Back to Journals » Journal of Asthma and Allergy » Volume 16
Characteristics of Allergic, Eosinophilic, and Overlapping Asthma Phenotypes Among Pediatric Patients with Current Asthma: A Cross-Sectional Study from Saudi Arabia
Authors Asseri AA
Received 7 September 2023
Accepted for publication 23 November 2023
Published 1 December 2023 Volume 2023:16 Pages 1297—1308
DOI https://doi.org/10.2147/JAA.S439089
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Ali Alsuheel Asseri
Department of Child Health, College of Medicine, King Khalid University, Abha, Saudi Arabia
Correspondence: Ali Alsuheel Asseri, Department of Child Health, College of Medicine, King Khalid University, Abha, Saudi Arabia, Tel +966500186013, Email [email protected]
Purpose: Asthma is one of the most common chronic diseases affecting 10%– 30% of children in Saudi Arabia. Although data exist on adult asthma phenotyping and endotyping in Saudi Arabia, little is known about asthma phenotypes in Saudi children.
Patients and Methods: This cross-sectional study enrolled pediatric patients diagnosed with bronchial asthma and followed in the pediatric pulmonology clinic of the Abha Maternity and Children Hospital between August 2021 and May 2023.
Results: A total of 321 children (aged 5– 14 years) were analyzed. The population was classified into allergic [169 (52.6%)], eosinophilic [144 (44.9%)], and overlapping allergic and eosinophilic asthma [97 (30.2%)] phenotypes. Regarding asthma severity, 35.5%, 50.2%, and 14.3% were classified as mild, moderate, and severe, respectively. Of the 321 patients in the study, 124 (38.6%) had at least one asthma exacerbation that required hospitalization. The number of reported missed school days in the previous year was 1571 days [190 (59.2%) patients reported at least one missed school day]. The factors associated with the likelihood of uncontrolled asthma for all study participants included: emergency room (ER) visit last year (OR = 3.7, 95% CI:0.6– 15.9]), overlapping eosinophilic and allergic (OR = 3.2, 95% CI = 1.8– 5.9), and allergic phenotype (OR = 2.7, 95% CI = 1.3– 5.4). The level of asthma control differed significantly among the three asthma phenotypes (p = 0.037).
Conclusion: Allergic asthma is the most prevalent asthma phenotype in this study, followed by the eosinophilic phenotype. The research has also shown that several factors predict uncontrolled asthma, including a family history of asthma, previous admission to the PICU, and previous hospitalization ever. There is, therefore, a definite need for multicenter cohort studies to better understand the phenotypes and endotypes of childhood asthma, as it could offer therapeutic and prognostic relevance.
Keywords: children, asthma, childhood asthma phenotypes, uncontrolled asthma, allergic asthma
Introduction
Asthma is a chronic respiratory disease affecting about 300 million people of all ages worldwide, with an estimated 250,000 deaths each year.1 It is one of the most common chronic diseases that afflicts an estimated 10%–30% of children in Saudi Arabia.2,3 Asthma is a very heterogeneous disease with regard to diagnosis, age of presentation, severity, underlying inflammatory process, triggers, natural history, and response to therapy. This heterogeneity complicates the understanding of the phenotypes and endotypes of childhood asthma. The availability of longitudinal data from several childhood asthma cohort studies exploring many aspects of this heterogeneity has opened many horizons for further research.4,5
Asthma is clinically characterized by recurrent episodes of coughing, wheezing, and dyspnea, pathophysiologically due to inflammation-driven variable expiratory airflow limitation.6,7 The type of airway inflammation in asthma patients varies between individuals and over time, and it could, if untreated, lead to airway remodeling. A precise understanding of these inflammatory pathways, as well as detailed clinical and physiological phenotyping of asthma, would help predict future exacerbations, guide the use of targeted therapy, and prevent airway remodeling.8,9 Based on the involved inflammatory cascade trigger, childhood asthma can be categorized into allergic and non-allergic asthma.9–11 Allergic asthma is characterized by airway inflammation triggered by aeroallergens. This inflammation is driven by T-helper cell type 2 (Th2) cytokines, such as interleukin (IL)-4, IL-5, and IL-13.11,12 These cytokines cause cascades of downstream events, including airway inflammation, airway eosinophilia, and further immunoglobulin E (IgE) synthesis.12,13 In addition to allergic phenotype, further categorization of childhood asthma has been proposed, including eosinophilic and overlapping allergic and eosinophilic asthma. Accurate phenotyping would help select the appropriate treatment and achieve asthma control. While progress has been made in phenotyping and endotyping adult asthma in Saudi Arabia,14 no studies have assessed asthma phenotypes in Saudi children. Asthma phenotypes in children may differ from those in adults, so pediatric asthma studies are needed to understand these differences better.
