Back to Journals » Integrated Pharmacy Research and Practice » Volume 11
Challenges Associated with Addressing Counterfeit Medicines in Nigeria: An Exploration of Pharmacists’ Knowledge, Practices, and Perceptions
Authors Adigwe OP , Onavbavba G , Wilson DO
Received 24 August 2022
Accepted for publication 9 November 2022
Published 16 December 2022 Volume 2022:11 Pages 177—186
DOI https://doi.org/10.2147/IPRP.S387354
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Jonathan Ling
Obi Peter Adigwe, Godspower Onavbavba, Diana Oyin-mieyebi Wilson
Office of the Director General, National Institute for Pharmaceutical Research and Development, Abuja, Federal Capital Territory, Nigeria
Correspondence: Obi Peter Adigwe, Office of the Director General, National Institute for Pharmaceutical Research and Development, Abuja, Federal Capital Territory, Nigeria, Email [email protected]
Introduction: Counterfeit medicines are substandard pharmaceutical products that are produced and sold with the intent to deceptively represent their authenticity, origin, or effectiveness. The risk of the existence of such products in healthcare provision remains a significant threat to public health. Pharmacists represent the most critical stakeholders in the supply, manufacture, purchase, and dispensing of pharmaceutical products, and as such can play critical roles in detecting and reducing the circulation of fake medicines. This study aimed to assess the knowledge and practices of pharmacists in Nigeria in relation to counterfeit medicines as well as the challenges associated with preventing and mitigating this menace in the country.
Methods: A cross-sectional study was undertaken to administer questionnaires to pharmacists across various sectors of practice in Nigeria. Data were analysed using Statistical Package for Social Sciences.
Results: A total of 390 valid responses were received. The respondents indicated that online drug commerce (72.68%), inadequate inspection (90.93%), inadequate legislation (88.83%), poor collaboration (89.94%), and poor cross-border enforcement (90.43%) were primary challenges to the mitigation of fake medicines circulation in the country. Whilst pharmacists were knowledgeable about counterfeit drugs, gaps were observed in their practices towards detection of these products, as about one-third (30.7%) of the sample indicated that their current knowledge and skills were inadequate to detect counterfeit medicines. Age, years of practice, and area of practice significantly influenced the abilities of the participants to detect counterfeit medicines.
Conclusion: Evidence from the study revealed that pharmacists had good knowledge of medicine counterfeiting in Nigeria. However, factors such as poor collaboration among regulatory agencies, inadequate inspection and legislation on the regulation of the pharmaceutical sector and online sales of medicines have contributed to the circulation of counterfeit medicines, and this has in turn affected healthcare services in the country.
Keywords: pharmacists, counterfeit medicines, knowledge, practices, challenges, Nigeria
Introduction
According to the World Health Organisation (WHO), spurious, falsified, falsely labeled, and counterfeit (SSFFC) medicines are all classified as substandard products; however, not all substandard products can be termed spurious, falsely labeled, falsified or counterfeits.1,2 Counterfeit medicines or substandard pharmaceutical products which are presented with the intention to mislead can pose a great risk to public health, especially since their authenticity, origin, and effectiveness are questionable.3 In this context, counterfeit medical products describe medications with a false representation of identity and/or source, whilst fake medicines refer to a term that best communicates drug counterfeiting to the public.1
Clearly, counterfeit or fake medicines may include products with wrong ingredients, without active ingredients, incorrect quantities of active ingredients, or with fake packaging.4 These medicines are below specified quality standards and have emerged as a significant threat to public health across Africa.5,6 In the last three decades, many developing nations have experienced a considerable increase in the circulation of counterfeit pharmaceutical products, and this has resulted in a lack of access to high-quality medicines.4,7,8 Available evidence also suggests that the sub-Saharan African region records an annual mortality rate of about 280,000 children as a result of counterfeit medications for malaria and pneumonia.2
Despite global efforts at eradicating drug counterfeiting, fake medicines still comprise an increasing percentage of the global drug market.9 In many industrialised nations, the rate of counterfeit medicines confiscation increased by 57% within the periods of 2007 and 2008 indicating a significant rise of its penetration into the global pharmaceutical supply chain.10 According to the Food and Drug Administration (FDA), counterfeit medicines account for more than 10% of the world drug market and pose a great challenge to eradication in both developed and developing nations.11,12
