Back to Journals » International Journal of Women's Health » Volume 16
Challenges in Using Progestin to Prevent Singleton Preterm Births: Current Knowledge and Clinical Advice
Received 9 June 2023
Accepted for publication 23 October 2023
Published 22 January 2024 Volume 2024:16 Pages 119—130
DOI https://doi.org/10.2147/IJWH.S394305
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
David B Nelson, Yevgenia Y Fomina
Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, University of Texas Southwestern Medical Center, Dallas, TX, USA
Correspondence: David B Nelson, Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, University of Texas Southwestern Medical Center, 5323 Harry Hines Boulevard, H6.106, Dallas, TX, 75235, USA, Email [email protected]
Abstract: Preterm birth is the leading cause of infant morbidity and mortality in children younger than 5 years old and accounts for approximately 35% of newborn deaths worldwide. The use of progestogen therapy for prevention of preterm birth has been one of the most controversial topics in modern obstetrics. Progestogens can be classified as natural or synthetic. Progesterone is a natural progestogen while progestins such as 17-alpha-hydroxyprogesterone caproate (17OHP-C) are synthetic steroid hormones. Evidence supporting the use of progestogens varies by formulation and populations studied. After more than a decade, the US Food and Drug Administration has withdrawn accelerated approval of 17OHP-C for the prevention of recurrent preterm birth in pregnant individuals with a singleton gestation. With this decision, there is no current FDA-approved treatment for prevention of spontaneous preterm birth. In this review, we provide a historical context behind the rise and fall of 17OHP-C clinical application, highlight the challenges behind the data supporting progestogen use, and offer suggestions on how to make an impact on preterm birth moving forward.
Keywords: accelerated approval, anxiety, FDA, healthcare costs, Makena®, National Institute of Health, neonatal morbidity and mortality, pregnancy, preterm birth, progestin, progestogen, progesterone, spontaneous preterm birth, 17-alpha-hydroxyprogesterone caproate
Burden of Preterm Birth
Preterm birth is defined by the World Health Organization (WHO) as delivery prior to 37 weeks of gestation based on the obstetric estimate.1 In the United States (US), 66% of infant deaths in 2018 occurred in preterm infants, with the infant mortality rate at less than 28 weeks 186 times higher than at 37 to 41 weeks.2,3 Infants born preterm with low-birthweight and survive have significantly higher risks of both short- and long-term morbidity and mortality.4 The frequency of these tragedies is inversely related to gestational age. For example, the incidence of cerebral palsy for infants born prior to 28 weeks is 15% as compared to 0.13% at term.5,6 Beyond the neonatal period, survivors of PTB also have increased risks of chronic diseases, such as elevated blood pressure, heart failure, chronic kidney disease, diabetes, and obstructive lung disease.5 Large cohort studies report that patients born preterm have approximately 30% to 50% higher all-cause mortality between 18 and 45 years than those born full-term.7 While early preterm births (<34 weeks) comprise the majority of severe neonatal complications, late preterm birth impacts a larger proportion of individuals and also carries neonatal risks. In a retrospective cohort study of 261,194 singletons born at 24 weeks or greater at Parkland Hospital, all measured adverse neonatal outcomes were significantly increased in the late preterm births as compared to “term” 39-week deliveries. The rate of aggregated morbidity was 34% of births at 34 weeks, as compared to 14% at 39 weeks.8
Taken together, preterm birth translates to substantial healthcare expenditures.9 The total societal burden of preterm birth was estimated at $25.2 billion based on the 2016 US birth cohort data.10,11 Beyond financial costs, the emotional burden of preterm birth to patients, families, communities, and societies cannot be overstated.
Trends in Preterm Birth
The rate of preterm birth in the US reached a nadir at 9.6% in 2014 but has been steadily rising since then to 10.5% in 2021 (Figure 1). The 2022 March of Dimes report card highlights the worsening state of maternal and infant health in the US and the increasing preterm birth rate.12 More than 70% of preterm births occur between 34 and 36 weeks of gestation. The recent increase in the overall preterm birth rate is largely driven by these late preterm deliveries that rose by an average annual rate of 2% from 2014 to 2019.13 The rate of early preterm birth, defined as births before 34 weeks of gestation, has remained unchanged at 2.8% since 2014.1
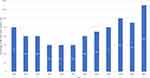 |
Figure 1 Preterm birth rate in the United States over time. Adapted from March of Dimes full report card; 2022. Available from: https://www.marchofdimes.org/peristats/data?top=3&lev=1&stop=60®=99&obj=1&slev=1.12 |
Preterm birth is classified as spontaneous or medically initiated for maternal or fetal indications. It is estimated that 20% of PTBs are medically induced, 20–30% are due to preterm premature rupture of membranes, 20–25% are due to infection or inflammation, and 25–30% are unexplained. One of the strongest predictors of spontaneous PTB is a history of prior spontaneous PTB. This is relevant as the indication for some progestin use has been prior spontaneous preterm birth. Other risk factors include extremes of age, low socioeconomic factors, limited prenatal care, tobacco use, substance use, stress, anemia, and lower genital tract infections.1 While a multitude of sociodemographic, biological and environmental factors have been associated with spontaneous preterm birth, the cause is not yet fully understood.
