedure every year. Establishment and maintenance of vascular access are important in the management of ESKD. Vascular complications that often arise due to hemodialysis are common and one of the main reasons for hospitalization. Cannulation complications ranged from minor hematomas to acute bleeding from pseudoaneurysms that required emergency surgical procedures. This study aims to assess the different clinicopathological characteristics of ESKD patients with vascular access cannulation complications and the surgical management related to the complications.
Materials and Methods: This research is a retrospective observational study. The research subjects in this study were ESKD patients in the vascular and endovascular surgery division of the tertiary hospital in West Java, Indonesia. There were 121 study subjects. Clinicopathological characteristics of vascular cannulation complications and surgical management are extracted from the medical record.
Results: Three major vascular complications were ruptured pseudoaneurysms 64/121 (52.9%), impending rupture pseudoaneurysms 28/121 (23.1%), and pseudoaneurysms 21/121 (17.4%). Common surgical procedures were ligation of the draining vein 47/121 (38.8%), arterial primary repair 28/121 (23.1%), and arterial patch repair 18/121 (14.9%). There was a significant relationship between symptoms of bleeding in ruptured pseudoaneurysms and bulging masses in pseudoaneurysms (p = 0.001). There was a significant relationship between the diameter of the vascular mass, vascular defect size, and hematoma and the type of surgical procedure taken (p < 0.010).
Conclusion: Ruptured, impending rupture, and pseudoaneurysms are major complications of vascular access in ESKD patients, and there was a significant relationship between the carried-out surgical procedure and the size of the vascular mass, defect, and hematoma.
Plain Language Summary: Chronic kidney failure is a particular challenge in terms of health problems in Indonesia. With the increase in kidney failure patients, the number of dialysis patients in this country has also sharply increased because blood dialysis is the main management for end-stage kidney failure. Dialysis treatment does not mean it is 100% safe; various complications can arise from the blood dialysis procedure. Many of the complications that arise are caused by problems with the blood vessels that serve as access to the dialysis machine. In this research, we aim to study the characteristics of blood dialysis complications related to blood vessel access. This study was conducted at one of the largest national hospitals in Indonesia, precisely in West Java province, which is the most populated province in Indonesia. In this study, we involved 121 end-stage kidney failure patients. This study is very important to carry out considering that around 30% of chronic kidney failure patients that were hospitalized have complications from blood vessels. We hope that this research can be used further in determining the best procedure for providing venous access for dialysis with all the limitations faced by developing countries.
Keywords: arteriovenous fistula, end-stage kidney disease, hemodialysis, pseudoaneurysm, vascular access
Introduction
End stage kidney disease (ESKD) is now widely acknowledged as one of the leading causes of death worldwide. Data from West Java, Indonesia, showed the 5-year survival rate for ESKD patients was 55%, and patients aged more than 55 years have a worse survival rate than younger patients.1 The survival rate of patients with ESKD can be increased by undergoing hemodialysis (HD); therefore, the establishment of vascular access is very important for good and efficient HD continuity.2–4 Arteriovenous grafts (AVG), central venous catheters (CVC), and arteriovenous fistulas (AVF) are the three types of vascular access accessible for haemodialysis.3,4 For individuals with limited access choices or in an emergency setting with difficulties establishing vascular access, the superficial femoral vein or common femoral vein (CFV) may be a helpful hemodialysis access conduit.5–7 The most frequently used permanent vascular access is an arteriovenous fistula. The creation and maintenance of an AVF are two major challenges for the majority of hemodialysis patients.2–4
Complications from vascular access are common and account for up to 36–39% of ESKD patients being hospitalized.2,3,8–10 The incidence of pseudoaneurysm formation is 5–60% in patients with AVF. Pseudoaneurysms can form at the artery, anastomosis site, or draining vein (matured vein in AVF). Pseudoaneurysms can form due to surgical procedures such as leakage, infection, repeated needle puncture, malpuncture of the artery, and inadequate pressure applied at the cannulation site after hemodialysis. Complications of ruptured pseudoaneurysms in AVF can cause bleeding and threaten the patient’s life.3,8,9,11,12 Surgical management of the vascular access complication varies depending on the type of complication.10,11,13–15 This study aims to assess the different clinicopathological characteristics of ESKD patients with vascular access complications and the surgical management related to the complications.
