Back to Journals » International Journal of Women's Health » Volume 16
Characteristics and Outcomes of Coronavirus Disease- 2019 Among Pregnant Women in Saudi Arabia; a Retrospective Study
Authors Abdelmola A, Albasheer O , Kariri AA, Akkam FM, Hakami RA, Essa SA, Jali FM
Received 21 October 2023
Accepted for publication 25 February 2024
Published 14 March 2024 Volume 2024:16 Pages 475—490
DOI https://doi.org/10.2147/IJWH.S445950
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Amani Abdelmola,1 Osama Albasheer,1 Atyaf A Kariri,2 Fatimah M Akkam,2 Rafeef A Hakami,2 Shahd A Essa,2 Fawziah M Jali2
1Department of Family and Community Medicine, Jazan University, Jazan, Saudi Arabia; 2Faculty of Medicine, Jazan University, Jazan, Saudi Arabia
Correspondence: Osama Albasheer, Department of Family and Community Medicine, Faculty of Medicine, Jazan University, Jazan, 45142, Saudi Arabia, Tel +966536827923, Email [email protected]
Background: Pregnancy-related coronavirus disease 2019 infection ranges from asymptomatic to very serious illness. This study aimed to determine the impact of the COVID-19 infection on pregnant women in the Jazan region of Saudi Arabia.
Methods: Retrospective observational study of women who had COVID-19 positive test in pregnancy admitted in King Fahd Hospital, Abu Arish General Hospital, and Sabya General Hospital, Jazan, Saudi Arabia during the period between March 2020 and March 2022. Data were extracted from the patient’s records. Frequency and percentage distributions were calculated for categorical variables. Descriptive studies and regression analysis were conducted to evaluate the association between selected variables and pregnancy outcomes.
Results: Of the 33 pregnant women with confirmed infection, the majority were in their second and third trimester, with approximately 42.4% requiring intensive care unit (ICU) admission and oxygen therapy. The most prevalent symptoms were high respiratory rate and low blood pressure, often accompanied by fever, cough, and shortness of breath. Live births resulted in 54.5% of the cases, while two maternal deaths were reported. Significant associations were found between the need for non-invasive ventilation and timing of infection (p = 0.026), the mode of delivery and timing of infection (p = 0.036), and the mode of delivery and body mass index (BMI) (p = 0.007).
Conclusion: COVID-19 poses significant risks to pregnant women, particularly in the third trimester, and emphasized the importance of early identification of high-risk pregnancies, strategic planning, and enhanced monitoring during antenatal care.
Keywords: COVID-19, pregnant women, maternal outcomes, SARS-CoV-2, Jazan
A Letter to the Editor has been published for this article.
Introduction
In December 2019, the world witnessed the emergence of a new coronavirus in Wuhan, China, leading to an ongoing global pandemic that has profoundly altered our way of life.1 The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19), a highly infectious disease that targets the respiratory system.2,3 Initial cases of COVID-19 presented with pneumonia coupled with clinical symptoms such as a dry cough, fever, and sneezing, which facilitated the virus’s spread through direct contact within proximity.4,5 Symptoms typically manifest within 5–14 days post-infection.6,7 As the disease progresses, it may lead to severe respiratory symptoms, hypoxia, multi-organ failure, and, in some cases, death due to (ARDS).8,9
People with underlying chronic conditions such as hypertension, chronic heart disease, and immunosuppressed patients are considered high-risk, with the infection potentially leading to fatal outcomes.10 Recent research suggests that individuals over 60 years are more susceptible to the infection, experiencing more severe symptoms than their younger counterparts, who typically exhibit mild to moderate symptoms or may even be asymptomatic.11 Since the pandemic’s onset, there has been a surge in confirmed cases, contributing to mortality and morbidity. In Saudi Arabia alone, the number of cases has exceeded 841,460.12 Pregnancy is a unique experience for women, yet requires diligent care and monitoring.13
The COVID-19 pandemic poses significant risks to pregnant women, underscoring the necessity for comprehensive research into the effects of infectious diseases like COVID-19 on pregnancy, transmission rates, and disease progression.14 Current studies indicate that pregnant women are at a higher risk of severe COVID-19 compared to non-pregnant women of the same age group due to physiological changes in their immune systems and the potential for severe complications.14,15