Several factors may explain the rising rates of PTB, including a growing prevalence of chronic medical conditions. Additionally, there are notable racial and ethnic disparities in the preterm birth rate in the United States. In 2021, 14.8% of births to non-Hispanic Black patients were preterm as compared to 9.5% in White and 10.2% in Hispanic patients.12 Inequalities in maternal and neonatal health outcomes of non-Hispanic Black pregnant individuals have persisted for decades. The causes behind these inequalities are complex but may in part be due to structural racism, distrust in the healthcare system, variation in quality of care, and inconsistent availability of community resources.14 The differing preterm birth rates across ethnicities are unacceptable and reflect the health disparities in the United States.
The reasons behind the decrease in preterm birth in the early part of the decade are multifactorial but may in part be due to differences in data ascertainment. In 2014, the National Center for Health Statistics transitioned to a new standard for estimating the gestational age of a newborn from the date of the last normal menses (LMP) to the obstetric estimate (OE) of gestation at delivery. The obstetric estimate is determined by considering all perinatal factors and assessments—including, but not limited to, ultrasound. This transition was made due to the greater validity of the OE as compared to LMP-based measures. When comparing these two methods using 2013 data, the mean OE based gestational age for all births was 38.5 weeks, slightly lower than the LMP-based average of 38.7 weeks. Births were somewhat less likely to be classified as preterm using the OE than the LMP classification (9.62% vs 11.39%), with the greatest difference in the late preterm subgroup (Figure 2). Overall, this difference translates to approximately 70,000 fewer OE-estimated preterm births. Interestingly the OE based infant mortality rate among preterm births was 19% higher than the LMP-based infant mortality rate. This difference may be attributed to an overestimation of LMP-based preterm birth rates. While the transition to the obstetric ultrasound should improve monitoring trends in gestational age, clinicians should take caution on how gestation length is defined when comparing rates of preterm birth over time—especially in the time period before OE was utilized.15
 |
Figure 2 OE- and LMP- based measures of gestational age for selected weeks: United States, 2013. Notes: OE is the obstetric estimate of the newborn; LMP is the date of the mother’s last normal menses. Reprinted from Martin JA, Osterman MJ, Kirmeyer SE, Gregory EC. Measuring Gestational Age in Vital Statistics Data: transitioning to the Obstetric Estimate. Natl Vital Stat Rep. 2015;64(5):1–20. Source: CDC.15 |
Role of Progestins in Preterm Birth
Preterm birth is not just labor that starts too soon. It is the result of a complex pathogenic process that inappropriately activates one or more components of the final common parturition pathway. There are a multitude of causes for this activation which include but are not limited to infection, stress, vascular disorders, cervical disease, and uterine overdistention (Figure 3).16
 |
Figure 3 Proposed mechanisms of disease implicated in spontaneous preterm labor. Created with BioRender.com. |
The role of progesterone in parturition physiology has long been an area of active research. Progestogens are either natural or synthetic. Progesterone is a natural progestogen while progestins are synthetic steroid hormones. In most laboratory animals, progesterone supplementation delays the onset of labor by decreasing myometrial activity and progesterone withdrawal initiates parturition.17 Similar results have not been observed in humans. While progesterones plays a key role in maintaining an early pregnancy, its withdrawal in term pregnancies does not initiate parturition.18 Circulating progesterone levels in patients during labor are similar to those measured one week before labor and remain elevated until after delivery of the placenta.19 Several mechanisms have been proposed to explain the quiescent effect of progesterones. Some studies have shown that progesterone supports late pregnancy by inhibiting expression of contraction associated protein (CAP) genes in the uterus myometrium.20 Other studies have shown that progesterone levels play a key role in the structural re-organization of collagen during cervical softening.21 Gene expression of the two distinct isoforms of the progesterone receptor (PR) – A and B, may also contribute to labor onset. PR-B regulates the oxytocin receptor pathway to suppress contractility while PR-A promotes myometrial gap junction coupling. The shift from PR-B to PR-A in late pregnancy may contribute to parturition.21 Additionally, the expression of PR-A in myometrial cells is increased by pro-inflammatory stimuli. These findings support inflammation as a driving mechanism of parturition. Selective progesterone receptor modulators (SPRMs) are one class of potential therapeutic candidates that are not currently approved for use but can block labor associated inflammatory activity by binding to and inhibiting PRs.22–24 SPRMs are just one example of how a better understanding of progesterone’s role in term and preterm physiology is essential to the development of effective preterm birth therapies.