Patients and Methods
Study Design and Setting
This research is a retrospective observational study in the West Java, Indonesian population, and follows the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines.16,17 The research subjects in this study were ESKD patients in the Vascular and Endovascular Surgery division of a tertiary general hospital in West Java from January 2018 to December 2022. The inclusion criteria for this study are ESKD patients with symptoms of an enlarged mass after cannulation at the vascular access site. We excluded vascular access complications from the arteriovenous fistula surgical procedure (leakage of anastomosis or failure of the AVF), vascular complications that were managed conservatively, patients who passed away before any surgical procedure, after intervention not related to hemodialysis, or central venous catheter (CVC) related complications. The study selection process is shown in Figure 1. The hospital ethics committee approved the study before it was conducted.
 |
Figure 1 The flowchart of the study selection process. |
Data Collection
Patient characteristics, clinicopathological data, and surgical procedure details were extracted from the medical record. Patient characteristics that were collected were age and sex. Clinicopathological data included: ESKD duration, risk factors or etiology for ESKD, hemodialysis time, subjective symptoms, diagnosis, the use of vascular access type, the location of the vascular complications, and the hemoglobin level and leucocyte count. Surgical procedure data were surgical procedure taken, vascular mass largest diameter, vascular defect size, and volume of the hematoma. The diagnoses that were obtained included: giant draining vein, ruptured pseudoaneurysm, impending ruptured pseudoaneurysm, pseudoaneurysm, and hematoma as shown in Figure 2.
Definition of Variables
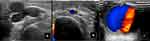
Some variable definitions in this study were as follows: The ESKD patient was diagnosed according to the Kidney Disease Improving Global Outcomes (KDIGO) Foundation 2012 guidelines, and end-stage kidney disease or renal failure was defined as a patient with a glomerular filtration rate less than 15 mL/min.18 The etiologies and risk factors (such as diabetes and hypertension) of the ESKD are based on the anamnesis and the nephrologist’s investigation of the patient’s history prior to the initiation of hemodialysis. Cannulation complications from vascular access in ESKD patients were diagnosed using the Marfen and Hapsari criteria, as shown in Table 1, with ultrasound criteria as shown in Figure 3. The surgical procedures that were used in the research subjects followed the Marfen and Hapsari flowchart of the surgical procedure for cannulation complications from vascular access in ESKD patients, as shown in Figure 4. Locations of the mass from patient complaints were divided according to the site of cannulation. The upper arm and antecubital fossa masses were caused by complications of brachiocephalic AVF, while the lower arm mass was caused by radiocephalic AVF. The vascular mass diameter was calculated from the size of the apparent mass using a caliper. The vascular defect size was measured during the operation by calculating the size of the defect vessel within a pseudoaneurysm using a sterile ruler. The volume of the hematoma was assessed by drawing the blood in the hematoma into a syringe.
 |
Table 1 Marfen and Hapsari Criteria for Cannulation Complications from Vascular Access in End-Stage Kidney Disease (ESKD) Patients |
 |
Figure 4 The Marfen and Hapsari flowchart of the surgical procedure for cannulation complications from vascular access in ESKD patients. |
Statistical Analysis
The statistical analysis of the variable was performed using SPSS 26 software (SPSS Inc., Chicago, IL, USA). Each quantitative variable is written as a percentage, and its numerical value is listed as the mean, standard deviation, median, minimum, and maximum. The Shapiro–Wilk test was used to determine the normality of the data distribution, and with a normal distribution, the comparison of the variables’ means was based on the ANOVA test. The variables with zero (0) values were treated as missing values and excluded from the analysis. The p value < 0.050 indicates a significant relationship.