The prevalence of fake medications in Nigeria has been attributed to inadequacy in drug regulation as well as lack of access to medicines in the healthcare delivery system.4,13 The situation is further heightened by a poor database on morbidity and mortality in the country, especially as it relates to substandard medicines.14 Between 2001 and 2005, drug regulatory agencies played active roles in reducing the circulation of counterfeit medicines from 40% to 17%, yet the issue continues to remain a major public health and socio-development burden, especially with essential medicines.15
The porosity at the different levels of the pharmaceutical supply chain has also paved the way for counterfeit medicine perpetrators.16 In 2018, data from the National Agency for Food and Drug Administration and Control (NAFDAC) revealed that the agency destroyed fake foods and drugs worth over 10 million US dollars in the country,17 yet the complexity in the circulation of counterfeit drugs means that this issue continues to pose significant risk to the Nation’s healthcare system.18,19
Internationally, evidence exists which links the incidents of counterfeit medicines with therapeutic failure and poor health outcomes.1 Available data suggest that pharmacists consider this as a major challenge to their profession and the credibility of the healthcare delivery system.20–23 Previous study amongst pharmacists in Sudan identified high cost of medicines to have a major role in the prevalence of drug counterfeiting.22 In a study undertaken in Lebanon, pharmacists attributed the circulation of counterfeit pharmaceutical products to increased demand for cheaper medicines.24 Despite the criticality of pharmacists in ensuring the supply, manufacture, purchase, and dispensing of medicines, the review of the literature reveals that there is a paucity of information on studies assessing the knowledge and practices of pharmacists in Nigeria regarding measures to control counterfeit medicines. An insight into pharmacists’ knowledge and practices can help develop strategies to aid prevention and control of counterfeit and fake drugs in healthcare provision. This study, therefore, aimed to address these gaps by assessing the knowledge and practices of pharmacists as it concerns counterfeit pharmaceuticals as well as the challenges associated with mitigating this menace in the country.
Methods
A cross-sectional survey of pharmacists in Nigeria was undertaken between March and May 2022. The data collection tool employed was a questionnaire that was designed in English language. The tool was developed following an extensive review of literature.4,7,13,24–26 An iterative process involving 3 faculty members with thematic research and teaching experience in this area was used to develop the questionnaire items. A draft version of all the items in the instrument was reviewed independently by each person, and they suggested changes, additions, and deletions. The revision process continued until a consensus was reached. The questionnaire items were structured to gain insights into the knowledge and practices of pharmacists regarding counterfeit medicines as well as the challenges of mitigating the circulation of fake drugs in Nigeria.
Face and content validation of the instrument was undertaken by an expert panel and was assessed for appropriateness, complexity, attractiveness, and relevance. Some of the statements were edited and reworded, whilst content validity was evaluated by quantitative method. Content validity ratio and content validity index were tested for each item, and only those that passed these tests were included in the final questionnaire. The questionnaire was pretested to ensure that its structure could assess the knowledge and practices of pharmacists on the circulation of counterfeit medicines as well as the challenges of mitigating the menace. The feedback received did not necessitate any major change.
The study was a national survey, and a sample size of 377 was computed for a population of 20,000 pharmacists in Nigeria at 95% confidence level, 5% margin of error, and 50% response distribution using the Epi Info software version 7. Participants were selected using a stratified multistage sampling method.27 Recruitment was undertaken from at least one state across the six geopolitical zones in Nigeria to enable relevant and even assessment of pharmacists across the country. Participants were therefore recruited randomly from the selected states.
Inclusion criteria for the study were licensed pharmacists who were willing to participate by providing informed consent, and currently practicing in Nigeria with at least one year of experience. Participants who did not meet these requirements were excluded from the study. Respondents were made up of pharmacists working in hospitals, industries, non-governmental organisations, administration, regulation, community, importation, and other relevant areas of pharmaceutical practice. Paper-based questionnaires were administered to the study participants physically.