As COVID-19 is characterized by a decrease in immune cells (lymphocytes), pregnant women with weakened immune systems are more susceptible to infection.14,16 Factors such as maternal age, health problems, complications during pregnancy, multiple pregnancies, genetic diseases, and pregnancy history contribute to an increased risk of infection.17 Pregnant women with underlying conditions, such as diabetes, obesity, or hypertension, and older mothers-to-be are at a higher risk of adverse maternal outcomes.18
Research conducted over the past two decades on previous coronaviruses, including severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) in 2003 and Middle Eastern respiratory syndrome-related coronavirus (MERS-CoV) in 2012, has discovered that pregnant women are more vulnerable to adverse outcomes such as endotracheal intubation, intensive care unit (ICU) hospitalization, and mortality.12,19 These viruses trigger cytokine storms within the body, initiating a series of immune responses and changes in peripheral leukocytes and immune system cells, which can lead to severe pregnancy complications.20
Infection during pregnancy also carries a slight risk of preterm birth, which may increase if the mother is infected at birth.21,22 While some studies suggest that COVID-19 may pose a heightened risk to pregnant women,14,15 others argue that the severity of the disease may not significantly differ in non-pregnant populations.23,24
This divergence underscores the necessity for further research, particularly in regions where data is lacking. In light of the limited research on COVID-19’s impact on pregnant women in Saudi Arabia, this study aims to evaluate the severity of COVID-19 among pregnant women admitted to hospitals in the Jazan region of Saudi Arabia and assess maternal outcomes and risk factors associated with the disease.
Our findings shed light on the impact of COVID-19 on pregnant women in Saudi Arabia, which can inform future clinical practice and public health interventions in the region.
Materials and Methods
Study Design
This research employed a multicenter retrospective study design, focusing on the Jazan region of Saudi Arabia. The study aimed to evaluate the impact of COVID-19 on the health of pregnant women, drawing data from three medical facilities: King Fahd Hospital, Abu Arish General Hospital, and Sabya General Hospital.
Participants
The study’s participants included all pregnant women (n=33), how had SARS-CoV-2 infection diagnosed at any point from the date of conception (2+0 weeks of gestation) to the date the pregnancy ended presented to Jazan hospitals during the period between March 2020 and March 2022. We examined maternal and pregnancy outcomes occurring after SARS-CoV-2 infection in pregnancy. The maternal outcome was admission to the intensive care (ICU) within 21 days of infection or death within 28 days of date of infection. Pregnancy outcomes were preterm delivery and fetal loss within 28 days of infection or vaginal or cesarean delivery after SARS-CoV-2 infection. Babies’ outcome is alive or death delivered to women after SARS-CoV-2 infection.
Data Collection
Participant details were systematically extracted from hospital medical records, ensuring strict adherence to confidentiality, privacy, and necessary approvals. The Data were organized within an Excel spreadsheet. This data collection process encompassed various factors, including demographic and anthropometric characteristics, medical and obstetric comorbidities, and epidemiological contacts with suspected or confirmed COVID-19 cases. The diagnosis of COVID-19 was confirmed via a reverse transcription polymerase chain reaction (RT-PCR) assay of a nasopharyngeal swab specimen. A comprehensive clinical evaluation of vital signs, symptoms, laboratory analysis, and chest assessment were documented upon admission.
Statistical Analysis
The Statistical Package for the Social Sciences (SPSS) was utilized for data analysis. Frequencies and percentages represented categorical variables. Descriptive studies were conducted for all demographic factors, admission vital signs, comorbidities supportive care measures, obstetric status, clinical characteristics, diagnoses, pregnancy complications, and outcomes.
A regression analysis evaluated the association between demographic factors and supportive care, clinical features, and delivery outcomes. Statistical significance was set at a P-value of less than 0.05.