The Rise and Fall of 17OHP-C
Historical Context
Despite an incomplete understanding of the mechanisms that drive parturition, clinical trials with various progestin formulations have dominated clinical preterm labor research for the last 50 years (Figure 4). The origins of 17OHP-C use for prevention of preterm labor can be traced to Johnson et al in 1975.25 This was a double-blind randomized control study with weekly 17OHP-C injections between 19 and 37 weeks of gestation. This study reported a mean duration of pregnancy in the 17OHP-C group of 38 weeks as compared to 35 weeks in the placebo group. There are several important caveats when analyzing the data from this study. First, it was a small study group of 48 patients. Second, there were no perinatal deaths in the 17OHP-C group as compared to 7 perinatal deaths in the placebo group (two neonatal deaths and 5 intrauterine deaths). Finally, seven patients also underwent concurrent cerclage placement (4 in the 17OHP-C group and 3 in the control group).25 Given the aforementioned confounders and small sample size, a randomized control study was done by Hauth et al in 1983.26 This study looked at the effect of 17OHP-C on pregnancy outcomes in an active-duty military population. Three groups of active-duty pregnant individuals were studied beginning at 16 and 20 weeks of gestation. Eighty pregnant patients were given weekly 17OHP-C injections, 88 received placebo, and 78 declined to participate. There were no significant differences between the three groups in the preterm birth rate or perinatal mortality.26 Due to conflicting results, Keirse et al did a meta-analysis of 7 clinical trials of progestin use in preterm birth prevention. They concluded that while progestin did not prevent miscarriage, it could reduce the occurrence of preterm birth without significantly reducing perinatal morbidity or mortality. This meta-analysis highlighted the notion that while some trials are statistically significant, this does not translate into clinical relevance or a tangible improvement in neonatal outcomes.27
 |
Figure 4 Clinical timeline of 17OHP-C trials and FDA decisions. |
Maternal-Fetal-Medicine Network Trial
Due to the widely disputed benefits of 17OHP-C, the National Institute of Child Health and Human Development Maternal-Fetal Medicine Unit Network (MFMU) set out to perform a double-blind, placebo-controlled trial in pregnant individuals with a history of preterm birth across 19 clinical centers in the United States. They enrolled 463 patients between 16 and 20 weeks gestation with a history of spontaneous preterm birth and randomized the patients in a 2:1 ratio of 17OHP-C weekly injections to placebo castor oil. In 2003, Meis et al published a statistically significant reduction in delivery at less than 37 weeks in the 17OHP-C group as compared to the control group (incidence of 36% in 17OHP-C group vs 54.9% in placebo group). They also reported significantly lower rates of necrotizing enterocolitis, intraventricular hemorrhage, and need for supplemental oxygen in the infants of patients in the 17OHP-C treatment group.28 A secondary analysis was performed to evaluate if 17OHP-C works preferentially depending on the gestational age of a previous preterm birth. Spong et al reported that patients with the earliest prior delivery <34 weeks delivered at significant more advanced gestational age if treated with 17OHP-C than with placebo. This difference was not appreciated if the prior preterm birth was >34 weeks.29 The results of the Meis et al study were challenged because of the unexpectedly high rate of preterm birth rate of 54.9% in the control group. This rate was well above the national average and was inconsistent with the authors own power calculations that predicted a PTB rate of 37% in the placebo group. It is also worth noting that the first phase of this study had a preterm delivery rate of 36% in the placebo group but was stopped prematurely due to problems with manufacturing of 17OHP-C.30 The high incidence of PTB in the control group may be explained by the significantly higher rate of recurrent preterm birth (27% in the progestin group vs 41% in the placebo group). Since a history of prior PTB is one of the strongest predictors for a recurrent PTB, this difference between the groups may have skewed the conclusions of the paper.
FDA Approval of 17OHP-C
In 2011, after the publication of the Meis study, the US Food and Drug Administration (FDA) approved intramuscular 17OHP-C for the sole indication of reduction of recurrent spontaneous PTB in pregnant people with a singleton gestation. Under a typical FDA approval process, at least two appropriately designed clinical trials must demonstrate efficacy for medication approval. Due to the public health burden of PTB and the lack of other effective interventions, the FDA granted 17OHP-C accelerated approval process under the Subpart H code of Federal Regulations.31 This regulatory pathway is used when a decision is made to grant temporary approval on the basis of a surrogate endpoint (<37 weeks of gestation), with the requirement that a second confirmatory study be conducted before full market approval is granted.
PROLONG Trial
The confirmatory study that met the FDA requirement for full market approval was called the Progestin’s Role in Optimizing Neonatal Gestation (PROLONG). This was a multinational study conducted at 93 sites across 9 countries, predominantly outside of the United States, between 2009 and 2018. The co-primary outcomes were PTB <35 weeks gestation and a composite of neonatal morbidity and mortality. The study protocol mirrored the original MFMU study. Despite these similarities, the PROLONG trial failed to demonstrate a reduction in spontaneous PTB or improvement in neonatal outcomes.32–34 The FDA explored differences that may explain the divergent results between the MFMU and PROLONG studies, identifying five demographic and baseline characteristics. These were (1) black race (2) history of more than one previous preterm birth (3) patient being single/without a partner (4) substance use in pregnancy and (5) less or equal to 12 years of formal education. A subgroup analysis of these five risk factors did not show a statistically significant difference in preterm birth rates <35 weeks or the neonatal composite index between the 17OHP-C and control groups (Figure 5).35 Importantly, this analysis refutes the argument that the low risk of preterm birth or neonatal events in the PROLONG trial explains the lack of 17OHP-C efficacy.36
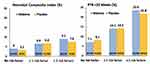 |
Figure 5 The PROLONG trial examining 5 demographic and baseline characteristics. Data from Nyugen CP.35 |
FDA Response
After the publication of the PROLONG trial, the FDA held an advisory committee hearing in October 2019 to determine if there remains substantial evidence of effectiveness for 17OHP-C in the management of preterm birth.37 The FDA reviewed the divergent results of the confirmatory study and ultimately voted 9–7 in favor of withdrawing 17OHP-C from the market.