Ethical approval was obtained from the Health Research and Ethics Committee of the National Institute for Pharmaceutical Research and Development before the commencement of data collection. Participation in the study was voluntary as informed consent was sought prior to the administration of questionnaires. Absolute confidentiality was maintained during the data collection process.
Following the importation of collected data into Statistical Package for Social Sciences software (SPSS) version 25, descriptive statistical analysis was carried out, and results were expressed in frequency and percentage. Inferential statistical analysis was undertaken, and chi-square test was used to determine the association between variables. A p-value of 0.05 or less was considered as the threshold for statistical significance.
Results
Demography and Response Rate
A total of 420 copies of questionnaires were administered, and 390 valid responses were received comprising 205 (52.6%) female and 185 (47.4%) male participants, a considerable proportion of respondents were below the age of 30 years (42.6%). Just over a third (37.4%) had been in pharmacy practice for less than 5 years, whilst respondents who had been in practice for over 15 years constituted the least proportion (16.7%). The majority of the study participants (71.3%) had a first degree as their highest level of qualification, whilst Ph.D. holders made up the smallest proportion (1.0%) of the sample. Further details about socio-demographic characteristics are presented in Table 1.
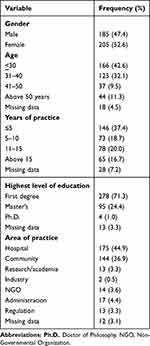 |
Table 1 Socio-Demographic Characteristics |
Knowledge of Drug Counterfeiting
Findings from this study showed that participants had a good knowledge of medicines counterfeiting with over 70% of the participants responding correctly to each item in this section. The study also revealed that a strong majority of pharmacists perceived that circulation of counterfeit medicines in the country was on the increase (97.2%) and almost all of them (99.5%) associated counterfeit pharmaceutical products as one of the causes of treatment failure. Further details relating to the knowledge of pharmacists on drug counterfeiting are presented in Table 2.
 |
Table 2 Knowledge of Drug Counterfeiting |
Practice Towards Counterfeit Medicines
Majority of the respondents in this study (92.4%) indicated that they checked batch number and expiry date of purchased medications on a regular basis; however, about a third of the sample (30.7%) indicated that their knowledge and skills were insufficient to detect counterfeit medicines. Although a considerable proportion of the participants (85.2%) responded that they do not obtain pharmaceuticals from unreliable sources, over a third of the respondents (39.6%) stated that they had unintentionally purchased counterfeit medicines in the course of their practice and 33.0% of them had unintentionally dispensed counterfeit medicines to patients. Table 3, provides more information on the practice of pharmacists towards counterfeit medicines.
 |
Table 3 Practice Towards Addressing Counterfeiting |
Challenges Associated with Mitigating Circulation of Counterfeit Medicines
Data obtained revealed that participants considered inadequate legislation on pharmaceuticals, inadequate inspection by regulatory agencies, online sales of drugs, poor collaboration among regulatory officials, as well as poor cross-border drug law enforcement as factors challenging the mitigation of counterfeit drug circulation in Nigeria. Figure 1 provides a graphical representation of the responses of the participants with respect to legislation and inspection.
 |
Figure 1 Legislation and inspection. |
A strong majority of the study participants (88.83%) indicated that inadequate legislation on pharmaceuticals in the country was a challenge to mitigating counterfeit drugs in this setting. More so, about 90.93% of the respondents were of the opinion that inadequate inspection activities by regulatory officials contribute to counterfeit medicines’ circulation.
As presented in Figure 2, poor collaboration among regulatory agencies as well as poor cross-border drug law enforcement were also regarded as challenges to the mitigation of counterfeit medicines’ circulation in the country. The majority of the study sample (52.66%) were in strong agreement about the culpability of cross-border law enforcement, as a contributor to the circulation of counterfeit medicines in Nigeria.
 |
Figure 2 Collaboration and cross-border law enforcement. |
Almost all respondents in the study (90.43%) agreed that poor enforcement of cross-border laws constituted a challenge to mitigating the menace of drug counterfeiting. A similar proportion (89.94%) also indicated poor collaboration among regulatory agencies as a serious challenge, whereas only a small proportion of the sample (3.72%) indicated this as an impediment towards addressing counterfeiting of medicines in Nigeria.