Additionally, the primary goal of asthma management is to achieve control of the disease and improve the quality of life for children and their families. This includes reducing school absenteeism, increasing productivity, and preventing emergency care and hospitalizations. Furthermore, understanding the relationship between asthma phenotyping and asthma control is essential, as the available biological therapies are largely based on precise asthma endotyping phenotyping classification.15 Targeting several underlying immunological pathways of asthma is the main mechanism of available asthma advanced therapeutic antibodies (biologics), including IgE (omalizumab), IL-5 (mepolizumab and reslizumab), IL-5 receptor (benralizumab), and IL-4/IL-13 receptor complex (dupilumab). Understanding the predictors of uncontrolled asthma and response to therapy is essential to identify and address modifiable risk factors of uncontrolled asthma and to target those who would benefit from available therapies. Several factors related to poor asthma control have been reported. A recent cross-sectional study from a single center in Saudi Arabia included 150 asthmatic children and found that children with multiple pediatric intensive care unit (PICU) admission were three times more likely to have uncontrolled asthma than children with no reported PICU admission.16 Another study from the Netherlands involving 408 children found that children with a family history of asthma were twice as likely to have uncontrolled asthma.17 Most research on asthma control has been conducted through surveys of parents reporting the asthma status or asthma control status of their child; studies on clinically-assessed asthma control in children with physician-diagnosed asthma are lacking.16,18 This study aimed to describe the characteristics of asthma phenotypes in children, including allergic, eosinophilic, and allergic eosinophilic asthma, and to identify potential factors contributing to uncontrolled asthma in this population.
Materials and Methods
Study Population and Setting
This cross-sectional study enrolled pediatric patients aged 5–14 years who were diagnosed with bronchial asthma and followed in the pediatric pulmonology clinic at the Abha Maternity and Children Hospital between August 2021 and May 2023. This hospital is considered a tertiary care and teaching hospital in the southwestern region of the Kingdom of Saudi Arabia. Physician-diagnosed asthma was defined according to the Global Initiative for Asthma (GINA), which includes episodes characterized by shortness of breath, cough, wheezing, or chest tightness following exposure to one or several triggers, either viral respiratory infection or allergen, with evidence of objective expiratory airflow limitation.19 Spirometry is not always feasible in confirming reversible airflow limitation in asthmatic children; hence, after excluding alternative diagnoses, asthma diagnosis was based on clinical response to trials of inhaled steroids after two months of treatment. Asthma severity was based on the dosing of inhaled corticosteroids and the worsening of symptoms with treatment discontinuation. This consistent with GINA criteria for childhood asthma management for mild, moderate, and severe asthma.19 Exclusion criteria included children with recurrent wheezing episodes under five and those with coexisting morbidities such as prematurity, cystic fibrosis, sickle cell disease, or other pulmonary diseases.
Study Variables
The data collection sheet consisted of three sections: clinical and demographic variables, laboratory variables, and spirometry measures. Clinical and demographic variables include age, gender, age at the first wheezy episode, allergic history, asthma severity, family history of asthma, asthma control over the last four weeks, school missing days over the last year, number of oral corticosteroids used over the last year, ED visits and admissions in the last year, any prior intubation, and any prior PICU admission. Body mass index (BMI; a measure of weight in kilograms divided by height in meters squared) was obtained from the body measurements file used to define obesity status. Children with a BMI in the >95th percentile was considered to be obese; those with a BMI between the 85th and 95th percentiles were considered to be overweight. These definitions were based on the corresponding age and sex reference groups.
Laboratory variables included baseline eosinophilic counts, total immunoglobulin E (IgE) measurement, and allergen-specific immunoglobulin E (IgE). A blood sample was collected at the time of the enrollment and processed at the laboratory department of the AMCH. An ELISA kit (Human Diagnostics, Germany, catalogue number #51015) was used to measure total IgE. For specific IgE assays, a panel of 20 common aeroallergens was tested. The panel comprised of pollens (Timothy grass, Cultivated rye, Alder, Birch, Oak, Olive tree, Ambrosia artemisiifolia, and Mugwort), house dust mites (Dermatophagoides pteronyssinus and Dermatophagoides farina), Cockroach (German), dander (Cat, Dog, Horse, and Camel), and mold spores (Penicillium notatum, Cladosporium herbarum, Aspergillus fumigatus, Candida albicans, and Alternaria alternata). These allergens were tested for patients who had a high total IgE level (> 100 IU/mL). The presence of serum-specific IgE (sIgE) of 3.5 IU/mL or more to at least one of the aforementioned allergens was considered positive sensitization.20
Spirometry (Jaeger Full Masterscreen PFT. Germany—2011. CD version 5.72.1.77) was performed pre- and post-bronchodilation according to European Respiratory Society (ERS)/American Thoracic Society (ATS) recommendations.21 We used an inline bacterial filter and the prediction equations from the Global Lung Initiative (GLI) 2012.22 A 12% or greater increase in forced expiratory volume in 1 s (FEV1) after bronchodilator inhalation is a diagnostic criterion for asthma.21
Study Variables Definition
Allergic rhinitis was defined as a chronic nasal disorder with one or more of the following symptoms: runny nose, stuffy nose, itchy nose, and sneezing. These symptoms can resolve, either spontaneously or with treatment, and they do not manifest as part of a cold. In addition to these symptoms, the attending physician confirmed the signs of nasal turbinate inflammation, including swelling, redness, and inferior turbinate secretions. The presence of two or more of these symptoms and signs is typically sufficient to confirm a diagnosis of allergic rhinitis.23,24
Asthma Phenotypes
Allergic asthma phenotype was defined as asthma associated with at least one allergic comorbid condition (allergic rhinitis, eczema, and food allergy) with > 100 IU/mL total serum IgE and sensitization to at least one aeroallergen.10,25,26
Eosinophilic asthma phenotype was defined as asthma with blood eosinophil counts ≥300 eosinophil cells/ μL.15 Because the standard definition for eosinophilic asthma in children is still evolving, a cutoff of ≥ 300 cells/mm3 was used as those cutoff has been mostly used for screening pediatric asthma patients into clinical trials.15,27 Patients with both allergic and eosinophilic asthma were considered to have an overlapping allergic and eosinophilic phenotype.7,28,29
Asthma Severity Assessment
According to the GINA guidelines, asthma severity was determined based on the frequency of symptoms and the use of controller therapies. Patients were classified as having mild, moderate, or severe persistent asthma. The mild persistent group had symptoms more than once a week but less than once a day, nocturnal symptoms more than twice a month, and pre-BD FEV1 (%) > 80%. The moderate-to-severe persistent asthma patients had symptoms daily, nocturnal symptoms more than once a week, and pre-BD FEV1 (%) < 80%.