Results
Socio-Demographic and Clinical Characteristics of Respondents
Table 1 represents the socio-demographic and clinical characteristics of the respondents involved in this study; A total of 33 pregnant women who had confirmed SARS-CoV-2 infection were included. Most participants (90%) were 20 to 39 years old. 39.4% were overweight or obese, according to the body mass index (BMI) ratio. Regarding gestational weeks, most women, 84.8% (n = 28), were in their 27th to 40th week of pregnancy.
 |
Table 1 Characteristics of Women with SARS-CoV-2 Infection During Pregnancy in the Period Between March 2020 and March 2022 Included in the Study (n=33) |
Approximately 12.1% (n = 4) were in their 14th to 26th week, while 3.0% (n = 1) were in their 1st to 13th week.
In terms of mode of delivery, 42.4% (n = 14) had a vaginal delivery, while 18.2% (n = 6) had a cesarean delivery. The number of fetuses for 60.6% (n = 20) was single, while it was unknown for 39.4% (n = 13) of the women.
Almost all (96.9%) pregnant women were infected with COVID-19 during the second or third trimester. For temperature, 72.7% (n = 24) had a normal temperature, 12.1% (n = 4) had a low temperature, and 6.1% (n = 2) had a high temperature. Regarding heart rate, 54.5% (n = 18) had a normal heart rate, but 33.3% (n = 11) had a high heart rate.
The respiratory rate was high at 72.7% (n = 24) but normal at only 3.0% (n = 1). Regarding blood pressure (BP), 42.4% had low BP, 36.4% were within the normal range of BP, and 15.2% had high BP.
Comorbidities of 33 Pregnant Women
The most frequent comorbidities were severe dehydration (21.2%) and malnutrition (12.1%), followed by hypertension and diabetes (9.1% and 6.1%, respectively), as shown in Figure 1.
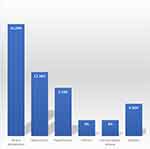 |
Figure 1 Comorbidities of pregnant women admitted between March 2020 and March 2022, Jazan, Saudi Arabia. |
Diagnostic Tests and Supportive Care That are Provided to the Pregnant Women
Table 2 represents the diagnostic tests used for diagnosis and the supported care provided to the respondents during hospital stay; As confirmed by diagnostic laboratory investigations, all pregnant women had positive corona virus tests. Of almost half who had a chest x ray performed, 24.2% presented infiltrates, while the remaining 18.2% did not.
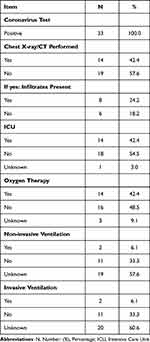 |
Table 2 The Diagnostic Tests and Supportive Care That are Provided to the Pregnant Women, Included in the Study (n=33) |
Regarding the supportive care provided to women regarding their medical status, almost half (42.4%) of the population was admitted to the ICU, and oxygen therapy was provided.
A third (33.3%) did not need non-invasive or invasive ventilation, with both non-invasive and invasive ventilation required by 6.1%, as shown below.
Pregnancy Status, Obstetric Signs, and Other Symptoms
According to their pregnancy status, as shown below (Figure 2), 39.4% of the population had abdominal pain, while 15.2% had uterine contractions and headaches.
 |
Figure 2 Pregnancy status, obstetric signs, and other symptoms upon admission of pregnant woman, Jazan, Saudi Arabia. |
Only 12.1% had watery vaginal discharge, and 9.1% showed signs of decreased or no fetal movement. A small number (3%) of the cases reported vaginal bleeding. Almost a third (27.3%) were in labor.
Clinical Features of Pregnant Women
As shown in Figure 3, the clinical characteristics of the pregnant women were primarily cough, shortness of breath, and fever (75.8%, 75.8%, and 39.40%, respectively).
 |
Figure 3 The Clinical Features of COVID-19 infected pregnant women included in the study, Jazan, Saudi Arabia. |
Less common were vomiting, diarrhea, and myalgia symptoms at 15.20%, 12.10%, and 21.20%, respectively.
Almost a fifth (18.20%) experienced chest pain, 6.10% experienced sputum production, and 9.10% had a sore throat. Loss of smell, loss of taste, and confusion were only present in 3% of each case.
Complications During Admission Among Pregnant Women
Pneumonia (15.2%) was the most reported complication, followed by ARDS (12.1%) and gestational diabetes, bleeding, anemia, and cardiac arrhythmia (each 6.1%), with car diac arrest, acute renal injury, and liver dysfunction only present in 3% of cases each (Figure 4).
