Back to Journals » Infection and Drug Resistance » Volume 17
Case Report: Encephalitis with Initial Manifestation of Orientia Tsutsugamushi Infection Detected by Metagenomic Next-Generation Sequencing
Authors Han S, Yang S, Wang Y, Xu Y
Received 29 November 2023
Accepted for publication 7 February 2024
Published 26 February 2024 Volume 2024:17 Pages 749—760
DOI https://doi.org/10.2147/IDR.S450693
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Song Han,1,* Suge Yang,1,* Yun Wang,2 Yingying Xu2
1The Second Hospital of Shandong University, Cheeloo College of Medicine of Shandong University, Shandong University, Jinan, 250033, People’s Republic of China; 2Department of Neurology Medicine, The Second Hospital of Shandong University, Cheeloo College of Medicine of Shandong University, Shandong University, Jinan, 250033, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yun Wang; Yingying Xu, Department of Neurology Medicine, The Second Hospital of Shandong University, Cheeloo College of Medicine of Shandong University, Shandong University, Jinan, 250033, People’s Republic of China, Email [email protected]; [email protected]
Purpose: Scrub typhus, caused by Orientia tsutsugamushi, is characterized by fever, eschars, lymphadenopathy, and rash. The absence of eschars in some cases makes it difficult to distinguish it from other diseases, complicating the diagnosis process. Atypical Scrub typhus is difficult to diagnose and often leads to delayed treatment. Therefore, early diagnosis and treatment through effective detection methods have high clinical value. Here, a case of scrub typhus with encephalitis symptoms is reported.
Patients and Methods: A 64-year-old man and mNGS testing.
Results: A 64-year-old man developed cough, headache, and fever, dismissing it as a respiratory tract infection. Initial treatment with cephalosporin antibiotics had minimal effect. Admission to the respiratory department showed inflammation in blood tests. Subsequent CT and further treatment provided no improvement. Multidisciplinary discussions and neurology department guidance were conducted to consider the suspected diagnosis of encephalitis in the patient. After improving the mNGS detection, the patient was diagnosed with “Orientia tsutsugamushi encephalitis”. After treatment with doxycycline, the patient’s symptoms were alleviated. He remained afebrile in follow-up and adhered well to medical advice.
Conclusion: Our case demonstrates that it is difficult to distinguish Orientia tsutsugamushi encephalitis from central nervous system infectious diseases such as meningitis and encephalitis using conventional diagnostic methods, which may affect the treatment plan for the disease. mNGS is a useful and valuable method for early diagnosis of scrub typhus.
Keywords: orientia tsutsugamushi infection, scrub typhus, encephalitis, metagenomics next-generation sequencing
Introduction
Scrub typhus, also known as Orientia tsutsugamushi infection, is an acute febrile infectious disease transmitted by arthropods (chiggers) through biting; its causative agent its causative agent is Orientia tsutsugamushi (formerly Rickettsia tsutsugamushi).1 This disease was initially described in Japan and historically was limited to the Asia-Pacific region, including rural and forested areas of India, Australia, Japan, and China. However, recent reports of locally acquired cases have also emerged in the Middle East and Latin America. It is estimated that at least one million people worldwide are infected annually, significantly impacting public health in Southeast Asia.2,3 In China, scrub typhus was first reported in Guangzhou in 1948, with the first outbreak occurring in Shandong in 1986. Research indicates that the incidence of scrub typhus has been increasing annually in various regions across China in recent years.4 Epidemiological statistics from 2006 to 2017 revealed over 120,000 cases of scrub typhus patients in 30 provinces in China.5
The main clinical manifestations of scrub typhus include fever, chills, rash, and eschars at the bite site, and may also involve headache, myalgia, cough, systemic lymphadenopathy, nausea, vomiting, and abdominal pain. In severe cases, it can lead to many complications in the lungs, brain, kidneys, meninges, and heart, such as acute respiratory distress syndrome, acute kidney failure, encephalitis, gastrointestinal bleeding, meningitis, myocarditis, and pneumonia.6–8 Without appropriate treatment, it can lead to severe multi-organ failure, with a mortality rate as high as 70%.9 Meningitis and/or encephalitis caused by Orientia tsutsugamushi often occur during disease exacerbation, leading to restlessness, confusion, and even epileptic seizures in patients.10 Although scrub typhus with encephalitis as the sole early symptom is rare, cases still exist.