Results
Study Population Characteristics
As described in Table 2, 121 patients were included in the study. There were 69 women and 52 men patients. The mean age was 46.1 ± 13.41 years. The ESKD duration mean was 25.02 ± 31.3 months. The majority etiology for the ESKD was hypertension (78.5%) and both hypertension and diabetes (11.7%). Hemodialysis is mostly scheduled for two times a week (86.8%). The symptoms for cannulation complications from vascular access were a bulging mass, a painful mass, and bleeding from the mass (100%, 89.2%, and 55.4%, respectively). Most of the diagnoses were ruptured pseudoaneurysms (52.9%), impending rupture pseudoaneurysms (23.1%), and pseudoaneurysms (17.4%). The vascular access that was used for hemodialysis was mostly from the arteriovenous fistula (73.6%), and the rest was from the common femoral vein (26.4%). The location of the vascular complications was mostly at the left forearm, left antecubital fossa, and left femoral (27.3%, 26.4%, and 14%, respectively). The mean hemoglobin level and leucocyte count were 7.27 ± 1.91 g/dL and 11,616 ± 6167 /μL. The majority of the surgical procedures for cannulation complications for vascular access were draining vein ligation, arterial primary repair, and arterial patch repair (38.8%, 23.1%, and 14.9%, respectively). The mean of the vascular mass largest diameter, the vascular defect, and the hematoma volume were 5.42 ± 3.38 cm, 8.79 ± 10.09 mm, and 87.93 ± 20 cc, respectively.
 |
Table 2 Characteristics of Research Subjects |
ANOVA Analysis of Diagnosis and Subjective Symptoms, Hemoglobin, and Leucocyte
In the ANOVA analysis among diagnosis and subjective symptoms, the mean duration of bleeding from the mass and bulging mass were statistically significant (p = 0.001) as shown in Table 3. The diagnosis of mean differences in hemoglobin showed a significant relationship (p = 0.001) as shown in Table 4.
 |
Table 3 ANOVA Analysis Between Diagnosis and Mean Differences of Subjective Symptoms (Days) |
 |
Table 4 ANOVA Analysis Between Diagnosis and Mean Differences of Hemoglobin and Leucocytes |
ANOVA Analysis of Surgical Procedure and Vascular Mass Diameter, Vascular Defect, and Hematoma Volume
In the ANOVA analysis among surgical procedure and vascular mass diameter, vascular defect, and hematoma volume. All of them showed a significant relationship (p < 0.010) as shown in Table 5.
 |
Table 5 ANOVA Analysis Between Surgical Procedure and Mean Differences of Vascular Mass Diameter, Vascular Defect, and Hematoma Volume |
Discussion
This is a single-center retrospective observational study describing and analyzing cannulation complications from vascular access in ESKD patients. The research subjects in this study were more women than men, and the mean age was 46 years. The data was similar in several studies that showed the prevalence of ESKD was higher in women than men, but the average was mostly in their fifties.19–22 Multicenter studies in Indonesia in 2018 and 2023 showed that the mean age of patients with ESKD was 44 years and the median was 52 years. There is an increasing trend in the younger population, and there were some statements that these facts correlated with higher risk in the obese population, physical inactivity, and metabolic disorders such as diabetes that are increasing in the younger population. Viral hepatitis is also one of the independent risk factors proposed to be linked with the increasing number of ESKD patients in the younger population in Indonesia.23,24
In this study, the duration of ESKD before the patient developed a cannulation complication from vascular access was 25 months, or two years. According to several studies with follow-up ranging from one year to five years, a vascular access complication may appear as early as three months, with an incidence of 0.03 to 0.24 events per 1000 days. A number of factors, such as patient comorbidities, surgical technique, vessel quality, vascular access care, and vascular access monitoring, are risk factors that increase the likelihood of vascular complications but are not frequently reported.11,12,25–28 Data from a global report and several studies show that the etiologies of ESKD are mostly diabetic (ranging from 30 to 50%) and hypertension (27%).29–33 In Indonesia, hypertension is still the most common etiology for ESKD (39%), followed by diabetes (22%). In this study, the major etiology for ESKD was hypertension (78.5%), followed by both hypertension and diabetes (11.7%).34 The majority of the subjects require HD twice a week (89.2%), while others require HD less than twice a week. This twice-a-week schedule usually occurs on Monday and Thursday, Tuesday and Friday, or Wednesday and Saturday. Conventional HD does not usually run-on Sunday, except for emergency ESKD patients. Other countries implement thrice-weekly HD as a regular treatment for ESKD patients, and several studies stated that more frequent dialysis correlated with better hypertension and hyperphosphatemia control and had a better effect on physical health and functioning.35–37 Other studies also reported that a higher frequency of hemodialysis was correlated with an increase in vascular access complications.38,39
The subjective symptoms of the subjects with cannulation complications from vascular access are a bulging mass, a painful mass, and bleeding from the mass (100%, 89.2%, and 55.4%, respectively). Thrombosis, oedema, bleeding, infection, clotting, pain, and an enlarged vessel (aneurysm) are common symptoms of vascular access complications.40–42 In this study, there was only one case of a giant draining vein. A giant draining vein is not showing signs of bleeding or pain, and there was no change in the hemoglobin. As seen in Table 4, the hemoglobin was 12 ± 0. The subjective symptoms of bulging mass were experienced by the patient long enough (a year) before they sought medication at the hospital.