Furthermore, the asthma severity was confirmed by the level of asthma treatment that control the symptoms: mild asthma requiring daily low-dose corticosteroids, moderate asthma necessitating low-dose ICS/LABA or medium-dose ICS, and severe asthma needing moderate- or high-dose ICS/LABA with or without montelukast.
Asthma Control Assessment
The level of asthma control was assessed using GINA asthma control guidelines, which evaluates the presence of four items in the past four weeks: diurnal and nocturnal symptoms, short-acting b2-agonists use, and activity limitation. The resulting level of asthma control is classified as (1) well-controlled if the child does not have any of the above symptoms, (2) partially controlled in the presence of one or two of the above symptoms, and (3) uncontrolled in the presence of at least three of the above symptoms. The asthma control levels (well, partially, and uncontrolled) were reported as numbers and percentages.
Statistical Analysis
The SPSS Software program version 29 (IBM SPSS Statistics for Windows, Armonk, NY: IBM Corp) was used for statistical analysis. We planned to include a sample of 384 children in the present study. The sample size was estimated based on an expected prevalence of 50% of allergic asthma among children with MD-asthma diagnosis,10,30–32 with an acceptable margin of error of 5% at a 95% confidence level (CI) and a design effect of 2. The sample size was determined using the Epi-Info 7 software. Normally distributed variables were represented as mean and standard deviation, while non-normally distributed variables were represented as median with interquartile range (IQR). The Shapiro–Wilk test and histogram data visualization were used to assess the normality of continuous variables. Counts and percentages were used to represent categorical variables. Differences between categorical variables were compared using Pearson’s Chi-square (χ²) and Fisher’s exact test, as appropriate. Univariable and multivariable binary logistic regression models were used to identify the factors associated with uncontrolled asthma. Results were reported as odds ratios (OR) and their 95% CI. The dependent variable was defined as having uncontrolled asthma. The independent variables included family history of asthma, any prior PICU admission, any prior asthma-related hospitalization ever, ER visit last year, severe asthma, and asthma phenotypes (allergic, eosinophilic, and overlapping allergic and eosinophilic). Significant differences were considered at p-values less than 0.05.
Results
Characteristics of the Study Population
Between August 2021 and May 2023, a total of 321 children aged 5–14 years who visited the AMCH pediatric pulmonology clinic were analyzed. The population was classified into allergic (n = 169, 52.6%), eosinophilic (n = 144, 44.9%), and overlapping allergic and eosinophilic asthma (n = 97, 30.2%) phenotypes (Figure 1). Table 1 summarizes the demographic and clinical characteristics of the patients by asthma phenotype. The median (IQR) ages of the subjects were 8 (6–11), 8 (6–10), and 8 (6–11) for allergic, eosinophilic, and overlapping allergic and eosinophilic phenotypes, respectively. Male patients represented 70.4% of allergic asthma, 65.3% of eosinophilic asthma, and 70.1% of the overlapping allergic and eosinophilic phenotype. The weight, height, and BMI did not differ significantly between the three phenotypes (data not shown). The prevalence of overweight and obesity (BMI percentile was >85% for overweight and >95% for obesity) in patients with allergic asthma, eosinophilic asthma, and the overlapping allergic and eosinophilic phenotype were (30.2%, n = 51), (27.8%, n = 40), and 33 (34.0%), respectively. The overall family history of asthma was 74.1% (238 out of 321). The family history of asthma among the allergic, eosinophilic, and overlapping allergic and eosinophilic phenotypes were 82.8%, 84.1%, and 86.6%, respectively. The most common associated atopic comorbidity was allergic rhinitis: 87.0%, 86.1%, and 89.7% among those with allergic asthma, eosinophilic asthma, and the overlapping allergic and eosinophilic phenotype, respectively. The median for the first wheezy episode among the three phenotypes was 12 months, while the median of MD-asthma diagnosis among the phenotypes was five years for allergic and overlapping allergic and eosinophilic phenotypes and 4.5 years for the eosinophilic phenotype. All patients were treated according to the GINA guidelines.19 High-dose steroid was used in 9 (2.8%) patients, 6 (66.7%) of whom had allergic asthma (data not shown). Montelukast was used as an add-on therapy in 185 (57.6%) patients, 59 (60.8%) of whom had overlapping allergic and eosinophilic asthma.