In December 2020 KV Pharmaceuticals requested another public hearing.38 The second hearing was held in October 2022 with public oral presentations. After an in-depth discussion of all the evidence, the FDA advisory panel concluded that Makena® has no confirmed clinical benefit on neonatal outcomes and recommended its withdrawal.39 Six months later, on April 5th 2023, the FDA finally issued a formal decision to withdraw 17OHP-C from the market.40
Healthcare Costs
During the 12 years that Makena® was on the market, a highly vulnerable population invested time, money, and hope into a drug that ultimately proved ineffective. Immediately after FDA approval, KV Pharmaceuticals manufactured and distributed 250 mg weekly injections of 17OHP-C under the brand name Makena® for pregnancies between 16 and 36 weeks of completed gestation. The original retail price was $1500 per injection, totaling a cost of over $30,000 per pregnancy.41 Adaptation of Makena® for all eligible pregnancies in the US would cost over $3 billion annually.41 Comparatively, the cost of the generic 17OHP-C equivalent was approximately $20 per injection, or $360 per pregnancy.42 Makena® cost was 75–150 times more than what formerly was being charged for the same medication at compounding pharmacies. The medical community raised a call-to-action urging the FDA to allow pharmacies to compound 17OHP-C to improve access for the general public.43
In response, the FDA released a statement on March 30th, 2011 stating,
…in order to support access to this important drug the FDA does not intend to take enforcement action against pharmacies that compound 17OHP-C.44
This statement allowed providers to compound 17OHP-C at a much lower cost and made subsequent studies of 17-OHCP across US hospitals financially feasible. One such study was a prospective cohort of 430 pregnant individuals with a prior history of PTB <35 weeks at Parkland Hospital in Dallas, Texas. It concluded that 17OHP-C was ineffective in prevention of recurrent PTB and was associated with an increased rate of gestational diabetes.45 The subsequent studies brought into question the reproducibility of the MFMU study results and paved the efforts for the eventual withdrawal of Makena® from the market.
Even after Makena® was shown to be ineffective in the confirmatory trial, there was a three-year delay in its withdrawal from the market. During this time patients, insurance companies, and the Centers for Medicare and Medicaid Services (CMS) continued to pay for a medication that had no clinical benefit. Between 2018 and 2021, CMS alone spent more than $700 million on Makena® injections.46 Beyond the financial burden, exposure to 17OHP-C may have had adverse long-term effects on the health of the offspring. 17OHP-C crosses the placental barrier, the fetus is capable of metabolizing it, and several embryo-fetal toxicity signals have been identified in laboratory studies. A Child Health and Development Studies of over 18,000 mother-child dyads reported an association between in-utero exposure to 17OHP-C and development of cancer in the offspring (adjusted hazard ratio 2.57 in the first trimester and 2.59 in the second-third trimester in the male offspring). The most prevalent cancers were colorectal, prostate, and pediatric brain.47 Thus, not only is administration of 17OHP-C ineffective for preterm management but it may have negative long-term outcomes for neonates, however no adverse events were noted by the FDA.34
From a public health perspective, discrepancies in study outcomes and national organization recommendations have caused confusion in a vulnerable patient population and resulted in public frustration. From a basic science perspective, the presence of a market approved drug discouraged other manufactures from developing a next generation of therapies to prevent preterm birth.48 Taken together, the Makena® story illustrates the real-life implications of using the FDA accelerated approval process for drugs with weak evidence of effectiveness.
A Place for 17OHP-C in Specialty Cases?
While 17OHP-C does not reproducibly decrease the rate of preterm births in singleton pregnancies, the question arises about the efficacy of 17OHP-C with pregnancies complicated by other conditions such as multiple gestations and preterm labor. Randomized control trials in twins and triplets report that 17OHP-C is not effective in reducing preterm birth in twin or triplet gestations.49,50 In pregnant individuals with preterm premature rupture of membranes or arrested preterm labor, weekly 17OHP-C injections did not extend the length of gestation as compared to controls.51,52 In nulliparous patients with a mid-trimester cervical length of less than 30 mm, 17OHP-C was also not effective in reducing the frequency of preterm birth.53 In pregnant individuals with tocolysis-arrested preterm labor, 17OHP-C did not significantly prolong labor.54 In summary, 17OHP-C has not been proven to be definitively effective in the aforementioned populations of preterm birth patients.
Vaginal and Oral Progestogen Formulations
Vaginal and oral progestogens have also been studied for their effect on preterm birth. The strength of evidence for vaginal progesterone is greatest for prevention of preterm birth in singletons with asymptomatic mid-trimester short cervix (<25 mm) on transvaginal ultrasound.1 The pregnant short cervix trial reported a 45% reduction in the rate of preterm birth before 33 weeks of gestation with improved neonatal outcomes.55 To evaluate if vaginal progesterone has a prophylactic effect on preterm birth, a randomized control trial in Europe called OPPTIMUM enrolled patients with any clinical risk factors of preterm birth (history of preterm birth, second trimester loss, preterm premature rupture of membranes, or history of cervical procedure) and prescribed them vaginal progesterone during the pregnancy course. This was a negative study reporting no associated reduced risk of preterm birth or improvement in neonatal adverse outcomes with vaginal progesterone in pregnant individuals at risk for preterm birth.56 Similarly, a study in the United States at Parkland Hospital also reported that vaginal progesterone did not reduce preterm birth in a cohort of patients with a prior history of preterm birth.57 In sum, these studies suggest that vaginal progesterone is only efficacious in a cohort of patients with a short cervix in the mid-trimester.55,58,59
Oral progestin use for the prevention of preterm birth is most commonly used in the first trimester to decrease the risk of pregnancy loss. The use of oral progestin for management of preterm birth is an area of active research. The SIPRO study is currently taking place in Hong Kong to determine whether early universal use of oral progestin before 14 weeks can prevent PTB in singleton pregnancies better than universal screening of cervical lengths in the second trimester.60
Current State of Preterm Birth
Preterm birth remains one of the most complex and important challenges in obstetrics. The sequelae of preterm birth extend into adulthood and present a substantial societal burden. With 17OHP-C rendered ineffective, the research focus has shifted to other treatment options such as prophylactic antibiotic use, vaginal progesterone, cerclages, and pessaries. Unfortunately, vaginal progesterone and cerclages show a reproducible benefit only for subgroups of the at-risk population.61 Despite initial enthusiasm, other initiatives such as activity restriction, vaginal pessaries, tocolytic agents, treatment of asymptomatic bacterial vaginosis, or administration of prophylactic antibiotics have not shown a reproducible improvement in neonatal outcomes.62,63 Presently, there is no FDA approved therapeutic option for patients at risk for preterm birth. While novel therapies like synthetic progesterone receptor modulators have promising results in delaying preterm birth in the mouse model, further studies are urgently needed to evaluate their therapeutic potential and possible risks in humans.