In addition, a considerable proportion of the participants were of the opinion that online sales of medicines have contributed to the circulation of counterfeit pharmaceuticals in Nigeria. Figure 3 provides the frequency distribution of the opinion of participants on the thematic impact of online medicines’ sales.
 |
Figure 3 Online sales of medicines. |
Also, slightly less than a quarter of the participants (19.09%) had a neutral disposition on the sales of medicines online as a contributor to the circulation of counterfeit drugs. However, about three-quarters of the sample (72.68%) indicated that this mode of commerce constituted a challenge affecting the mitigation of fake pharmaceuticals.
Further statistical analyses were undertaken to determine the association between variables and socio-demographic characteristics. Table 4 represents a cross-tabulation between the demography of the study participants and their abilities to detect counterfeit medicines in the course of practice. There was no significant relationship between participants’ level of education and their abilities to distinguish counterfeit medicines from genuine products (p = 0.531). However, findings revealed that 92.2% of the participants who had practiced pharmacy for more than 15 years indicated that they had detected counterfeit medicines in the course of their work, whereas only 61.1% of respondents with practice experience of 5 years and below affirmed to have identified counterfeit medicines (p = 0.001).
 |
Table 4 Cross-Tabulation of Demography with Detection of Counterfeit Medicines in the Course of Practice |
Furthermore, a statistically significant association emerged with respect to the adequacy of inspection by regulatory agencies in the country and in the aspects age and area of practice of the study participants. Respondents practicing in regulatory sectors seemed more inclined to disagree that poor inspection by regulatory agencies was a challenge to the mitigation of counterfeit medicine’s circulation in Nigeria (p < 0.001). Similarly, older pharmacists were more likely to disagree with this factor as a challenge to the mitigation of counterfeit medicines’ circulation compared to the younger respondents (p = 0.020). These findings are presented in Table 5.
 |
Table 5 Cross-Tabulation of Demography with Inadequacy of Inspection by Regulatory Agencies as a Challenge to the Mitigation of Counterfeit Medicines |
Discussion
The findings from this study revealed that majority of the participants had good knowledge of counterfeit medicines and their menace to the society. This was expected, as respondents in the study were trained and licensed pharmacists. Pharmacists are drug experts who play critical roles in the health system in ensuring the rational use of medicines.28,29 Given their advanced training, the knowledge of the dangers of drug counterfeiting and measures to mitigate the phenomenon is expected. The findings of this study are therefore of critical importance, as an empirical review of the extant knowledge of drug counterfeiting can control the supply in the value chain. Almost all the participants agreed that counterfeit medicines have been a serious threat to healthcare in the country. They were also of the opinion that counterfeit medicines can further contribute to drug resistance and increased morbidity and mortality. This finding is consistent with a similar study30 and signifies that pharmacists understood the extent of severity caused by counterfeit medicines to public health.
However, despite understanding the menace of counterfeit drugs to the healthcare system, up to one-third of the sample indicated that their current knowledge and skills were inadequate to detect counterfeit medicines in circulation. According to the WHO's global surveillance monitoring system, detecting falsified medical products for front-line workers requires keen awareness of the likely risk factors, adverse drug events, as well as visual detection skills such as packaging, misspellings, tablet colour and frailty, or unexpected expiry dates.31 The deficiency of skills reported in this study could be due to the professional’s inability to identify counterfeiting through visual inspection of instruments only such as seals, embossments, the character of the print, and the colour of the product or packaging.24 Though this group constituted the minority, the criticality of the subject renders this finding important. Older participants were more likely to have detected counterfeit medicines in the course of their practice. This corroborates previous findings,32 which reported that pharmacists with practice experience of over two decades were more capable of detecting counterfeit medicines through visual scrutiny. Furthermore, pharmacists practicing in community settings, non-governmental organisations, industries, or regulatory sectors were more likely to detect counterfeit medicines. This can be attributed to the frequency of their exposure to products.33 This finding, therefore, suggests the need for prioritisation of hands-on evidence-based techniques for the detection of counterfeit medicines in the continuous development programme (CPD) organised by the regulators of pharmacy practice. Innovative tools such as simulation exercises can also help increase younger pharmacists’ exposure to products. Improving relevant knowledge in counterfeit detection will better protect patients’ and consequently improve healthcare delivery in Nigeria.