 |
Table 1 Characteristics of the Enrolled Patients Stratified According to the Asthma Phenotype |
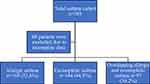 |
Figure 1 Flowchart of the study population. n = number. |
Asthma Severity Among the Phenotypes
Table 2 classifies asthma severity by the allergic, eosinophilic, and overlapping allergic and eosinophilic phenotypes. Regarding asthma severity, 35.5%, 50.2%, and 14.3% were classified as mild, moderate, and severe asthma, respectively. Moderate asthma was the most represented class (161/321, 50.2%). Asthma severity was based on symptoms and level of asthma medication use. Of the 321 patients in the study, 124 (38.6%) had at least one asthma exacerbation that required hospitalization ever. The overall median number of hospitalizations (IQR) was 4 days (0–10), and the median number (IQR) of ER visits of the last year was 2 visits (1–3). A total of 74 (23.1%) patients had PICU admission ever: 49 (29%) had allergic asthma, 39 (27.1%) had eosinophilic asthma, and 30 (30.9%) had the overlapping allergic and eosinophilic phenotype. The number of reported missed school days in the previous year was 1571 days: 190 (59.2%) patients reported at least one missed school day, and the median (IQR) was 5 days (2–10). Asthma control was assessed using the GINA guidelines. In total, 144 (44.9%) patients had well-controlled asthma, and 16.5% had uncontrolled asthma. The spirometry assessments were available for 57 patients (Figure 2). The overall median predicted percentage of forced expiratory volume in 1 second (FEV1%) of 88 (75.5–103). The predicted FVC% and predicted FEV1% did not differ by asthma severity (data not shown).
 |
Table 2 Asthma Severity Among the Enrolled Patients Stratified by Asthma Phenotypes |
Factors Associated with Uncontrolled Asthma
Table 3 shows the factors associated with uncontrolled asthma. The following factors were associated with uncontrolled asthma: a family history of asthma, previous admission to the PICU, asthma-related hospitalization ever, ER visit in the past year, severe asthma, eosinophilic phenotype, overlapping allergic and eosinophilic phenotype, and allergic phenotype (all p-values less than 0.05). Results of the univariable and multivariable logistic regression are reported in Table 4. The logistic regression was performed to ascertain the effects of a family history of asthma, PICU admission ever, asthma-related hospitalization ever, ER visit last year, severe asthma, and asthma phenotypes (allergic, eosinophilic, and overlapping allergic and eosinophilic) on the likelihood that participants have uncontrolled asthma. The logistic regression model was statistically significant (χ2(8) = 19.431, p = 0.013). The model explained 11.1% (Nagelkerke R2) of the variance in uncontrolled asthma and correctly classified 80.9% of cases. Factors associated with the likelihood of uncontrolled asthma for all study participants, from highest to lowest odds ratio were: ER visit last year (OR = 3.7, 95% CI = 0.6–15.9), overlapping eosinophilic and allergic (OR = 3.2, 95% CI = 1.8–5.9), allergic phenotype (OR = 2.7, 95% CI = 1.3–5.4), PICU admission ever (OR = 2.6, 95% CI = 1.4–4.9), hospitalization last year (OR = 2.6, 95% CI = 1.4–4.9), severe asthma (OR = 2.3, 95% CI = 1.2–4.8), a family history of asthma (OR = 2.1, 95% CI = 1.1–6.0), and eosinophilic phenotype (OR = 2.0, 95% CI = 1.1–3.6). The level of asthma control differed significantly among the three asthma phenotypes (p = 0.037). Additionally, there was a statistically significant difference between the uncontrolled asthma of the three asthma phenotypes (p = 0.004) (Figure 3).
 |
Table 3 Factors Associated with Uncontrolled Asthma |
 |
Table 4 Logistic Regression Showing Unadjusted and Adjusted Odds Ratios for Patients with Uncontrolled Asthma |
Discussion
Research continues to study the different phenotypes of childhood asthma to better understand the underlying pathogenesis of the disease and lead to more targeted and personalized approaches to asthma therapy.33,34 Recently, several randomized clinical trials have demonstrated the efficacy and safety of monoclonal antibodies that target key asthma mediators, particularly in severe childhood asthma.15 This cross-sectional study indicated that among Saudi children with current asthma who were followed up in a pediatric pulmonology clinic, the allergic asthma phenotype was the most common phenotype (52.6%), followed by the eosinophilic asthma phenotype (44.9%). Additionally, several clinical factors predicted uncontrolled asthma, such as family history of asthma, previous admission to the PICU, asthma-related hospitalization ever, ER visit in the past year, severe asthma, and overlapping allergic and eosinophilic and allergic phenotypes. To the best of the author’s knowledge, the present study is the first to describe the phenotypic characteristics of asthmatic children in Saudi Arabia.
