Back to Journals » Infection and Drug Resistance » Volume 17
Case Report: Pneumonia Caused by Chlamydia Psittaci and Cryptococcus Co-Infection
Authors Zhang A, Lao X, Liang J, Xia X, Ma L, Liang J
Received 31 October 2023
Accepted for publication 28 February 2024
Published 6 March 2024 Volume 2024:17 Pages 845—849
DOI https://doi.org/10.2147/IDR.S445920
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Anbing Zhang,1,* Xiaoli Lao,1,2,* Jinguang Liang,3 Xiuqiong Xia,1 Lei Ma,4 Jianping Liang1,2
1Department of Respiratory and Critical Care Medicine, Zhongshan People’s Hospital, Zhongshan, People’s Republic of China; 2Guangdong Medical University, Zhanjiang, Guangdong, People’s Republic of China; 3Department of Respiratory and Critical Care Medicine, Zhongshan Huangpu People’s Hospital, Zhongshan, People’s Republic of China; 4Department of Respiratory and Critical Care Medicine, Fuyang Second People’s Hospital, Fuyang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianping Liang, Department of Respiratory and Critical Care Medicine, Zhongshan People’s Hospital, No. 2, Sunwen East Road, Zhongshan, 528400, People’s Republic of China, Tel +86 15989107884, Fax +86 760-89880255, Email [email protected]
Abstract: This study presents a rare case of pneumonia caused by a co-infection of Chlamydia psittaci and Cryptococcus, confirmed by metagenomic next-generation sequencing (mNGS). The patient, who had underlying chronic hepatitis B, had adopted a stray pigeon before the onset of the disease. The primary symptoms were fever, and a productive cough. The patient recovered following treatment with moxifloxacin and itraconazole. C. psittaci and Cryptococcus infections may both have been transmitted from the stray pigeon. This report highlights the potential for infections caused by multiple zoonotic pathogens and the value of mNGS for making the diagnosis of these infections.
Keywords: pneumonia, Chlamydia psittaci, Cryptococcus, co-infection, metagenomic next-generation sequencing
Introduction
Previous studies have reported mixed infections of Chlamydia psittaci with viruses or bacteria in avian species,1,2 as well as co-infections of Cryptococcus and Pneumocystis carinii in humans.3 However, to our knowledge, co-infections of C. psittaci and Cryptococcus have not been reported previously. Bird exposure as a potential source of infection is frequently overlooked during medical history-taking. This, along with the challenges in culturing C. psittaci and Cryptococcus, can lead to delayed or missed diagnosis of such conditions. Here, we present a case of pulmonary infection caused by C. psittaci and Cryptococcus in a patient with a history of bird exposure to raise awareness of such diseases among clinicians.
Case Presentation
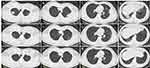
The patient, a 41-year-old male farmer, had a 20-year history of chronic hepatitis B infection, which was managed with traditional Chinese medicine. Additionally, he had a 10-year history of fatty liver disease. Five days prior to admission, he had developed a fever, with a peak body temperature of 39.9°C and symptoms of a respiratory infection, including a sore throat, cough, and yellow sputum. He self-medicated with ibuprofen for the fever; however, he experienced recurrent fever and therefore sought treatment at our hospital. Blood tests on admission showed a white blood cell count of 9.65×109/L, neutrophil count of 7.37×109/L, neutrophil percentage of 76.3%, lymphocyte count of 1.24×109/L, and lymphocyte percentage of 12.8%. The C-reactive protein (CRP) and procalcitonin levels were 189.28 mg/L and 0.09 ng/mL, respectively. Arterial blood gas analysis showed a pH of 7.458, partial pressure of oxygen (pO2) of 70.5 mmHg, partial pressure of carbon dioxide (pCO2) of 35.2 mmHg, and HCO3− of 24.9 mmol/L, which gave an oxygenation index of 214 mmHg. The hepatitis B surface antigen test was positive, with alanine aminotransferase (ALT), aspartate aminotransferase (AST), and creatine kinase (CK) levels of 128 U/L, 57.8 U/L, and 336 U/L, respectively (see Table 1 for details). The cryptococcal antigen test was positive. The β-D-glucan test for fungal infections was at 45.54 pg/mL and the aspergillus antigen test 0.2 μg/L. Influenza A and B antigen tests, and the dengue NS1 antigen test, were negative. Chest computed tomography (CT) revealed bilateral pneumonia (Figure 1A–D). The patient was diagnosed with pulmonary infection and treated with intravenous moxifloxacin (0.4 g daily), carbocisteine for expectoration, compound methoxyphenamine capsules for cough relief, physiotherapy for sputum clearance, and sodium glucuronic acid injection and compound glycyrrhizin capsules as adjuvant treatments for hepatitis B. On the third day of hospitalization, fiberoptic bronchoscopy was performed due to persistent fever, and bronchoalveolar lavage was conducted in the right lower lobe bronchus. A total of 20 mL of lavage fluid was sent to Guangzhou Huayin Medical Laboratory Center for metagenomic next-generation sequencing (mNGS) analysis. The mNGS detected C. psittaci (read count: 3820) and Cryptococcus neoformans (read count: 51). A separate mNGS test of a throat swab also revealed C. neoformans (read count: 1). However, mNGS of a blood sample did not detect any pathogens. On further inquiry into the patient’s history, he recalled having adopted a stray pigeon 2 weeks before the symptom onset. Based on these findings, he was diagnosed with pneumonia caused by C. psittaci and C. neoformans co-infection. He was treated with itraconazole for Cryptococcus (200 mg orally twice a day) because our hospital does not stock fluconazole, and the moxifloxacin administration was continued to treat the C. psittaci infection. The patient’s temperature dropped down to normal on the third day after starting itraconazole treatment. After antimicrobial treatment, the patient’s inflammatory indices and liver transaminase levels improved. Follow-up chest CT 2 weeks after completing treatment showed significant alleviation of lung inflammation (Figure 1E–H). His hematology parameters as well as CRP and liver transaminase levels also showed improvement.