This study only describes the three major symptoms of ESKD patients experiencing vascular access cannulation complications; even with this limitation, the data show that these three symptoms are associated with each other. The first stage in the formation of a pseudoaneurysm after injury to the vascular wall is hematoma formation with turbulent blood flow, which will lead to the bulging mass whether or not it hurts. If the blood flow does not cease on its own, a wall made of the by-product of the clotting cascade will form, and if this wall ruptures, bleeding will manifest as a symptom.43 In a pseudoaneurysm, necrotic cells, macrophages, and other inflammatory cells surround what’s left of the intimal layer. In an aneurysm, there are three layers of the vessel wall: the intimal layer, the mid-layer (media), and the adventitia, as shown in Figure 5.
The ANOVA analysis showed that the mean (days) of the ESKD patients experiencing the symptom of bleeding had a significant difference with the diagnosis of hematoma and ruptured pseudoaneurysm (p = 0.001), this showed that patients with ruptured pseudoaneurysm came earlier to the hospital than patients with hematoma. While the mean (days) of the symptom of bulging mass had a significant difference with the diagnosis of pseudoaneurysm than other diagnoses (p = 0.001), this showed that patients with pseudoaneurysm who only experienced bulging mass without bleeding or pain took a longer time to seek treatment than patients with hematoma, ruptured, or impending ruptured pseudoaneurysm as shown in Table 3. The analysis between the mean differences of hemoglobin and leucocytes and the diagnosis of vascular complications is shown in Table 4. There were significant differences between the means of hemoglobin. A patient with a ruptured pseudoaneurysm had lower hemoglobin due to bleeding than other diagnoses (p =0.001).
This study reported that more than a quarter of the subjects received HD through the CFV as their regular vascular access (26.4%). The use of the CFV is usually combined with the use of the upper limb’s superficial veins, such as the cephalic vein at the antecubital fossa. The blood to the dialysis machine comes from the CFV cannulation, and the blood from the dialysis machine returns back to the body from the cephalic vein,44 as shown in Figure 6. The use of the CFV as hemodialysis access is not recommended for regular use. However, in emergency settings where establishing vascular access through a central venous catheter seems difficult, the CFV can be used.5,45 In West Java, the CFV is usually used as vascular access for HD in the emergency patient, but previous reports showed that patients who were against the surgical procedure for the creation of an AVF or the insertion of a CVC preferred to use the CFV as routine HD access.46
The most common diagnosis of cannulation complications in this study combined from the AVF and CFV cannulation was ruptured pseudoaneurysm (52.9%); the second was impending rupture pseudoaneurysm (23.1%); and then pseudoaneurysm (17.4%). In our knowledge, this is the first report that categorized the diagnosis of pseudoaneurysm from vascular access into three categories: ruptured, impending rupture, and pseudoaneurysm, as described in Table 1. The purpose of the categorization was to determine the emergency procedure that needed to be taken and the possible complications that might arise for ruptured and impending rupture. Hemodialysis and surgical procedures need to be done in an emergency, while for pseudoaneurysms, the procedure can be done electively. The relationship between low hemoglobin levels and the diagnosis of ruptured pseudoaneurysm and hematoma was significant (p = 0.001).