Until recently, asthma has been deemed to be a disease with one phenotype. However, several studies have disproved this notion and demonstrated that asthma is a heterogeneous disease with different clinical phenotypes and multiple inflammatory endotypes.28,35–38 Allergic asthma is the most prevalent phenotype of childhood asthma that develops earlier in life and is associated with other atopic comorbid disorders.29,31,39,40 Meanwhile, other asthma endotypes, such as eosinophilic and neutrophilic asthma, have been identified.39,41,42 Our descriptive analysis revealed that the median age of asthma diagnosis was 5 years, and 52.6% of the enrolled subjects had allergic asthma. In accordance with the present results, previous studies have demonstrated that allergic asthma starts in childhood.10,34,43 In a comparative cross-sectional analysis of subjects with severe asthma, Miranda et al concluded that severe allergic asthma starts at a younger age with significant allergen sensitization compared to late-onset severe asthma (98% vs 76%, P < 0.007).44 Additionally, they reported that eczema is a common comorbid allergic disorder associated with early-onset allergic asthma.44 A recent review of longitudinal cohort studies of childhood asthma found that school-age children with mild-to-moderate asthma have a predominance of type 2 inflammation, as evidenced by elevated levels of interleukin 5 and 13.13,45 Because it is difficult to biopsy the airways directly to confirm the presence of this inflammatory endotype, indirect measures of inflammation have been used to confirm this endotype, such as exhaled nitric oxide and type 2 biological markers.10,43,46,47 One can, therefore, conclude that allergic asthma is common in children with predominant type 2 inflammation endotype, independent of asthma severity. However, further research is needed to clearly define these phenotypes and identify simple and feasible biomarkers of the underlying endotypes.
In the present study, the definition of eosinophilic phenotype was only based on a blood eosinophil count of ≥ 300 cells/mm.3 We found that eosinophilic asthma prevalence was 44.9%, and 67.4% of them overlapped with allergic asthma. Additionally, among patients with allergic asthma, 57.4% had eosinophilic asthma. Overall, 30.2% of the participants in this study had the overlapping asthma phenotype. Although both the allergic and eosinophilic asthma phenotypes are mechanistically driven by type 2 (T2) inflammation, they are two distinct clinical phenotypes,11,32 consistent with previous studies. Tran et al studied 269 children with asthma and found that among children with eosinophilic asthma, 81% also had atopic asthma, and the percentage of children with both eosinophilic and atopic asthma increased with an increase in the eosinophil cutoff.11 Furthermore, another pediatric study found that 41% of children with severe asthma and 36% of children with mild-moderate asthma had eosinophilic asthma. The investigators in this study used sputum eosinophil count for eosinophilic asthma phenotyping. They found that the inflammatory phenotyping of the studied group was unstable, regardless of asthma severity or inhaled steroid use. Overall, the phenotyping and endotyping of childhood asthma are complex and dynamic. Further research is needed to better understand this complex and heterogeneous disease, which will help personalize therapy, especially with the development of new asthma biologic agents.
Asthma control and overall asthma severity are two distinct concepts that are sometimes used interchangeably. International and national asthma guidelines recommend that asthma control and level of asthma severity should be assessed with each visit, given the complexity and dynamic nature of the asthma severity.19,48 Asthma control, however, is affected by the caregiver’s level of education and the assessment tool used.18 In the current study, the GINA asthma severity and level of control were used. The overall distribution of asthma severity among the studied patients was as follows: mild (35.5%), moderate (50.2%), and severe (14.3%). About 64.5% of the participants had combined moderate-severe asthma. The prevalence of moderate-to-severe asthma in this study was higher compared to other pediatric asthma studies. For instance, Lee et al studied 288 steroid-naive asthmatic children and found that 31% of those with eosinophilic asthma had moderate-to-severe asthma, compared to 20% of those with non-eosinophilic asthma.29 The increased prevalence of moderate-severe asthma in our study is likely because the patients were recruited from a specialized clinic and may not be representative of all children with asthma. In the present study, the asthma severity was determined using symptoms and medication use according to the recent GINA guidelines.19 Previous studies have shown that there is no single, definitive tool that can be used to determine asthma severity. Furthermore, the correlation between asthma symptoms and predicted FEV1% is weak,49–51 although several spirometry measures, particularly predicted FEV1% and maximal mid-expiratory flow at 25%–75%, have been shown to predict future asthma exacerbations.52,53 Our findings were similar to those of Bacharier et al (29), who reported comparable percentages of asthma categories in asthmatic children at subspecialty care clinics: 27.9% mild persistent, 22.4% moderate persistent, and 42.9% severe persistent. In this study, 59.2% of children missed at least one school day due to asthma in the past 12 months. Additionally, 38.8% of the enrolled patients were ever hospitalized due to severe asthma exacerbation. These findings are in concordance with the prior literature that proved that asthma is associated with school absence,54,55 repeated presentation to the emergency department,56 and even poor academic performance.57 A large survey study of children with current asthma in 35 US states and the District of Columbia found that 51% of children missed at least one school day due to asthma in the past 12 months.54 Taken together, data on moderate-severe asthma among children in Saudi Arabia are scarce. The results of this study highlight the need for further research to study the prevalence of moderate and severe childhood asthma in Saudi Arabia, as well as the contributing factors to asthma severity. The present findings could also have important consequences for developing school and public health asthma programs to reduce school absenteeism and the economic impact of childhood asthma.