 |
Table 1 Laboratory Test Results of the Patient |
The patient was treated with moxifloxacin for 2 weeks. Following discharge, the patient continued taking itraconazole (200 mg twice a day). At the 4-week follow-up, chest CT revealed that the lesions in both lungs had healed (Figure 1I–L). His hematology results and CRP levels returned to normal, and his liver transaminase levels showed further improvement. The patient was treated with itraconazole for 3 months.
Discussion
Psittacosis is a zoonotic disease caused by C. psittaci. Humans can contract this disease either via direct contact or by inhaling particles of excreta and respiratory secretions of infected birds.4 C. neoformans is an opportunistic fungal pathogen. Individuals with compromised immune systems, such as those with HIV infection, are at increased risk of infection.5 As with C. psittaci, Cryptococcus can be isolated from bird feces.6 Peralta et al7 reported a case of cryptococcal meningitis in a patient who had contact with a parrot and was suspected to have contracted the infection by zoonotic transmission of Cryptococcus from the parrot. The patient suffered neurological sequelae due to delayed diagnosis and treatment.
It is unclear whether our patient was infected with Cryptococcus or C. psittaci first. The patient had a definitive history of exposure to pigeons, potentially the source of both the C. psittaci and Cryptococcus infection. Individuals with compromised immunity are more susceptible to Cryptococcus infection. Chronic hepatitis B virus infection can induce T-cell and natural killer cell dysfunction.8,9 The patient’s chronic hepatitis B virus infection may have caused immunodeficiency, thus increasing his risk of Cryptococcus infection.10 We speculate that the patient might have initially been infected with C. neoformans, which has the capacity to induce apoptosis in macrophages via its capsular polysaccharides, predominantly galactoxylomannan and glucuronoxylomannan.11,12 Macrophages play an important role in the immune response against chlamydial infection,13 a reduction in macrophages could enhance susceptibility to C. psittaci infection. Another study suggested a synergistic interaction between adenovirus and C. psittaci in affected parrots, in which the adenovirus induced immune suppression in the host, resulting in an increase in the C. psittaci bacterial load.1
Awareness of psittacosis is limited owing to its nonspecific clinical manifestations such as fever and cough.14 Additionally, routine diagnostic tests, including serological testing and culture, have limitations in diagnosing psittacosis.15 These factors make diagnosing psittacosis challenging. Pet birds are frequently overlooked as a potential source of Cryptococcus infection7 and clinicians often do not consider the possibility of this infection. Therefore, infection by rare pathogens should be considered during the differential diagnosis in patients exhibiting atypical symptoms of pulmonary infection, with negative results for routine pathogens and no improvement after empirical antibiotic treatment. In such cases, a timely bronchoalveolar lavage for mNGS should be considered.
A 2021 report showed that compared with culture and serological testing, mNGS offered a sensitivity of 81–86% and specificity of 91–95% for bacterial detection, and a sensitivity of 63–70% and specificity of 92–96% for fungal detection.16 In addition, mNGS can identify C. psittaci more rapidly and exhibits distinct advantages for detecting mixed pathogen infections.14 Therefore, mNGS is a valuable for diagnosing co-infections with C. psittaci and other respiratory pathogens.
C. psittaci can compromise the functionality of chicken macrophages, thereby facilitating the invasion of the H9N2 avian influenza virus.13 In birds co-infected with C. psittaci and H9N2, C. psittaci has been shown to suppress the host’s immune response by inhibiting humoral immunity and altering the Th1/Th2 balance, leading to an elevated mortality rate in the host.17 Based on these findings, we speculate that co-infections of C. psittaci and Cryptococcus could exacerbate the disease severity, resulting in enhanced damage and an increased mortality rate. In this case, the patient received prompt fiberoptic bronchoscopy after admission, and the collected bronchoalveolar lavage fluid was sent for mNGS analysis. With early diagnosis and treatment, the patient made a good recovery, and further progression of the disease was prevented.
Conclusion
Pneumonia caused by a co-infection of C. psittaci and Cryptococcus is rare and has no specific clinical manifestations. Metagenomic next-generation sequencing and detection of serum cryptococcal antigen are valuable tools for accurate diagnosis.