Modifiable Risk Factors
We encourage clinicians to focus on modifiable risk factors of preterm birth. We can make a tangible impact on preterm birth rates by providing early and reliable prenatal care, encouraging tobacco cessation, expanding maternal opioid treatment programs, addressing social determinants of health, and screening for mental health disorders. Today, more than 2.2 million people of childbearing age live in maternity deserts. Each year 150,000 babies are born to patients living in community without a hospital offering obstetric care.64 Increasing access to inpatient obstetric services and qualified obstetric provides is critical to improving outcomes and decreasing the rates of preterm birth. Pregnant individuals need affordable, high- quality health insurance prior to pregnancy so that clinicians can implement preventative care. Nationally, we need to support a legislative call to action for healthcare policies that expand medical coverage and extend the length of coverage beyond 60 days postpartum.
Maternal Stress and Anxiety
Maternal stress has been identified in more than half of patients experiencing a preterm delivery.65 The neuroendocrine pathway involving corticotropin releasing hormone and cortisol as mediators of a “placental clock” is a potential mechanism for premature delivery.66–69 The American College of Obstetrics and Gynecology encourages providers to complete a full assessment of mood and emotional well-being for all patients at least once in the perinatal period.70 Providers should coordinate access to mental health counselors, domestic violence shelters, and substance use disorder clinics. Importantly, integrating mental health services into routine prenatal care by co-locating mental health counselors and obstetrics providers at the same clinics can significantly increase utilization of these resources.71 Targeting maternal mental health by incorporating regular screenings into routine peripartum care, increasing accessibility to behavioral health services, and raising community awareness may help reverse the rising preterm birth rate.
Lack of Funding to Answer Important Questions
Looking forward, in order to effectively prevent preterm birth, we must first understand the basic biologic mechanisms that cause it. Unfortunately, pregnancy research at the basic science and clinical level is underfunded in the United States. The National Institutes of Health (NIH) is one of the main funding source for health research. Unlike other diseases such as cancer and heart disease, reproductive sciences do not have an independent research institute or center whose sole mission is to make advancements in the field. Instead, obstetrics and gynecology is part of the National Institute of Child Health and Human Development (NICHD), a center dedicated to both child and maternal health. The NIH allocates a disproportionately small amount of funding to the NICHD given the burden of the diseases that this center encompasses in the pediatric and obstetric populations.72 Last year, of the $43 billion annual NIH budget, $1.59 billion was allocated to the NICHD, only $635 million of which was ultimately granted to pregnancy-related projects (Figure 6).73 Despite preterm birth alone affecting 1 in 10 pregnancies in the United States and the national rising maternal-perinatal mortality rates, the NIH budget only allocated 1.4% of its total budget to pregnancy-related research in 2022.73 This data represents an alarming under-representation of obstetrics related research on the national platform and highlights an urgent need for increased funding. Greater NIH support of research in early human development and pregnancy is essential for the advancement of our field and the health of our society.
 |
Figure 6 NIH funding allocation in 2022. Notes: The red rectangle highlights the NIH funding allocated to the National Institute of Child Health and Human Development in 2022. Adapted from NIH Reporter. Fiscal year 2022. Available from: https://reporter.nih.gov/search/fUY0rBAOHUmfky5HZooIlw/projects/charts. 73 |
Conclusion
Preterm birth remains the principal unsolved problem in obstetrics. After more than a half century of clinical trials, 17OHP-C has proven to be ineffective and has been taken off the market by the FDA. Today, care for patients with a history of preterm birth should focus on interventions that decrease risk factors. As a society, we need to prioritize public health initiatives that expand perinatal care. Collectively, we need to allocate more resources to research that focuses on understanding of the pathophysiology and mechanisms of preterm birth. Only then, can we make a tangible impact on a disease that affects one in ten American newborns.
Disclosure
The authors report no conflicts of interest in this work.
References
1. American College of O, Gynecologists’ Committee on Practice B-O. Prediction and prevention of spontaneous preterm birth: ACOG Practice Bulletin, Number 234. Obstet Gynecol. 2021;138(2):e65–e90. doi:10.1097/AOG.0000000000004479
2. Ely DM, Driscoll AK. Infant mortality in the United States, 2020: data from the period linked birth/infant death file. Natl Vital Stat Rep. 2022;71(5):1–18.