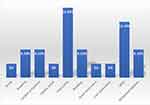 |
Figure 4 Pregnancy related complications of COVID-19 infected pregnant women included in the study, Jazan, Saudi Arabia. |
Delivery Status and Outcomes of COVID-19 Infected Pregnant Women
Table 3 reported the mode of delivery and the maternal and neonatal outcomes of the COVID-19 infected pregnant women; 16 (48.5%) women delivered during hospital admission, and 12 (36.4%) did not deliver during admission. Regarding the mode of delivery, 14 (42.4%) had a vaginal delivery, and 7 (21.2%) had a caesarean delivery. For the onset of labor, 6 (18.2%) entered spontaneous labor, 3 (9.1%) were induced, 7 (21.2%) had a caesarean section before labor onset, and 5 (15.2%) had an unknown labor onset. In terms of results of admissions, 31 (93.9%) were discharged alive, and 2 (6.1%) resulted in death.
 |
Table 3 Mode of Delivery, Maternal and Neonatal Outcomes of Pregnant Women with SARS-CoV-2 Infection During Pregnancy in the Period Between March 2020 and March 2022 Included in the Study |
For pregnancy outcomes, 12 (36.4%) did not deliver during admission, 2 (6.1%) had a spontaneous abortion, and 18 (54.5%) had a live birth. Two (6.1%) maternal deaths occurred. The underlying causes of these deaths were 1 (3.0%) indirect maternal death and 1 (3.0%) death from a coincidental cause.
Eighteen (54.5%) newborns were discharged healthy, two (6.1%) died, and one (3.0%) had an unknown outcome.
Regression Analysis
Regression Analysis of Some Demographic Factors with Supportive Care
A linear regression analysis assessed the relationship between maternal age, infection time, and BMI as independent variables and supportive care factors as dependent variables, were shown in Table 4. The admission to the ICU was not significantly associated with any of the demographic factors examined, including maternal age (t = −0.950, p = 0.350), infection time (t = 0.703, p = 0.488), and BMI (t = −1.548, p = 0.132).
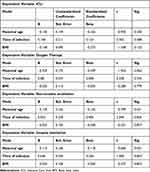 |
Table 4 The Regression Analysis of Demographic Factors and Supportive Care of Women with SARS-CoV-2 Infection During Pregnancy in the Period Between March 2020 and March 2022 Included in the Study |
Likewise, oxygen therapy was not significantly associated with demographic factors. Maternal age (t = −1.942, p = 0.062), time of infection (t = 0.328, p = 0.745), and BMI (t = −0.283, p = 0.779) all showed non-significant relationships with oxygen therapy. Noninvasive ventilation was significantly associated with infection time (t = 2.346, p = 0.026). An increase in infection time was associated with an increase in non-invasive ventilation. However maternal age (t = −0.799, p = 0.431) and BMI (t = −0.221, p = 0.827) did not correlate significantly with non-invasive ventilation. Invasive ventilation was not significantly associated with any of the demographic factors examined. Maternal age (t = −0.682, p = 0.501), infection time (t = 1.905, p = 0.067), and BMI (t = −0.227, p = 0.822) all had non-significant relationships with invasive ventilation.
Regression Analysis of Some Demographic Factors with Clinical Features
A linear regression analysis analyzed factors associated with clinical features of COVID-19 in pregnant women, including cough, shortness of breath, and fever and maternal age, infection time, and BMI, as shown in Table 5. The results showed that maternal age, infection time, and BMI were not significantly associated with cough, fever, or shortness of breath. The cough was not significantly associated with any of the demographic factors examined, including maternal age (t = −0.825, p = 0.416), infection time (t = −1.590, p = 0.123), and BMI (t = 1.181, p = 0.247). Similarly, shortness of breath was not significantly associated with any demographic factors. Maternal age (t = −1.293, p = 0.206), infection time (t = −1.843, p = 0.076), and BMI (t = 0.161, p = 0.873) showed non-significant relationships with shortness of breath. Fever was also not significantly associated with any of the demographic factors examined. Maternal age (t = −1.466, p = 0.153), infection time (t = −1.033, p = 0.310), and BMI (t = 0.698, p = 0.491) all had nonsignificant relationships with fever.