As shown in Figure 4, the diagnosis of vascular complications, size, and site of the defect greatly affect the surgeon’s choice of surgical procedure. Small defects in the artery or vein were repaired with simple repair, but large defects in the artery need to be repaired with bypass or ligation, while large defects in veins need to be ligated. Table 5 shows an analysis of the mean differences in vascular mass diameter, vascular defect, hematoma volume, and the surgical procedures done for the vascular complications. The analysis showed that hematoma evacuation has a significant relationship with the largest mean of vascular mass diameter (p = 0.002), the arterial bypass with vein graft procedure has a significant relationship with the largest mean of vascular defect (p = 0.001), and hematoma evacuation has a significant relationship with the largest mean of hematoma volume (p = 0.001). The mean of vascular diameter for vein ligation procedure is larger than other procedures, this mean that defect of the vein might resulted in larger bulging mass than the arterial defect.
The strength of this study is that, to our knowledge, this is the first study to describe the use of common femoral veins as routine vascular access in developing countries. The limitations of the study are that the data on the patient factors such as comorbidities of the patients was not collected and described, especially the comorbid of atherosclerosis and infection that greatly affected the formation of pseudoaneurysm; the data from the follow-up after the surgical procedure was not collected; and the vascular access that was used after the surgical procedure was not described. The use of the common femoral vein as vascular access needs to be studied more, as in West Java, Indonesia, it still remains an option for hemodialysis access.
Conclusion
Ruptured, impending rupture, and pseudoaneurysms are major complications of vascular access in ESKD patients, with three main symptoms: bulging mass, pain, and bleeding. The diagnosis of vascular complications is important to determine the surgical procedure and technique to repair and preserve the vascular access.
Data Sharing Statement
All data and tables used to support the findings of this study are included within the article and available upon request to the corresponding author.
Ethical Declaration
This research was carried out in accordance with the Declaration of Helsinki, and the Dr. Hasan Sadikin Hospital ethics committee approved the study with No. Ethical Approval LB.02.01/X.6.5/202/2020.
Provenance and Peer Review
Not commissioned, externally peer-reviewed.
Statements and Declarations
The authors received no funding for this paper.
Acknowledgments
We thank all the trainees and surgical residents who helped carry out this study, and Bethy Suryawathy Hernowo and Hermin Aminah Usman for providing the histological pictures used in this report.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors affirm that they have no known financial or interpersonal conflicts that would have appeared to have an impact on the study presented.
References
1. Afiatin A, Agustian D, Wahyudi K, Riono P, Roesli RM. Survival analysis of chronic kidney disease patients with hemodialysis in West Java. Indonesia, year 2007–2018. Majalah Kedokteran Bandung. 2020;52(3):172–179. doi:10.15395/mkb.v52n3.2124
2. Arasu R, Jegatheesan D, Sivakumaran Y. Overview of hemodialysis access and assessment. Can Fam Physician. 2022;68(8):577–582. doi:10.46747/cfp.6808577
3. Lawson JH, Niklason LE, Roy-Chaudhury P. Challenges and novel therapies for vascular access in haemodialysis. Nat Rev Nephrol. 2020;16(10):586–602. doi:10.1038/s41581-020-0333-2
4. Allon M. Vascular access for hemodialysis patients: new data should guide decision making. Clin J Am Soc Nephrol. 2019;14(6):954–961. doi:10.2215/CJN.00490119
5. Huber TS, Ozaki CK, Flynn TC, Ross EA, Seeger JM. Use of superficial femoral vein for hemodialysis arteriovenous access. J Vascular Surg. 2000;31(5):1038–1041. doi:10.1067/mva.2000.104587
6. Ribeiro MM, Rodrigues E, Bezerra A, et al. Superficial femoral vein transposition as a solution for hemodialysis vascular access. J Vasc Brasileiro. 2022;21. doi:10.1590/1677-5449.202101352