3. Chawanpaiboon S, Vogel JP, Moller AB, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7(1):e37–e46. doi:10.1016/S2214-109X(18)30451-0
4. Eichenwald EC, Stark AR. Management and outcomes of very low birth weight. N Engl J Med. 2008;358(16):1700–1711. doi:10.1056/NEJMra0707601
5. Himpens E, Van den Broeck C, Oostra A, Calders P, Vanhaesebrouck P. Prevalence, type, distribution, and severity of cerebral palsy in relation to gestational age: a meta-analytic review. Dev Med Child Neurol. 2008;50(5):334–340. doi:10.1111/j.1469-8749.2008.02047.x
6. American College of Obstetricians and Gynecologists. Executive summary: neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Obstet Gynecol. 2014;123(4):896–901. doi:10.1097/01.AOG.0000445580.65983.d2
7. Crump C. An overview of adult health outcomes after preterm birth. Early Hum Dev. 2020;150:105187. doi:10.1016/j.earlhumdev.2020.105187
8. McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111(1):35–41. doi:10.1097/01.AOG.0000297311.33046.73
9. Nelson DB, Spong CY. Seasonality associated with preterm birth-are we borrowing from our children? JAMA Netw Open. 2022;5(2):e2145808. doi:10.1001/jamanetworkopen.2021.45808
10. Frey HA, Klebanoff MA. The epidemiology, etiology, and costs of preterm birth. Semin Fetal Neonatal Med. 2016;21(2):68–73. doi:10.1016/j.siny.2015.12.011
11. Waitzman NJ, Jalali A, Grosse SD. Preterm birth lifetime costs in the United States in 2016: an update. Semin Perinatol. 2021;45(3):151390. doi:10.1016/j.semperi.2021.151390
12. March of Dimes full report card; 2022.Available from: https://www.marchofdimes.org/peristats/data?top=3&lev=1&stop=60®=99&obj=1&slev=1.
13. Hamilton BE, Martin JA, Osterman MJK. Births: provisional data for 2021. In: Vital Statistics Rapid Release. Hyattsville, MD: National Center for Health Statistics; 2022:20.
14. Culhane JF, Goldenberg RL. Racial disparities in preterm birth. Semin Perinatol. 2011;35(4):234–239. doi:10.1053/j.semperi.2011.02.020
15. Martin JA, Osterman MJ, Kirmeyer SE, Gregory EC. Measuring Gestational Age in Vital Statistics Data: transitioning to the Obstetric Estimate. Natl Vital Stat Rep. 2015;64(5):1–20.
16. Buhimschi CS, Schatz F, Krikun G, Buhimschi IA, Lockwood CJ. Novel insights into molecular mechanisms of abruption-induced preterm birth. Expert Rev Mol Med. 2010;12:e35. doi:10.1017/S1462399410001675
17. Norwitz ER, Robinson JN, Challis JR. The control of labor. N Engl J Med. 1999;341(9):660–666. doi:10.1056/NEJM199908263410906
18. Csapo AI, Pulkkinen M. Indispensability of the human corpus luteum in the maintenance of early pregnancy. Luteectomy evidence. Obstet Gynecol Surv. 1978;33(2):69–81. doi:10.1097/00006254-197802000-00001
19. Hanssens MC, Selby C, Symonds EM. Sex steroid hormone concentrations in preterm labour and the outcome of treatment with ritodrine. Br J Obstet Gynaecol. 1985;92(7):698–702. doi:10.1111/j.1471-0528.1985.tb01451.x
20. Peavey MC, Wu SP, Li R, et al. Progesterone receptor isoform B regulates the Oxtr-Plcl2-Trpc3 pathway to suppress uterine contractility. Proc Natl Acad Sci U S A. 2021;118(11):e2011643118.
21. Nadeem L, Shynlova O, Mesiano S, Lye S. Progesterone via its type-a receptor promotes myometrial gap junction coupling. Sci Rep. 2017;7(1):13357. doi:10.1038/s41598-017-13488-9
22. Merlino AA, Welsh TN, Tan H, et al. Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J Clin Endocrinol Metab. 2007;92(5):1927–1933. doi:10.1210/jc.2007-0077
23. Condon JC, Hardy DB, Kovaric K, Mendelson CR. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol. 2006;20(4):764–775. doi:10.1210/me.2005-0242
24. Shynlova O, Nadeem L, Dorogin A, Mesiano S, Lye SJ. The selective progesterone receptor modulator-promegestone-delays term parturition and prevents systemic inflammation-mediated preterm birth in mice. Am J Obstet Gynecol. 2022;226(2):249 e1–249 e21. doi:10.1016/j.ajog.2021.08.013
25. Johnson JW, Austin KL, Jones GS, Davis GH, King TM. Efficacy of 17 alpha-hydroxyprogesterone caproate in the prevention of premature labor. N Engl J Med. 1975;293(14):675–680. doi:10.1056/NEJM197510022931401
26. Hauth JC, Gilstrap LC, Brekken AL, Hauth JM. The effect of 17 alpha-hydroxyprogesterone caproate on pregnancy outcome in an active-duty military population. Am J Obstet Gynecol. 1983;146(2):187–190. doi:10.1016/0002-9378(83)91051-7
27. Keirse MJ. Progestogen administration in pregnancy may prevent preterm delivery. Br J Obstet Gynaecol. 1990;97(2):149–154. doi:10.1111/j.1471-0528.1990.tb01740.x
28. Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348(24):2379–2385. doi:10.1056/NEJMoa035140
29. Spong CY, Meis PJ, Thom EA, et al. Progesterone for prevention of recurrent preterm birth: impact of gestational age at previous delivery. Am J Obstet Gynecol. 2005;193(3 Pt 2):1127–1131. doi:10.1016/j.ajog.2005.05.077
30. Romero R, Stanczyk FZ. Progesterone is not the same as 17 alpha-hydroxyprogesterone caproate: implications for obstetrical practice. Am J Obstet Gynecol. 2013;208(6):421–426. doi:10.1016/j.ajog.2013.04.027
31. Department of Health and Human Services, Food and Drug Administration. NDA 021945. Accelerated approval. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2011/021945s000ltr.pdf. Accessed May 15, 2023.