Regression Analysis of Some Demographic Factors with Delivery and Outcomes
The results of the linear regression analysis between maternal age, infection time, and BMI as independent variables and mode of delivery, pregnancy outcome, maternal death, and newborn outcome factors as dependent variables, were presented in Table 6. The delivery method was significantly associated with two demographic characteristics: infection time (t = 2.278, p = 0.036) and BMI (t = 3.082, p = 0.007). Specifically, an increase in infection time was associated with an increase in the mode of delivery of 0.537 units, and an increase in BMI was associated with a 0.290-unit rise in the delivery method. However, maternal age did not show a significant association with the mode of delivery (t = 0.980, p = 0.341).
The pregnancy outcome was significantly associated with the infection time (t = 2.692, p = 0.012). An increase in infection time was associated with a 2.969-unit increase in pregnancy outcome. Neither maternal age (t = 0.342, p = 0.735) nor BMI (t = 0.761, p = 0.453) were significantly associated with pregnancy outcomes.
None of the demographic factors examined showed a significant association with maternal death. Maternal age (t = −0.713, p = 0.481), infection time (t = −0.901, p = 0.375), and BMI (t = −1.315, p = 0.199) had non-significant relationships with maternal death.
Similarly, none of the demographic factors showed a significant association with neonatal outcomes. Maternal age (t = −0.464, p = 0.649), infection time (t = −1.639, p = 0.120), and BMI (t = 0.942, p = 0.359) all had nonsignificant relationships with the neonatal outcome.
Discussion and Limitations
Discussion
In this retrospective study, we evaluated the characteristics and outcomes of COVID-19 in 33 pregnant women admitted to hospitals in Jazan, Saudi Arabia. It was observed that a majority of these women (96.9%) contracted the virus in the second or third trimester, with only one instance of hospitalization in the first trimester. BMI was a significant factor, with 39.4% of women classified as overweight or obese.
This study underlines the profound impact of COVID-19 on the health of pregnant women, aligning with previous research.25–27 COVID-19 is associated with preterm delivery, implying that these women are more likely to give birth before the 37th week of pregnancy.28
The most frequently reported symptoms were cough, shortness of breath, and fever, with prevalence rates of 75.8%, 75.8%, and 3.4%, respectively. This is consistent with other studies that found fever and cough to be the most common symptoms.24,29,30
However, our study reported less frequent occurrences of other symptoms like chest pain, diarrhea, and vomiting, which aligns with further research.30 Some patients developed pneumonia, necessitating ICU admission, oxygen therapy, and non-invasive or invasive ventilation, indicating potentially severe disease, especially in the third trimester, as reported elsewhere.31–33
However, a third of the patients did not require any respiratory support. A significant proportion (63.6%) reported minor health and neonatal complications.
Nevertheless, there were two instances of mortality and two spontaneous abortions, with 21.2% of the women undergoing cesarean sections before labor. Pregnant women with severe COVID-19 infection were more likely to have a cesarean delivery, deliver prematurely, experience mortality around the time of birth, or suffer from severe illnesses such as hypertension or postpartum hemorrhage.34
The most common comorbidities were dehydration (21.2%) and malnutrition (12.1%), followed by hypertension and diabetes. These comorbidities during pregnancy, which could enhance the inflammatory response and affect the immunological response, may also increase COVID-19-related maternal mortality.35 Diabetes, hypertension, and heart disease in pregnant women increased the likelihood of severe COVID-19 outcomes, maternal morbidities, and unfavorable delivery outcomes.36 Chest X-rays revealed infiltrates in 24.2% of the patients, similar to studies reporting worse radiographic and laboratory indicators of severity.37 Still, over a third did not deliver.
Pregnant individuals with COVID-19 may require additional precautions during delivery to minimize the risk of transmission to healthcare workers and newborns.38
We found no correlation between age, time of infection, BMI, ICU admission, oxygen therapy, invasive ventilation, or symptoms. However, a longer infection duration was associated with the necessity for non-invasive ventilation, suggesting that escalating support might be needed as the disease progresses. The mode and delivery outcomes were observed to be correlated with longer infection times and a higher BMI, potentially impacting decisions and outcomes.