7. Gradman WS, Cohen W, Haji-Aghaii M. Arteriovenous fistula construction in the thigh with transposed superficial femoral vein: our initial experience. J Vascular Surg. 2001;33(5):968–975. doi:10.1067/mva.2001.115000
8. Çakıcı M, Yaman ND, Özçınar E, et al. Surgical interventions for arteriovenous fistula aneurysms: our single-center experience. Turkish J Vasc Surg. 2017;26(1):12–17. doi:10.5606/tjvs.2017.5
9. Sahasrabudhe P, Dighe T, Panse N, Patil S. Retrospective analysis of 271 arteriovenous fistulas as vascular access for hemodialysis. Indian J Nephrol. 2013;23(3):191. doi:10.4103/0971-4065.111845
10. Belli S, Yabanoglu H, Aydogan C, et al. Surgical interventions for late complications of arteriovenous fistulas. Int Surg. 2014;99(4):467–474. doi:10.9738/INTSURG-D-14-00012.1
11. Stolic R. Most important chronic complications of arteriovenous fistulas for hemodialysis. Med Princ Pract. 2013;22(3):220–228. doi:10.1159/000343669
12. Al-Jaishi AA, Liu AR, Lok CE, Zhang JC, Moist LM. Complications of the arteriovenous fistula: a systematic review. J Am Soc Nephrol. 2017;28(6):1839–1850. doi:10.1681/ASN.2016040412
13. Aljuaid MM, Alzahrani NN, Alshehri AA, et al. Complications of arteriovenous fistula in dialysis patients: incidence and risk factors in Taif city, KSA. J Fam Med Primary Care. 2020;9(1):407. doi:10.4103/jfmpc.jfmpc_848_19
14. Demir D, Ceviker K, Aydin MS, Sahinalp S. Complications of arteriovenous fistula with polytetraflouroethylen grafts in hemodialysis patients. Niger J Clin Pract. 2015;18(1):120–123. doi:10.4103/1119-3077.146993
15. Staaf K, Fernström A, Uhlin F. Cannulation technique and complications in arteriovenous fistulas: a Swedish Renal Registry-based cohort study. BMC Nephrol. 2021;22(1):1–2. doi:10.1186/s12882-021-02458-z
16. Vandenbroucke JP, Elm EV, Altman DG, et al.; Strobe Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Internal Med. 2007;147(8):W163. doi:10.7326/0003-4819-147-8-200710160-00010-w1
17. Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(Suppl 1):S31. doi:10.4103/sja.SJA_543_18
18. Hashmi MF, Benjamin O, Lappin SL. End-stage renal disease. In: StatPearls [Internet]. StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499861/.
19. Carrero JJ, Hecking M, Chesnaye NC, Jager KJ. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol. 2018;14(3):151–164. doi:10.1038/nrneph.2017.181
20. Tomlinson LA, Clase CM. Sex and the incidence and prevalence of kidney disease. Clin J Am Soc Nephrol. 2019;14(11):1557–1559. doi:10.2215/CJN.11030919
21. Lewandowski MJ, Krenn S, Kurnikowski A, et al. Chronic kidney disease is more prevalent among women but more men than women are under nephrological care: analysis from six outpatient clinics in Austria 2019. Wiener klinische Wochenschrift. 2023;135(3–4):89–96. doi:10.1007/s00508-022-02074-3
22. Harris RC, Zhang MZ. The role of gender disparities in kidney injury. Ann Translat Med. 2020;8(7):514. doi:10.21037/atm.2020.01.23
23. Hustrini NM, Susalit E, Rotmans JI. Prevalence and risk factors for chronic kidney disease in Indonesia: an analysis of the National Basic Health Survey 2018. J Global Health. 2022;12. doi:10.7189/jogh.12.04074
24. Hustrini NM, Susalit E, Lydia A, et al. The etiology of kidney failure in Indonesia: a multicenter study in Tertiary-Care Centers in Jakarta. Ann Global Health. 2023;89(1):36. doi:10.5334/aogh.4071
25. Lin CC, Yang WC, Chen MC, Liu WS, Yang CY, Lee PC. Effect of far infrared therapy on arteriovenous fistula maturation: an open-label randomized controlled trial. Am J Kidney Dis. 2013;62(2):304–311. doi:10.1053/j.ajkd.2013.01.015
26. Lok CE, Sontrop JM, Faratro R, Chan CT, Zimmerman DL. Frequent hemodialysis fistula infectious complications. Nephron extra. 2014;4(3):159–167. doi:10.1159/000366477
27. Olsha O, Hijazi J, Goldin I, Shemesh D. Vascular access in hemodialysis patients older than 80 years. J Vascular Surg. 2015;61(1):177–183. doi:10.1016/j.jvs.2014.07.005
28. Lok CE, Oliver MJ, Su J, Bhola C, Hannigan N, Jassal SV. Arteriovenous fistula outcomes in the era of the elderly dialysis population. Kidney Int. 2005;67(6):2462–2469. doi:10.1111/j.1523-1755.2005.00355.x