32. Blackwell SC, Gyamfi-Bannerman C, Biggio JR, et al. PROLONG clinical study protocol: hydroxyprogesterone caproate to reduce recurrent preterm birth. Am J Perinatol. 2018;35(12):1228–1234. doi:10.1055/s-0038-1642062
33. Blackwell SC, Gyamfi-Bannerman C, Biggio JR, et al. 17-OHPC to prevent recurrent preterm birth in singleton gestations (PROLONG Study): a multicenter, international, randomized double-blind trial. Am J Perinatol. 2020;37(2):127–136. doi:10.1055/s-0039-3400227
34. The Food and Drug Administration (FDA). Final decision on the proposal to withdraw approval of Makena; 2023. Available from: https://www.regulations.gov/document/FDA-2020-N-2029-0385.
35. Nyugen CP. Makena (hydroxyprogesterone caproate injection). New drug application 021945/supplement 023; 2019. Available from: https://www.fda.gov/media/132431/download.
36. Nelson DB, McIntire DD, Leveno KJ. A chronicle of the 17-alpha hydroxyprogesterone caproate story to prevent recurrent preterm birth. Am J Obstet Gynecol. 2021;224(2):175–186. doi:10.1016/j.ajog.2020.09.045
37. Department of Health and Human Sergices, Food and Drug Administration. FDA Briefing Document NDA 021945. Hydroxyprogesterone caproate injection. BRUDAC meeting; 2019. Available from: https://www.fda.gov/media/132003/download.
38. Covis Pharma GmbH’s briefing materials in response to the center for drug research and evaluation’s notice of opportunity for a hearing and proposal to withdraw approval of Makena. Docket No FDA-2020-N-2029; 2022. Available from: https://pink.pharmaintelligence.informa.com/-/media/supporting-documents/pink-sheet/2022/10/covis_briefing_document.pdf?rev=874e6596db1344698aff43d4e9b89e22&hash=8F1553842F1864F84C17066D498A9411.
39. Department of Health and Human Sergices, Food and Drug Administration. Public hearing announcement involving the obstetrics, reproductive, and urologic drugs advisory committee; 2022. Available from: https://www.fda.gov/advisory-committees/advisory-committee-calendar/updated-public-participation-information-october-17-19-2022-hearing-announcement-involving.
40. Department of Health and Human Sergices, Food and Drug Administration. FDA commissioner and chief scientist announce decision to withdraw approval of Makena; 2023. Available from: https://www.fda.gov/news-events/press-announcements/fda-commissioner-and-chief-scientist-announce-decision-withdraw-approval-makena.
41. Armstrong J. Unintended consequences--the cost of preventing preterm births after FDA approval of a branded version of 17OHP. N Engl J Med. 2011;364(18):1689–1691. doi:10.1056/NEJMp1102796
42. Cohen AW, Copel JA, Macones GA, Menard MK, Riley L, Saade GR. Unjustified increase in cost of care resulting from U.S. Food and Drug Administration approval of Makena (17 alpha-hydroxyprogesterone caproate). Obstet Gynecol. 2011;117(6):1408–1412. doi:10.1097/AOG.0b013e31821c2d75
43. Silver RM, Cunningham FG. Deus ex Makena? Obstet Gynecol. 2011;117(6):1263–1265. doi:10.1097/AOG.0b013e31821c6fb1
44. US Food and Drug Administration. Makena (hydroxyprogesterone caproate injection) Information. Makena (hydroxyprogesterone caproate injection) Information. Available from: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/makena-hydroxyprogesterone-caproate-injection-information#:~:text=FDA%20withdrew%20approval%20of%20Makena,longer%20shown%20to%20be%20effective.
45. Nelson DB, McIntire DD, McDonald J, Gard J, Turrichi P, Leveno KJ. 17-alpha Hydroxyprogesterone caproate did not reduce the rate of recurrent preterm birth in a prospective cohort study. Am J Obstet Gynecol. 2017;216(6):600 e1–600 e9. doi:10.1016/j.ajog.2017.02.025