Findings from this study confirmed that majority of pharmacists purchased medicines from reliable sources, and this is in line with previous study.32 However, given that over a third of the research participants affirmed to have unintentionally purchased or dispensed counterfeit medicines in the course of their practice, this is clear evidence of existing risk factors in the drug supply chain. Strengthening of all aspects of the pharmaceutical supply chain is therefore critical in the overarching strategy aimed at reducing fake medicines’ circulation.
Although counterfeit medicines can infiltrate legitimate drug supply chain, a strong prevention strategy entails pharmacists restricting their medicines’ purchase to reliable sources such as manufacturers, distributors, and sales representatives.9 The majority of the respondents in this study agreed that online sales of drugs can increase the circulation of counterfeit medicines. This finding corroborates evidence from other studies that reported increase in internet commerce of medicines as a factor compromising the integrity of the medicine supply chain.34–36 To curb the proliferation of counterfeit pharmaceuticals via this mode of commerce, it is important that contextual regulations, policy and practice guidelines be responsive to this and other related technological advances.
Older pharmacists as well as those working in the regulatory sector were less likely to indicate the adequacy of inspection as a barrier to tackling counterfeit medicines in Nigeria. The majority of the study participants were, however, of opinion that the circulation of counterfeit medicines in the country can be attributed to the inadequacy of inspection by the regulatory sector. It is therefore evident that there is a need for the provision of enhanced regulatory services, inspection, and pharmacovigilance as well as up-scaling of adequate human resources to enable the regulatory sector meet its responsibilities.37,38
Respondents also reported that poor cross-border enforcement of drug laws was a challenge to the reduction of imported fake drugs in the country. In Nigeria, about seventy percent of medicines are primarily imported from other countries.39 This situation not only threatens Medicines’ Security,40 but also implies that poor cross-border enforcement of the existing drug laws has the potential to contribute to the infiltration of counterfeit medicines in this setting. Pharmacists in this study further indicated that poor collaboration among regulatory agencies in the country was a challenge to the mitigation of counterfeit medicines. This finding exacerbates the risk of cross-border infiltration expressed by the study participants, given the role of synergy in border enforcement activities. Therefore, in articulating strategies for developing robust systems that can prevent medicines’ counterfeiting, it is critical that strategic collaboration be encouraged amongst regulatory agencies.
Few pharmacists who practiced in the academic, industry, and administration settings responded to the questionnaire, and this emerged as a limitation to the study. Although a random sampling strategy was employed, it is important to note that pharmacists who work in the indicated thematic area comprise only about ten percent of the Nigerian pharmacy workforce.41 The majority of recruited participants were therefore community and hospital pharmacists. This weakness did not, however, impede the relevance of the findings, as the study participants’ knowledge, experience and exposure in the thematic areas provided novel insights that can inform practice and policy reforms.
Conclusion
This study revealed that the participants had good knowledge and practice with respect to medicine counterfeiting, as well as regarding strategies to mitigate and control the menace. However, factors such as poor collaboration among regulatory agencies, inadequate legislation on regulation of the pharmaceutical sector, poor enforcement of existing laws, and online sales of pharmaceutical products emerged as challenges to the control of counterfeit medicines. It is therefore important that government and policymakers develop contextual strategies, underpinned by these findings to undertake relevant policy and practice reforms.
In undertaking its policymaking and regulatory responsibilities, government needs to strengthen the legislation and inspection of imported products as well as work towards encouraging a strategic increase in local pharmaceutical manufacturing. This is key to reducing the burden of fake drug importation into the system. Similarly, deficiencies in the identification of counterfeit medicines by pharmacists can be curbed by improvement in the curricula of training for pharmacists within institutions of learning, as well as during continuous professional development. Further studies are required to deepen emergent findings, as well as to explore these phenomena amongst practitioners in industry, administration and the academia.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Isles M. What’s in a word? Falsified/counterfeit/fake medicines – the definitions debate. Med Access @ Point Care. 2017;1(1):e40–e48. doi:10.5301/maapoc.0000008
2. Shrivastava SR, Shrivastava PS, Ramasamy J. Public health measures to fight counterfeit medicine. Int J Prev Med. 2013;5(3):370.