This study revealed that 49.5% of the patients had asthma that was either partially controlled or uncontrolled, and 16.5% had uncontrolled asthma. The results of several studies have been inconsistent.17,58 In line with the findings of this study, a previous study found that 15% of patients had uncontrolled asthma.58 By contrast, another previous study found that 47% of patients had uncontrolled asthma.17 In this study, we found that the following factors were associated with uncontrolled asthma: a family history of asthma, admission to the PICU ever, asthma-related hospitalization ever, ER visits in the past year, severe asthma, eosinophilic phenotype, overlapping allergic and eosinophilic phenotype, and allergic phenotype. These variables were not statistically significant after combining them in one model, although the trend of associations for all these factors remained consistent, except for eosinophilic asthma. Previous studies have shown that a family history of asthma is an independent risk factor for uncontrolled asthma in children.17,55 The small sample size of this study may have contributed to the lack of statistical significance of these factors in predicting uncontrolled asthma, especially when combined.
This study has several limitations. First, it had a small sample size, which may have limited the ability of our analysis to predict the patients who would have uncontrolled asthma. Second, the study was conducted at a subspecialty pediatric pulmonology clinic, which may have introduced selection bias and limited the generalizability of the findings to all childhood asthmatics. Third, the study was cross-sectional, which means that it only measured asthma control at a single point in time. Finally, it only used the GINA asthma control tool, which limits the ability to clearly define the severity and level of asthma control, as it is recommended to use multiple assessment tools and longitudinal assessment. Despite its limitations, this study certainly adds to our understanding of the asthma phenotypes among Saudi children and the potential predictors of uncontrolled asthma.
Conclusion
This study aimed to characterize different asthma phenotypes among children attending an asthma clinic at a tertiary care center in Saudi Arabia and demonstrates that allergic asthma is the most prevalent asthma phenotype, followed by the eosinophilic phenotype. The research has also shown that an overlap between these two phenotypes is common. The second major finding was that several factors could predict uncontrolled asthma, including a family history of asthma, previous admission to the PICU, and previous hospitalization ever. There is, therefore, a definite need for multicenter cohort studies to better understand the phenotypes and endotypes of childhood asthma, as it could provide therapeutic and prognostic insights.
Ethics Approval and Informed Consent
The study was approved by the Research Ethical Committee (REC), General Directorate of Health Affairs-Aseer, Abha, Saudi Arabia (approval number REC#5-10-2019). The study was conducted according to the Declaration of Helsinki. Informed consent was obtained from all parent or legal guardian of the patients involved in the study.
Acknowledgments
The authors extend their appreciation to the Ministry of Education in KSA for funding this research work through the project number (KKU-IFP2-H-13). In addition, the author is thankful for the study participants for their time and effort in participating in this research.
Disclosure
The author declares no competing interests in this work.
References
1. Bousquet J, Mantzouranis E, Cruz AA, et al. Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on Severe Asthma. J Allergy Clin Immunol. 2010;126(5):926–938. doi:10.1016/j.jaci.2010.07.019
2. Alahmadi TS, Banjari MA, Alharbi AS. The prevalence of childhood asthma in Saudi Arabia. Int J Pediatr Adolesc Med. 2019;6(2):74–77. doi:10.1016/j.ijpam.2019.02.004
3. Al-Moamary MS, Alhaider SA, Alangari AA, et al. The Saudi Initiative for Asthma - 2021 Update: guidelines for the diagnosis and management of asthma in adults and children. Ann Thorac Med. 2021;16(1):4–56. doi:10.4103/atm.ATM_697_20
4. Bisgaard H, Bønnelykke K. Long-term studies of the natural history of asthma in childhood. J Allergy Clin Immunol. 2010;126(2):187–197. doi:10.1016/j.jaci.2010.07.011