46. Perrone M. FDA panel backs removal of unproven pregnancy drug. ABC News. ABC News Network; 2022.
47. Murphy CC, Cirillo PM, Krigbaum NY, Cohn BA. In utero exposure to 17 alpha-hydroxyprogesterone caproate and risk of cancer in offspring. Am J Obstet Gynecol. 2022;226(1):132 e1–132 e14. doi:10.1016/j.ajog.2021.10.035
48. Aaron DG, Cohen IG, Adashi EY. The FDA struggle to withdraw Makena: problems with the accelerated approval process. JAMA. 2022;328(24):2394–2395. doi:10.1001/jama.2022.22986
49. Rouse DJ, Caritis SN, Peaceman AM, et al. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med. 2007;357(5):454–461. doi:10.1056/NEJMoa070641
50. Caritis SN, Rouse DJ, Peaceman AM, et al. Prevention of preterm birth in triplets using 17 alpha-hydroxyprogesterone caproate: a randomized controlled trial. Obstet Gynecol. 2009;113(2 Pt 1):285–292. doi:10.1097/AOG.0b013e318193c677
51. Briery CM, Veillon EW, Klauser CK, et al. Women with preterm premature rupture of the membranes do not benefit from weekly progesterone. Am J Obstet Gynecol. 2011;204(1):54 e1–5. doi:10.1016/j.ajog.2010.08.022
52. Briery CM, Klauser CK, Martin RW, Magann EF, Chauhan SP, Morrison JC. The use of 17-hydroxy progesterone in women with arrested preterm labor: a randomized clinical trial. J Matern Fetal Neonatal Med. 2014;27(18):1892–1896. doi:10.3109/14767058.2014.892922
53. Grobman WA, Thom EA, Spong CY, et al. 17 alpha-hydroxyprogesterone caproate to prevent prematurity in nulliparas with cervical length less than 30 mm. Am J Obstet Gynecol. 2012;207(5):390 e1–8. doi:10.1016/j.ajog.2012.09.013
54. Rozenberg P, Chauveaud A, Deruelle P, et al. Prevention of preterm delivery after successful tocolysis in preterm labor by 17 alpha-hydroxyprogesterone caproate: a randomized controlled trial. Am J Obstet Gynecol. 2012;206(3):206 e1–9. doi:10.1016/j.ajog.2011.12.026
55. Hassan SS, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38(1):18–31. doi:10.1002/uog.9017
56. Norman JE, Marlow N, Messow CM, et al. Vaginal progesterone prophylaxis for preterm birth (the OPPTIMUM study): a multicentre, randomised, double-blind trial. Lancet. 2016;387(10033):2106–2116. doi:10.1016/S0140-6736(16)00350-0
57. Nelson DB, Lafferty A, Venkatraman C, et al. Association of vaginal progesterone treatment with prevention of recurrent preterm birth. JAMA Netw Open. 2022;5(10):e2237600. doi:10.1001/jamanetworkopen.2022.37600
58. McKay LA, Holford TR, Bracken MB. Re-analysis of the PREGNANT trial confirms that vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix. Ultrasound Obstet Gynecol. 2014;43(5):596–597. doi:10.1002/uog.13331
59. Romero R, Conde-Agudelo A, Da Fonseca E, et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol. 2018;218(2):161–180. doi:10.1016/j.ajog.2017.11.576
60. Cheung KW, Seto MTY, Ng EHY. Early universal use of oral progesterone for prevention of preterm births in singleton pregnancy (SINPRO study): protocol of a multicenter, randomized, double-blind, placebo-controlled trial. Trials. 2020;21(1):121. doi:10.1186/s13063-020-4067-z
61. Hoffman MK. Prediction and prevention of spontaneous preterm birth: ACOG Practice Bulletin, Number 234. Obstet Gynecol. 2021;138(6):945–946. doi:10.1097/AOG.0000000000004612
62. Yoon BH, Romero R, Park JY, et al. Antibiotic administration can eradicate intra-amniotic infection or intra-amniotic inflammation in a subset of patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2019;221(2):142 e1–142 e22. doi:10.1016/j.ajog.2019.03.018
63. Kenyon SL, Taylor DJ, Tarnow-Mordi W, Group OC. Broad-spectrum antibiotics for preterm, prelabour rupture of fetal membranes: the ORACLE I randomised trial. ORACLE Collaborative Group. Lancet. 2001;357(9261):979–988. doi:10.1016/s0140-6736(00)04233-1
64. March of Dimes. Nowhere to Go: maternity care deserts across the U.S. (2022 Report). Available from: https://www.marchofdimes.org/research/maternitycare-deserts-report.aspx.
65. Manuck TA, Esplin MS, Biggio J, et al. The phenotype of spontaneous preterm birth: application of a clinical phenotyping tool. Am J Obstet Gynecol. 2015;212(4):487.e1–487.e11. doi:10.1016/j.ajog.2015.02.010
66. Wadhwa PD, Porto M, Garite TJ, Chicz-DeMet A, Sandman CA. Maternal corticotropin-releasing hormone levels in the early third trimester predict length of gestation in human pregnancy. Am J Obstet Gynecol. 1998;179(4):1079–1085. doi:10.1016/s0002-9378(98)70219-4
67. Holzman C, Jetton J, Siler-Khodr T, Fisher R, Rip T. Second trimester corticotropin-releasing hormone levels in relation to preterm delivery and ethnicity. Obstet Gynecol. 2001;97(5 Pt 1):657–663. doi:10.1016/s0029-7844(00)01209-6
68. Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol. 1999;180(1 Pt 3):S257–S263. doi:10.1016/s0002-9378(99)70712-x
69. Mancuso RA, Schetter CD, Rini CM, Roesch SC, Hobel CJ. Maternal prenatal anxiety and corticotropin-releasing hormone associated with timing of delivery. Psychosom Med. 2004;66(5):762–769. doi:10.1097/01.psy.0000138284.70670.d5
70. American College of Obstetricians and Gynecologists. Screening for perinatal depression. ACOG Committee Opinion No. 757. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132(5):e208–212. doi:10.1097/AOG.0000000000002927
71. Rodriguez AN, Holcomb D, Fleming E, et al. Improving access to perinatal mental health services: the value of on-site resources. Am J Obstet Gynecol MFM. 2021;3(6):100456. doi:10.1016/j.ajogmf.2021.100456
72. Rice LW, Cedars MI, Sadovsky Y, et al. Increasing NIH funding for academic departments of obstetrics and gynecology: a call to action. Am J Obstet Gynecol. 2020;223(1):79 e1–79 e8. doi:10.1016/j.ajog.2020.03.022
73. NIH Reporter. Fiscal year 2022. Available from: https://reporter.nih.gov/search/fUY0rBAOHUmfky5HZooIlw/projects/charts.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