The study underscores that the probability of severe COVID-19 increases with higher BMI, maternal age, and duration of infection, echoing other research.39
The underlying factors could include age, BMI, ethnicity, chronic diseases, and pregnancy disorders.40 Comparative studies have reported higher risks of maternal death, preeclampsia, preterm birth, and neonatal complications.41 The CDC also indicates that the duration of infection, severity, and comorbidities affect outcomes.42,43
This study underscores the significant impact of COVID-19 on pregnant women, particularly those in the later stages of pregnancy. It frequently results in respiratory and obstetric complications. The severity of the disease and outcomes are associated with underlying health conditions and the duration of infection. However, the majority of women and neonates survived with appropriate care. Additional research on this vulnerable group is necessary to inform management and policy.
Limitations
The limitations of this study include a small sample size, reduced statistical power, and generalizability. The retrospective design may introduce bias through missing or inaccurate data. Limited data on disease progression and outcomes restrict the understanding of long-term effects. The focus on a single region also limits generalizability. The exact cause of maternal mortality could not be definitively determined. Data supporting the neonatal outcome like; Apgar score and cord pH were missing in the records. Studies that are more extensive are needed to validate these findings.
Conclusions
The impact of COVID-19 on pregnant women can be severe, leading to critical respiratory symptoms, pneumonia, ARDS, and other complications that pose risks to both mother and child. However, favorable outcomes are achievable for most pregnant women and newborns with appropriate supportive care and management.
In this study, most pregnant women with COVID-19 were in their third trimester, primarily exhibiting symptoms such as cough, shortness of breath, and fever. Concurrently, comorbidities such as hypertension, diabetes, and malnutrition were prevalent.
Supportive care interventions, such as oxygen therapy and ICU admission, were frequently required. Some women also need invasive or non-invasive ventilation. The timing of infection during pregnancy and the mother’s BMI surfaced as significant predictors of the delivery mode, with a later infection and higher BMI associated with an increased likelihood of a cesarean section. The timing of infection also significantly impacted pregnancy outcomes, with later infections correlating with poorer outcomes. Importantly, there was no correlation between maternal age, time of infection, and BMI with the primary symptoms of COVID-19 in pregnant patients, such as cough, breathlessness, and fever.
Most newborns born to COVID-19-positive mothers in this study were discharged in good health, although a small proportion did experience neonatal death.
Comprehensive monitoring of all health aspects is critical for a healthy delivery during pregnancy, especially for pregnant women battling COVID-19. This includes robust awareness during antenatal care follow-ups campaigns and frequent counseling sessions on the pandemic and preventive precautions. This study serves as a foundation for future research to evaluate the broader impact of COVID-19 on pregnant women. This demographic represents an understudied and vulnerable group whose unique needs and experiences are vital for informing vaccine strategies and other preventive measures. Further research is required to delve deeper into the effects of COVID-19 on this population.
Data Sharing Statement
The raw data that support the results of this study is available from the corresponding author and will be provided upon reasonable request.
Ethical Considerations
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Standing Committee for Scientific Research - Jazan University ((HAPO-10-Z-001)), Saudi Arabia. (Reference No.: 2223/351, Date of decision: 13 March 2022).
Informed Consent Statement
Patient consent was waived due to data collected from hospital medical records, ensuring strict adherence to confidentiality, privacy, and necessary approvals.