5. Ernando F, Artinez DM, Nne A, et al. Asthma and Wheezing in the First Six Years of Life. N Eng J Med. 1995;332;133.
6. Hough KP, Curtiss ML, Blain TJ, et al. Airway remodeling in asthma. Front Med Lausanne. 2020;7:191. doi:10.3389/fmed.2020.00191
7. Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180(5):388–395. doi:10.1164/rccm.200903-0392OC
8. Saglani S, Lloyd CM. Novel concepts in airway inflammation and remodelling in asthma. Eur Respir J. 2015;46(6):1796–1804. doi:10.1183/13993003.01196-2014
9. Just J, Bourgoin-Heck M, Amat F. Clinical phenotypes in asthma during childhood. Clin Exp Allergy. 2017;47(7):848–855. doi:10.1111/cea.12939
10. Akar-Ghibril N, Casale T, Custovic A, Phipatanakul W. Allergic endotypes and phenotypes of asthma. J Allergy Clin Immunol Pract. 2020;8(2):429–440. doi:10.1016/j.jaip.2019.11.008
11. Tran TN, Zeiger RS, Peters SP, et al. Overlap of atopic, eosinophilic, and TH2-high asthma phenotypes in a general population with current asthma. Ann Allergy Asthma Immunol. 2016;116(1):37–42. doi:10.1016/j.anai.2015.10.027
12. Lambrecht BN, Hammad H, Fahy JV. The cytokines of asthma. Immunity. 2019;50(4):975–991. doi:10.1016/j.immuni.2019.03.018
13. Fahy JV. Type 2 inflammation in asthma-present in most, absent in many. Nat Rev Immunol. 2015;15(1):57–65. doi:10.1038/nri3786
14. Al-Jahdali H, Wali S, Albanna AS, et al. Prevalence of eosinophilic, atopic, and overlap phenotypes among patients with severe asthma in Saudi Arabia: a cross-sectional study. BMC Pulm Med. 2022;22(1):67. doi:10.1186/s12890-022-01856-9
15. Bacharier LB, Maspero JF, Katelaris CH, et al. Dupilumab in children with uncontrolled moderate-to-severe asthma. N Engl J Med. 2021;385(24):2230–2240. doi:10.1056/NEJMoa2106567
16. Mazi A, Madani F, Alsulami E, et al. Uncontrolled asthma among children and its association with parents’ asthma knowledge and other socioeconomic and environmental factors. Cureus. 2023;15(2):e35240. doi:10.7759/cureus.35240
17. Kansen HM, Le TM, Uiterwaal CSPM, et al. Prevalence and Predictors of Uncontrolled Asthma in Children Referred for Asthma and Other Atopic Diseases. J Asthma Allergy Meijer. 2020;13:67–75. doi:10.2147/JAA.S231907
18. Al-Zalabani AH, Almotairy MM. Asthma control and its association with knowledge of caregivers among children with asthma: a cross-sectional study. Saudi Med J. 2020;41(7):733–739. doi:10.15537/smj.2020.7.25167
19. G. I. N. A. Global Initiative for Asthma. 2023 GINA Report-Global Strategy for Asthma Management and Prevention. Available: https://ginasthma.org/wp-content/uploads/2023/07/GINA-2023-Full-report-23_07_06-WMS.pdf.
20. Amarasekera M. Immunoglobulin E in health and disease. Asia Pac Allergy. 2011;1(1):12–15. doi:10.5415/apallergy.2011.1.1.12
21. Stanojevic S, Kaminsky DA, Miller MR, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022;60(1):56.
22. Graham BL, Steenbruggen I, Barjaktarevic IZ, et al. Standardization of spirometry 2019 update an official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med. 2019;200(8):E70–E88. doi:10.1164/rccm.201908-1590ST
23. Schuler CF, Montejo JM. Allergic rhinitis in children and adolescents. Pediatr Clin North Am. 2019;66(5):981–993. doi:10.1016/j.pcl.2019.06.004
24. Bousquet J, Van Cauwenberge P, Khaltaev N; Aria Workshop Group, World Health Organization. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(5):S147–S334. doi:10.1067/mai.2001.118891
25. Froidure A, Mouthuy J, Durham SR, Chanez P, Sibille Y, Pilette C. Asthma phenotypes and IgE responses. Eur Respir J. 2016;47(1):304–319. doi:10.1183/13993003.01824-2014
26. Busse WW, Morgan WJ, Gergen PJ, et al. Randomized Trial of Omalizumab (Anti-IgE) for Asthma in Inner-City Children Abstract. The New England Journal of Medicine. 2011;364(11):1005–1015. doi:10.1056/NEJMoa1009705
27. Perikleous EP, Steiropoulos P, Nena E, Paraskakis E. Biologic therapies in pediatric asthma. J Pers Med. 2022;12(6):999. doi:10.3390/jpm12060999
28. Castro-Rodriguez JA, Ramirez AM, Toche P, et al. Clinical, functional, and epidemiological differences between atopic and nonatopic asthmatic children from a tertiary care hospital in a developing country. Ann Allergy Asthma Immunol. 2007;98(3):239–244. doi:10.1016/S1081-1206(10)60712-0
29. Lee YJ, Kim KW, Choi BS, Sohn MH, Kim KE. Clinical characteristics of eosinophilic and noneosinophilic asthma in children. Acta Paediatr. 2013;102(1):53–57. doi:10.1111/apa.12046
30. Pakkasela J, Ilmarinen P, Honkamäki J, et al. Age-specific incidence of allergic and non-allergic asthma. BMC Pulm Med. 2020;20(1):9. doi:10.1186/s12890-019-1040-2
31. Schatz M, Rosenwasser L. The allergic asthma phenotype. J Allergy Clin Immunol Pract. 2014;2(6):645–8; quiz 649. doi:10.1016/j.jaip.2014.09.004
32. Holstege J, Jahn A, Schulz C, et al. Patients with allergic and eosinophilic asthma in the German severe asthma registry. Eur Respir J. 2015;46.
33. Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18(5):716–725. doi:10.1038/nm.2678
34. Pembrey L, Barreto ML, Douwes J, et al. Understanding asthma phenotypes: the world asthma phenotypes (WASP) international collaboration. ERJ Open Res. 2018;4(3):00013–2018. doi:10.1183/23120541.00013-2018
35. Bush A. Pathophysiological mechanisms of asthma. Front Pediatr. 2019;7:68. doi:10.3389/fped.2019.00068
36. Howard R, Rattray M, Prosperi M, Custovic A. Distinguishing asthma phenotypes using machine learning approaches. Curr Allergy Asthma Rep. 2015;15(7):38. doi:10.1007/s11882-015-0542-0
37. Cowan K, Guilbert TW. Pediatric asthma phenotypes. Curr Opin Pediatr. 2012;24(3):344–351. doi:10.1097/MOP.0b013e32835357ab
38. Lødrup Carlsen KC, Pijnenburg M. Identification of asthma phenotypes in children. Breathe. 2011;8:38–44. doi:10.1183/20734735.004611
39. Licari A, Castagnoli R, Brambilla I, et al. Asthma endotyping and biomarkers in childhood asthma. Pediatr Allergy Immunol Pulmonol. 2018;31(2):44–55. doi:10.1089/ped.2018.0886
40. Oksel C, Haider S, Fontanella S, Frainay C, Custovic A. Classification of pediatric asthma: from phenotype discovery to clinical practice. Front Pediatr. 2018;6:258. doi:10.3389/fped.2018.00258
41. Porcaro F. Difficult and severe asthma in children. Children. 2020;7. doi:10.3390/children8010007
42. Carr TF, Zeki AA, Kraft M. Eosinophilic and noneosinophilic asthma. Am J Respir Crit Care Med. 2018;197(1):22–37. doi:10.1164/rccm.201611-2232PP
43. Conrad LA, Cabana MD, Rastogi D. Defining pediatric asthma: phenotypes to endotypes and beyond. Pediatr Res. 2021;90(1):45–51. doi:10.1038/s41390-020-01231-6
44. Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113(1):101–108. doi:10.1016/j.jaci.2003.10.041
45. Just J, Saint Pierre P, Amat F, et al. What lessons can be learned about asthma phenotypes in children from cohort studies? Pediatric Allergy and Immunology. 2015;26:300–305. doi:10.1111/pai.12359
46. Di Cicco M, Peroni DG, Ragazzo V, Comberiati P. Application of exhaled nitric oxide (FeNO) in pediatric asthma. Curr Opin Allergy Clin Immunol. 2021;21(2):151–158. doi:10.1097/ACI.0000000000000726
47. Popović-Grle S, Štajduhar A, Lampalo M, Rnjak D. Biomarkers in different asthma phenotypes. Genes. 2021;12(6):801. doi:10.3390/genes12060801
48. Al-Moamary MS, Alhaider SA, Alangari AA. The Saudi Initiative for Asthma-2021 Update Guidelines for the Diagnosis and Management of Asthma in Adults and Children. Ann Thoracic Med. 2021;16(1):4.
49. Murray C, Foden P, Lowe L, Durrington H, Custovic A, Simpson A. Diagnosis of asthma in symptomatic children based on measures of lung function: an analysis of data from a population-based birth cohort study. Lancet Child Adolesc Health. 2017;1(2):114–123. doi:10.1016/S2352-4642(17)30008-1
50. Schifano ED, Hollenbach JP, Cloutier MM. Mismatch between asthma symptoms and spirometry: implications for managing asthma in children. J Pediatr. 2014;165(5):997–1002. doi:10.1016/j.jpeds.2014.07.026
51. Bacharier LB, Strunk RC, Mauger D, White D, Lemanske RF, Sorkness CA. Classifying asthma severity in children: mismatch between symptoms, medication use, and lung function. Am J Respir Crit Care Med. 2004;170(4):426–432. doi:10.1164/rccm.200308-1178OC
52. Lazova S, Priftis S, Petrova G, NasevaE, VelikovaT. MMEF25-75 may predict significant BDR and future risk of exacerbations in asthmatic children with normal baseline FEV1. Int J Physiol Pathophysiol Pharmacol. 2022;14(1):33–47.
53. Fuhlbrigge AL, Kitch BT, Paltiel AD, et al. FEV1 is associated with risk of asthma attacks in a pediatric population. J Allergy Clin Immunol. 2001;107(1):61–67. doi:10.1067/mai.2001.111590
54. Hsu J, Qin X, Beavers SF, Mirabelli MC. Asthma-related school absenteeism, morbidity, and modifiable factors. Am J Prev Med. 2016;51(1):23–32. doi:10.1016/j.amepre.2015.12.012
55. Zhao J, He Q, Zhang G, et al. Status of asthma control in children and the effect of parents’ knowledge, attitude, and practice (KAP) in China: a multicenter study. Ann Allergy Asthma Immunol. 2012;109(3):190–194. doi:10.1016/j.anai.2012.07.005
56. Soril LJJ, Leggett LE, Lorenzetti DL, Noseworthy TW, Clement FM. Reducing frequent visits to the emergency department:A systematic review of interventions. PLoS One. 2015;10(4):e0123660. doi:10.1371/journal.pone.0123660
57. Fleming M, Fitton CA, Steiner MFC, et al. Educational and health outcomes of children treated for asthma: Scotland-wide record linkage study of 683716 children. Eur Respir J. 2019;54(3):1802309. doi:10.1183/13993003.02309-2018
58. Sasaki M, Yoshida K, Adachi Y, et al. Factors associated with asthma control in children: findings from a national web-based survey. J Allergy Clin Immunol. 2015;135:AB175.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