29. Eknoyan G, Lameire N, Eckardt K, et al. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3(1):5–14.
30. Vaidya SR, Aeddula NR. Chronic renal failure. In: The Scientific Basis of Urology.
31. Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. 2022;12(1):7–11. doi:10.1016/j.kisu.2021.11.003
32. Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA. 2019;322(13):1294–1304. doi:10.1001/jama.2019.14745
33. Fraser SD, Blakeman T. Chronic kidney disease: identification and management in primary care. Pragmat Observatl Res. 2016;Volume 7:21–32. doi:10.2147/POR.S97310
34. Indonesia PN. 11th Report of Indonesian renal registry 2018. Jakarta: Perhimpunan Nefrologi Indonesia; 2018.
35. Daugirdas JT. Hemodialysis treatment time: as important as it seems? Semin Dial. 2017;30(2):93–98. doi:10.1111/sdi.12575
36. Chien CW, Huang CJ, Chao ZH, Huang SK, Chen PE, Tung TH. Hemodialysis interval and its association with emergency care and mortality: a nationwide population-based cohort study. Medicine. 2019;98(10):e14816. doi:10.1097/MD.0000000000014816
37. Hall YN, Larive B, Painter P, et al.; Frequent Hemodialysis Network Trial Group. Effects of six versus three times per week hemodialysis on physical performance, health, and functioning: frequent Hemodialysis Network (FHN) randomized trials. Clin J Am Soc Nephrol. 2012;7(5):782–794. doi:10.2215/CJN.10601011
38. Mendonca S, Bhardwaj S, Sreenivasan S, Gupta D. Is twice-weekly maintenance hemodialysis justified? Indian J Nephrol. 2021;31(1):27. doi:10.4103/ijn.IJN_338_19
39. Dai L, Lu C, Liu J, et al. Impact of twice-or three-times-weekly maintenance hemodialysis on patient outcomes: a multicenter randomized trial. Medicine. 2020;99(20):e20202. doi:10.1097/MD.0000000000020202
40. Adib-Hajbagheri M, Molavizadeh N, Alavi NM, Abadi MH. Factors associated with complications of vascular access site in hemodialysis patients in Isfahan Aliasghar hospital. Iranian J Nurs Midwifery Res. 2014;19(2):208. PMID: 24834093.
41. Ravani P, Quinn R, Oliver M, et al. Examining the association between hemodialysis access type and mortality: the role of access complications. Clin J Am Soc Nephrol. 2017;12(6):955–964. doi:10.2215/CJN.12181116
42. Hemachandar R. Analysis of vascular access in haemodialysis patients-single center experience. J Clin Diagn Res. 2015;9(10):OC01. doi:10.7860/JCDR/2015/13342.6611
43. Ramírez S, Bastidas N, Lozano N, Casas D, Hernández M. Pseudoaneurysm and post-traumatic arteriovenous fistula in pulmonary artery branch: case report and review of the literature. Radiol Case Rep. 2023;18(2):476–480. doi:10.1016/j.radcr.2022.10.087
44. Murdeshwar HN, Anjum F. Hemodialysis. In: A Medication Guide to Internal Medicine Tests and Procedures. Elsevier Health Sciences; 2023: 149–152. Available from: https://www.ncbi.nlm.nih.gov/books/NBK563296/.
45. Gołębiowski T, Letachowicz K, Letachowicz W, et al. Urgent hemodialysis with the use of peripheral veins to avoid catheter insertion. Hemodialysis Int. 2016;20(4):E4–E6. doi:10.1111/hdi.12426
46. Kalitouw F. P2B2 PABI XVI Manado 2019 continuing professional development in surgery: the patients and surgeon in Universal Health Coverage Era. Bali Med J. 2019;8(2):1–65. doi:10.15562/bmj.v8i1.1540
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.