Acknowledgments
We would like to express our sincere gratitude to all those who have supported and contributed to this research. Special thanks go to our colleagues and mentors for their valuable insights, constructive feedback, and unwavering encouragement throughout the development of this paper. We also extend our appreciation to the Deanship of Scientific Research, Jazan University, for supporting this research through the Research Unit Support Program (Support Number: ISP23-47).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by Deputyship for Research and Innovation, Ministry of Education in KSA (grant number ISP23-47).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Cheng ZJ, Shan J. 2019 Novel coronavirus: where we are and what we know. Infection. 2020;48(2):155–163. doi:10.1007/s15010-020-01401-y
2. Hu B, Guo H, Zhou P, Shi Z-L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141–154. doi:10.1038/s41579-020-00459-7
3. Synowiec A, Szczepański A, Barreto-Duran E, Lie LK, Pyrc K. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): a Systemic Infection. Clin Microbiol Rev. 2021;34. doi:10.1128/CMR.00133-20
4. Allam Z. The First 50 days of COVID-19: a Detailed Chronological Timeline and Extensive Review of Literature Documenting the Pandemic. In: Surveying the Covid-19 Pandemic and Its Implications. Elsevier; 2020:1–7. doi:10.1016/B978-0-12-824313-8.00001-2
5. Ren -L-L, Wang Y-M, Z-Q W, et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J. 2020;133:1015–1024. doi:10.1097/CM9.0000000000000722
6. Wu Y, Kang L, Guo Z, Liu J, Liu M, Liang W. Incubation Period of COVID-19 Caused by Unique SARS-CoV-2 Strains. JAMA Network Open. 2022;5:e2228008. doi:10.1001/jamanetworkopen.2022.28008
7. Lauer SA, Grantz KH, Bi Q, et al. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: estimation and Application. Ann Intern Med. 2020;172:577–582. doi:10.7326/M20-0504
8. Verity R, Okell LC, Dorigatti I, et al. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669–677. doi:10.1016/S1473-3099(20)30243-7
9. Lu S, Huang X, Liu R, et al. Comparison of COVID-19 Induced Respiratory Failure and Typical ARDS: similarities and Differences. Front Med. 2022:9. doi:10.3389/fmed.2022.829771
10. People with Certain Medical Conditions | CDC. Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html.
11. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi:10.1056/NEJMoa2001316
12. World Health Organization Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data. Available from: https://covid19.who.int/table.
13. Ryu D, Kim DH, Price JT, et al. Comprehensive pregnancy monitoring with a network of wireless, soft, and flexible sensors in high- and low-resource health settings. Proc Natl Acad Sci. 2021:118. doi:10.1073/pnas.2100466118
14. Wei SQ, Bilodeau-Bertrand M, Liu S, Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. Can Med Assoc J. 2021;193:E540–8. doi:10.1503/cmaj.202604
15. Zambrano LD, Ellington S, Strid P, et al. Update: characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status — United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641–1647. doi:10.15585/mmwr.mm6944e3
16. Rad HS, Röhl J, Stylianou N, et al. The Effects of COVID-19 on the Placenta During Pregnancy. Front Immunol. 2021:12. doi:10.3389/fimmu.2021.743022
17. Phoswa WN, Khaliq OP. Is pregnancy a risk factor of COVID-19? Eur J Obstet Gynecol Reprod Biol. 2020;252:605–609. doi:10.1016/j.ejogrb.2020.06.058
18. Berghella V, Hughes BL, COVID-19: pregnancy issues and antenatal care; 2021.
19. Wong SF, Chow KM, Leung TN, et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191:292–297. doi:10.1016/j.ajog.2003.11.019
20. Wenling Y, Junchao Q, Xiao Z, Ouyang S. Pregnancy and COVID-19: management and challenges. Rev Inst Med Trop Sao Paulo. 2020;62. doi:10.1590/s1678-9946202062062
21. Gurol-Urganci I, Jardine JE, Carroll F, et al. Maternal and perinatal outcomes of pregnant women with SARS-CoV-2 infection at the time of birth in England: national cohort study. Am J Obstet Gynecol. 2021;225:522.e1–522.e11. doi:10.1016/j.ajog.2021.05.016
22. Aljohani MA, Albalawi FM, Albalawi BM, et al. Consequences of SARS-CoV-2 Infection in Pregnant Women and Their Infants: a Systematic Review. Cureus. 2022. doi:10.7759/cureus.32787