3. World Health Organization (WHO). A study on the public health and socioeconomic impact of substandard and falsified medical products. Available from: https://apps.who.int/iris/handle/10665/331690.
4. Erhun WO, Babalola OO, Erhun MO. Drug regulation and control in Nigeria: the challenge of counterfeit drugs. J Health Popul Dev Count. 2001;4(2):23–34.
5. Atholl J, David WH. Substandard drugs: a potential crisis for public health. Br J Clin Pharmacol. 2014;78(2):218–243. doi:10.1111/bcp.12298
6. Ekeh CM, Adekoya HO. Awareness and adoption of drug mobile authentication service: a conscious approach in eradication of fake and counterfeit drugs in Nigeria. KIU J Soc Sci. 2021;7(1):43–51.
7. Osibo OO. Faking and counterfeiting of drugs. West Afr J Pharm. 1998;12:53–57.
8. Glass BD. Counterfeit drugs and medical devices in developing countries. Res Rep Trop Med. 2014;5:11–22. doi:10.2147/RRTM.S39354
9. Blackstone EA, Fuhr JJP, Pociask S. The health and economic effects of counterfeit drugs. Am Health Drug Benefits. 2014;7(4):216–224.
10. Delepierre A, Gayot A, Carpentier A. Update on counterfeit antibiotics worldwide; public health risks. Med Mal Infect. 2012;42(6):247–255. doi:10.1016/j.medmal.2012.04.007
11. Jha AK, Prasopa-Plaizier N, Larizgoitia IA, Bates DW. Patient safety research: an overview of the global evidence. BMJ Qual Saf. 2010;19(1):42–47. doi:10.1136/qshc.2008.029165
12. Cockburn R, Newton PN, Agyarko EK, Akunyili D, White NJ. The global threat of counterfeit drugs: why industry and governments must communicate the dangers. PLoS Med. 2005;2(4):100. doi:10.1371/journal.pmed.0020100
13. Okereke M, Anukwu I, Solarin S, Ohuabunwa MS. Combatting substandard counterfeit medicines in the Nigerian drug market: how industrial pharmacists can rise up to the challenge. commentary. Pharm Based Pract Pract Based Res. 2021;12(3):15. doi:10.24926/iip.v12i3.4233
14. Akunyili D. The challenges faced by NAFDAC in the national regulatory process as it relates to essential drugs for prevention of maternal and newborn deaths in Nigeria. Trop J Obstet Gynaecol. 2010;27(1):81–87.
15. Ogbonna BO, Ilika AL, Nwabueze SA. National drug policy in Nigeria, 1985–2015. World J Pharm Res. 2015;4(6):248–264.
16. Erhun WO. Maintaining qualitative pharmacy practice in a depressed economy. Niger J Pharm. 1996;27(2/3):9–13.
17. Obinna C. Nigeria losing war against fake drugs. Vanguard Nigeria; 2019. Available from: https://www.vanguardngr.com/2019/03/nigeria-losing-war-against-fake-drugs-experts/.
18. Garuba HA, Kohler JC, Huisman MA. Transparency in Nigerian public pharmaceutical sector: perceptions from policymakers. Glob Health. 2009;5(1):1–13. doi:10.1186/1744-8603-5-14
19. Akinyadenu O. Counterfeit drugs in Nigeria: a threat to public health. Afr J Pharm Pharmacol. 2013;7(36):2571–2576. doi:10.5897/AJPP12.343
20. Law E, Youmans SL. Combating counterfeit medications: the California pharmacist perspective. J Pharm Prac. 2011;24:114–121. doi:10.1177/0897190010380745
21. Taleb YA, Madadha RA. Pharmacists’ awareness about counterfeit medications in Jordan. J Roy Med Serv. 2013;20:57–70. doi:10.12816/000007
22. Alfadl AA, Hassali MA, Ibrahim MI. Counterfeit drug demand: perceptions of policy makers and community pharmacists in Sudan. Res Social Adm Pharm. 2013;9:302–310. doi:10.1016/j.sapharm.2012.05.002
23. Al-Worafi YM. Pharmacy practice and its challenges in Yemen. Australas Med J. 2014;7:17. doi:10.4066/AMJ.2014.1890
24. Sholy L, Gard P, Williams S, MacAdam A. Pharmacist awareness and views towards counterfeit medicine in Lebanon. Int J Pharm Pract. 2018;26(3):273–280. doi:10.1111/ijpp.12388
25. Bashir A, Galal S, Ramadan A, Wahdan A, El-Khordangui L. Community pharmacists’ perceptions, awareness and practices regarding counterfeit medicines: a cross-sectional survey in Alexandria, Egypt. East Mediterr Health J. 2020;26(5):556–564. doi:10.26719/emhj.19.058
26. Yakubu O An analysis of loopholes in the pharmaceutical supply chain, and methods for improving control of counterfeit drugs in Nigeria [Research Thesis]. Dublin: Griffith College; 2020. Available from: https://go.griffith.ie/446/1/3280_Ojima_Precious_Yakubu_Ojima_Precious_Yakubu_Final_Dissertation_Copy_3970_607779346.pdf.