The metagenomic next-generation sequencing (mNGS) technology, with its high efficiency, accuracy, and comprehensiveness, has been used for the diagnosis of clinical rare, novel, and atypical infectious diseases. It holds significant diagnostic value for scrub typhus without eschars as the main manifestation solely presenting as encephalitis.
We report a case of Orientia tsutsugamushi encephalitis, which presented with severe headache, persistent fever, ineffectiveness of antibiotic treatment, delayed reactions, indifference, and neurological symptoms, without eschar formation. These symptoms easily lead to confusion with other infectious diseases. In the absence of a definitive diagnosis using routine diagnostic methods, metagenomic next-generation sequencing (mNGS) was employed to confirm the disease as Orientia tsutsugamushi encephalitis. This method offers advantages such as rapidity and accuracy, and holds high diagnostic value in clinical practice.
Materials and Methods
We collected and analyzed detailed medical records of the patient, including admission information, past medical history, comprehensive neurological examinations, neuroimaging studies, echocardiography, serum laboratory investigations, routine cerebrospinal fluid analysis, and cytological examinations. This study was conducted following the principles of the Helsinki Declaration and obtained informed consent from the patient. Approval for this study was obtained from the Research Ethics Committee of the Second Hospital of Shandong University.
mNGS Testing Method (The Experiments and Quality Testing Were Assisted by Dingjing Biotechnology (Jinan, China))
Sample Processing and Nucleic Acid Extraction
Samples were taken and homogenized by shaking with glass beads. DNA was extracted using column-based extraction method for library construction.
Library Construction and Sequencing
DNA libraries were constructed using end repair method, with specific tag sequences introduced at the ends of each library. The concentration of libraries was determined using Qubit 4.0 Fluorometer (Q33226, Thermo Fisher, USA) in conjunction with Qubit® dsDNA HS Assay Kit (Q32854, Thermo Fisher, USA). The size of insert fragments in the libraries was detected using Agilent 2100 Bioanalyzer (G2939BA, Agilent, USA). DNA nanoballs were prepared using the One-Step DNB Preparation Kit (1000025076, MGI Tech Co., Ltd., Shenzhen) and then sequenced on the MGISEQ-200 sequencer.
Bioinformatics Analysis
The raw sequencing data were initially processed to eliminate sequences containing sequencing adapters, low quality, short length (<35bp), and low complexity. Subsequently, the remaining sequences were aligned to the human reference genome (hg38) using STAR software (v2.7.1a) to remove host sequences. The non-host sequences were then matched against a custom-built microbial genome database containing bacteria, fungi, parasites, and viruses. The coverage value was 0.004977633, with a depth of 1X. Each non-host sequence was aligned with the DNA sequences in the database using the principle of optimal similarity, and species information features from the database DNA sequences were utilized to summarize and merge the number of non-host sequences matched to different species. The pathogenic microbial reference genomes used for classification were downloaded from the National Center for Biotechnology Information (NCBI) (ftp://ftp.ncbi.nlm.nih.gov/genomes/) and other public databases.
Results
The patient was a 64-year-old male farmer who frequently works in the fields. After a day of work in the field, the patient started coughing, producing yellow sputum. He also experienced headaches characterized by throbbing pain, fever with a maximum temperature of approximately 39°C, along with chills and fatigue. Additionally, they had bloating, loss of appetite, and no other discomforts. The patient sought medical attention at a local clinic, where “acute bronchitis” was considered, and he was treated with cephalosporins and ambroxol, but with no significant improvement in symptoms. To undergo further systematic assessment and treatment, the patient visited the respiratory department of our hospital. After initial examination in the outpatient clinic, the attending physician noticed slight redness on the patient’s legs, resembling insect bite marks, but no rash was observed. The possibility of a severe infection was considered, and hospitalization was recommended for further treatment.