23. Asghar MS, Siddiqui MA, Iqbal S, et al. COVID-19 infection among pregnant and non-pregnant women: comparison of biochemical markers and outcomes during COVID-19 pandemic, A retrospective cohort study. Ann Med Surg. 2022:76. doi:10.1016/j.amsu.2022.103527
24. Molteni E, Astley CM, Ma W, et al. Symptoms and syndromes associated with SARS-CoV-2 infection and severity in pregnant women from two community cohorts. Sci Rep. 2021;11:6928. doi:10.1038/s41598-021-86452-3
25. Adhikari EH, Moreno W, Zofkie AC, et al. Pregnancy outcomes among women with and without severe acute respiratory syndrome coronavirus 2 infection. JAMA Network Open. 2020;3:e2029256. doi:10.1001/jamanetworkopen.2020.29256
26. Bermas BL, Gianfrancesco M, Tanner HL, et al. COVID-19 in pregnant women with rheumatic disease: data from the COVID-19 global rheumatology alliance. J Rheumatol. 2022;49:110–114. doi:10.3899/jrheum.210480
27. Conde-Agudelo A, Romero R. SARS-CoV-2 infection during pregnancy and risk of preeclampsia: a systematic review and meta-analysis. Am J Obstet Gynecol. 2022;226:68–89.e3. doi:10.1016/j.ajog.2021.07.009
28. Smith LH, Dollinger CY, VanderWeele TJ, Wyszynski DF. Hernández-Díaz S: timing and severity of COVID-19 during pregnancy and risk of preterm birth in the international registry of coronavirus exposure in pregnancy. BMC Pregnancy Childbirth. 2022;22:775. doi:10.1186/s12884-022-05101-3
29. Allotey J, Stallings E, Bonet M, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020:m3320. doi:10.1136/bmj.m3320
30. Chao M, Menon C, Elgendi M. A ranking of the most common maternal COVID-19 symptoms: a systematic review. Front Med. 2022;9. doi:10.3389/fmed.2022.865134
31. Dadhwal K, Stonham R, Breen H, Poole S, Saeed K, Dushianthan A. Severe COVID‐19 pneumonia in an intensive care setting and comparisons with historic severe viral pneumonia due to other viruses. Clin Respir J. 2022;16:301–308. doi:10.1111/crj.13482
32. Laino ME, Ammirabile A, Lofino L, et al. Prognostic findings for ICU admission in patients with COVID-19 pneumonia: baseline and follow-up chest CT and the added value of artificial intelligence. Emerg Radiol. 2022;29:243–262. doi:10.1007/s10140-021-02008-y
33. Mobeireek A, AlSaleh S, Ezzat L, et al. Risk factors for intensive care admission in patients with COVID-19 pneumonia: a retrospective study. J Infect Public Health. 2023;16:1230–1235. doi:10.1016/j.jiph.2023.05.027
34. NIH-funded study suggests COVID-19 increases risk of pregnancy complications | National Institutes of Health (NIH). Available from: https://www.nih.gov/news-events/news-releases/nih-funded-study-suggests-covid-19-increases-risk-pregnancy-complications.
35. López-Rodríguez G, Galván M, Galván Valencia O. Comorbidities associated with maternal mortality from COVID-19 in Mexico. Gac Med Mex. 2023;157. doi:10.24875/GMM.M21000623
36. Smith ER, Oakley E, Grandner GW, et al. Clinical risk factors of adverse outcomes among women with COVID-19 in the pregnancy and postpartum period: a sequential, prospective meta-analysis. Am J Obstet Gynecol. 2023;228:161–177. doi:10.1016/j.ajog.2022.08.038
37. Hazari KS, Abdeldayem R, Paulose L, et al. Covid-19 infection in pregnant women in Dubai: a case-control study. BMC Pregnancy Childbirth. 2021;21:658. doi:10.1186/s12884-021-04130-8
38. Pregnancy | COVID-19 Treatment Guidelines. Available from: https://www.covid19treatmentguidelines.nih.gov/special-populations/pregnancy/.
39. Buonsenso D, Raffaelli F, Tamburrini E, et al. Clinical role of lung ultrasound for diagnosis and monitoring of COVID‐19 pneumonia in pregnant women. Ultrasound Obstet Gynecol. 2020;56:106–109. doi:10.1002/uog.22055
40. Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 Infection. JAMA Pediatr. 2021;175:817. doi:10.1001/jamapediatrics.2021.1050
41. Papageorghiou AT, Deruelle P, Gunier RB, et al. Preeclampsia and COVID-19: results from the INTERCOVID prospective longitudinal study. Am J Obstet Gynecol. 2021;225:289.e1–289.e17. doi:10.1016/j.ajog.2021.05.014
42. Investigating the Impact of COVID-19 during Pregnancy | CDC. Available from: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/special-populations/pregnancy-data-on-covid-19/what-cdc-is-doing.html.
43. Pregnant and Recently Pregnant People | CDC. Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/pregnant-people.html.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