27. Parsons VL. Stratified sampling. Wiley StatsRef. 2014;1–11. doi:10.1002/9781118445112.stat05999.pub2
28. Alfa J, Adigwe OP. Rational use of medicines in Nigeria: a critical review. J Biol Agric Healthc. 2014;4:89–99.
29. Toklu HZ, Mensah E. Why do we need pharmacists in pharmacovigilance systems? Online J Public Health Inform. 2016;8(2). doi:10.5210/ojphi.v8i2.6802
30. Akunyili D. Strategies employed in combatting drug counterfeiting in Nigeria.
31. World Health Organization. WHO global surveillance and monitoring system for substandard and falsified medical products; 2017. Available from: https://apps.who.int/iris/bitstream/handle/10665/326708/9789241513425-eng.pdf.
32. Odili VU, Osemwenkha S, Eke EU, Okeri HA. Identification of counterfeit drugs by community pharmacists in Lagos State. Trop J Pharm Res. 2006;5(1):545–550. doi:10.4314/tjpr.v5i1.14631
33. Mhando L, Jande MB, Liwa A, Mwita S, Marwa KJ. Public awareness and identification of counterfeit drugs in Tanzania: a view on antimalarial drugs. Adv Public Health. 2016;1–8. doi:10.1155/2016/6254157
34. Aminu NA, Gwarzo MS. The eminent threats of counterfeit drugs to quality health care delivery in Africa: updates on consequences and way forward. Asian J Pharma Clin Res. 2017;10(7):82–86. doi:10.22159/ajpcr.2017.v10i7.18384
35. Sipahi H, Peltekoglu K, Sencan N. Adverse effects of counterfeit drugs on public health. J Fac Pharm Ist Univ. 2014;44(1):89–99.
36. Shipalana P, Matema T, Van Der Westhuizen H. Counterfeit pharmaceuticals: a major threat to public health. South African Institute of international affairs; 2020. Available from https://www.jstor.org/stable/resrep29593.
37. Hepler CD, Strand LM. Opportunities and responsibilities in pharmaceutical care. Am J Hosp Pharm. 1990;47:533–543. doi:10.1093/ajhp/47.3.533
38. Esposito M. Strategies to combat counterfeit drugs and other pharma supply chain threats. Guest column; 2018. Available from: https://www.pharmaceuticalonline.com/doc/strategies-to-combat-counterfeit-drugs-and-other-pharma-supply-chain-threats-0001.
39. Akande-Sholabi W, Adebisi YA. The impact of COVID-19 pandemic on medicine security in Africa: Nigeria as a case study. The Pan African Medical Journal. 2020;35(Suppl2). doi:10.11604/pamj.supp.2020.35.2.23671
40. Adigwe OP. Stakeholders’ perspective of role of policy and legislation in achieving medicines’ security. Int J World Pol Dev Stud. 2020;6(6):66–73. doi:10.32861/ijwpds.66.66.73
41. Ekpenyong A, Udoh A, Kpokiri E, Bates I. An analysis of pharmacy workforce capacity in Nigeria. J Pharm Policy Pract. 2018;11(1):1–9. doi:10.1186/s40545-018-0147-9
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