Data Sharing Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
Ethics Approval and Informed Consent
The studies involving human participants were reviewed and approved by the Ethics Review Committee of Zhongshan People’s Hospital. The patient provided his written informed consent to participate in this study.
Acknowledgments
The authors thank the patient for his cooperation during the diagnostic process.
Funding
This study was supported by the Science and Technology Plan Project of Zhongshan (grant no. 2020B1113).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. To KK, Tse H, Chan WM, et al. A novel psittacine adenovirus identified during an outbreak of avian chlamydiosis and human psittacosis: zoonosis associated with virus-bacterium coinfection in birds. PLoS Negl Trop Dis. 2014;8(12):e3318. doi:10.1371/journal.pntd.0003318
2. De Boeck C, Kalmar I, Dumont A, Vanrompay D. Longitudinal monitoring for respiratory pathogens in broiler chickens reveals co-infection of Chlamydia psittaci and Ornithobacterium rhinotracheale. J Med Microbiol. 2015;64(5):565–574. doi:10.1099/jmm.0.000047
3. Huang J, Weng H, Lan C, Li H. Pulmonary co-infection with Cryptococcus species and Pneumocystis jirovecii in an old patient without a previous predisposing illness. Mycopathologia. 2022;187(5–6):613–616. doi:10.1007/s11046-022-00651-8
4. Balsamo G, Maxted AM, Midla JW, et al. Compendium of measures to control Chlamydia psittaci infection among humans (psittacosis) and pet birds (avian chlamydiosis), 2017. J Avian Med Surg. 2017;31(3):262–282. doi:10.1647/217-265
5. Zhao Y, Ye L, Zhao F, et al. Cryptococcus neoformans, a global threat to human health. Infect Dis Poverty. 2023;12(1):20. doi:10.1186/s40249-023-01073-4
6. Anacona C, González CFE, Vásquez LR, Escandón P. First isolation and molecular characterization of Cryptococcus neoformans var grubii in excreta of birds in the urban perimeter of the Municipality of Popayán, Colombia. Rev Iberoam Micol. 2018;35(3):123–129. doi:10.1016/j.riam.2018.01.005
7. Peralta DP, Najjar H, Garcia-Chan J. Cryptococcal meningitis in an immunocompetent man exposed to a pet cockatoo: an overlooked zoonosis. Cureus. 2022;14(8):e28122.
8. Fisicaro P, Barili V, Rossi M, et al. Pathogenetic mechanisms of T cell dysfunction in chronic HBV infection and related therapeutic approaches. Front Immunol. 2020;11:849. doi:10.3389/fimmu.2020.00849
9. Lunemann S, Malone DF, Hengst J, et al. Compromised function of natural killer cells in acute and chronic viral hepatitis. J Infect Dis. 2014;209(9):1362–1373. doi:10.1093/infdis/jit561
10. Spies FS, de Oliveira MB, Krug MS, et al. Cryptococcosis in patients living with hepatitis C and B viruses. Mycopathologia. 2015;179(3–4):307–312. doi:10.1007/s11046-014-9843-4
11. Villena SN, Pinheiro RO, Pinheiro CS, et al. Capsular polysaccharides galactoxylomannan and glucuronoxylomannan from Cryptococcus neoformans induce macrophage apoptosis mediated by Fas ligand. Cell Microbiol. 2008;10(6):1274–1285. doi:10.1111/j.1462-5822.2008.01125.x
12. Chiapello LS, Baronetti JL, Garro AP, Spesso MF, Masih DT. Cryptococcus neoformans glucuronoxylomannan induces macrophage apoptosis mediated by nitric oxide in a caspase-independent pathway. Int Immunol. 2008;20(12):1527–1541. doi:10.1093/intimm/dxn112
13. Chu J, Guo Y, Xu G, et al. Chlamydia psittaci triggers the invasion of H9N2 avian influenza virus by impairing the functions of chicken macrophages. Animals. 2020;10(4):722. doi:10.3390/ani10040722
14. De Gier B, Hogerwerf L, Dijkstra F, van der Hoek W. Disease burden of psittacosis in the Netherlands. Epidemiol Infect. 2018;146(3):303–305. doi:10.1017/S0950268817003065
15. Huang W, Wang F, Cai Q, et al. Epidemiological and clinical characteristics of psittacosis among cases with complicated or atypical pulmonary infection using metagenomic next-generation sequencing: a multi-center observational study in China. Ann Clin Microbiol Antimicrob. 2023;22(1):80. doi:10.1186/s12941-023-00631-w
16. Gu W, Deng X, Lee M, et al. Rapid pathogen detection by metagenomic next-generation sequencing of infected body fluids. Nat Med. 2021;27(1):115–124. doi:10.1038/s41591-020-1105-z
17. Chu J, Zhang Q, Zhang T, et al. Chlamydia psittaci infection increases mortality of avian influenza virus H9N2 by suppressing host immune response. Sci Rep. 2016;6(1):29421. doi:10.1038/srep29421
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.