After the patient was admitted, a detailed medical history was obtained. There was no history of chronic conditions such as hypertension, diabetes, or coronary heart disease. The patient denied any history of infectious diseases such as hepatitis or tuberculosis, as well as any close contact with individuals affected by these diseases. There was no surgical history, trauma, or blood transfusions. The patient had no allergies to food or medications and received vaccinations according to the schedule. The patient had a regular lifestyle and denied any history of insect bites, but had been smoking for over 30 years at a rate of approximately 20 cigarettes per day, and had quit smoking for 1 year. He was no history of alcohol consumption, drug abuse, or other harmful habits. The patient was mentally and psychologically stable, had two daughters, and both the spouse and daughters were in good health. The patient’s parents had passed away, and there was no known family history of similar diseases.
Upon physical examination by the respiratory department, no significant abnormalities were observed. Coarse breath sounds were heard on auscultation of both lungs, and no dry or wet rales were detected. After the initial inquiry following the patient’s admission, we initially considered infectious diseases and then needed to confirm the pathogen. It was also important to differentiate from non-inflammatory diseases that can cause fever (Table 1).
 |
Table 1 Differential Diagnosis, Exclusion of Causes, and Subsequent Treatment Plans for Febrile Illnesses |
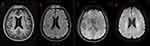
After the patient’s admission, a comprehensive set of laboratory tests and pathogen detection results were obtained (Table 2). There were some abnormal laboratory findings, including a high white blood cell count of 10.94 × 10^9/L, a low lymphocyte percentage of 14.2%, and a high neutrophil percentage of 82.4%. In addition, the CRP level was elevated at 52.69mg/L, and the PCT level was high at 0.998ng/mL. Liver and kidney function tests revealed elevated ALT (74U/L) and AST (50U/L) levels and decreased albumin levels at 28.7g/L. The patient had hyponatremia with a sodium level of 125.0mmol/L, and clotting function tests indicated elevated PT (15.1 seconds), APTT (54.2 seconds), fibrinogen degradation product (17.14ug/mL), and D-Dimer (4490.00ng/mL) levels. Serologic tests for Brucella, Mycoplasma pneumoniae, Legionella, Leptospira, T-SPOT, Aspergillus antigen, and other infectious diseases series revealed no significant abnormalities. The patient completed the Weil-Felix test, and the result was negative. Imaging studies suggested the presence of pneumonia without any abnormal masses. Brain MRI revealed multiple ischemic lesions, bilateral mastoiditis, and slight bleeding in the left frontal lobe, with no significant abnormality on DWI (Figure 1).
 |
Table 2 Laboratory Tests and NGS Results of the Pathogen for Patient’s Hospital Admission and Follow-Up Visits |
Based on the above findings, we believed that the patient was suffering from inflammation and infection, electrolyte imbalance, and the main diagnosis upon admission was: 1. Pneumonia, 2. Hypokalemia. The patient had commenced symptomatic supportive treatment in the respiratory department, including sputum culture and antimicrobial therapy. The antibiotics were switched from cefminox and levofloxacin to meropenem, levofloxacin, and acyclovir. In view of the fact that the current antibiotic used for the patient is not effective, we have upgraded the antibiotic and covered the treatment of viral infection. After treatment, the patient’s cough and sputum production improved compared to previously.
Unfortunately, the patient continues to experience headache during the treatment process, and persistent high fever with a temperature of 38–39°C, accompanied by delayed response, and drowsiness. A hospital-wide consultation was requested by the respiratory department, and the neurology department performed a comprehensive neurological examination. The patient appears lethargic, with slightly cloudy consciousness, coherent in conversation, occasionally making mistakes in recalling breakfast content. No obvious abnormalities were found in cognitive ability related to calculation, spatial orientation, and time recognition. Pupils are 2mm bilaterally, with a normal response to light. The mouth and tongue are centered, with no evident abnormal manifestations in the remaining cranial nerves. Muscle strength is at level V, and muscle tone is normal, with no abnormal deep or superficial reflexes. Both sides show negative pathological signs, and the neck is soft, with a negative Kernig’s sign; however, the coordination of movements is uncooperative. Due to the patient’s prolonged high fever and the ineffectiveness of general antibiotic treatment, the possibility of concurrent encephalitis infection is considered. Therefore, it is recommended that the patient be transferred to the neurology department for further investigation, including a comprehensive examination of cerebrospinal fluid to establish a clear diagnosis.
On the day of admission to the neurology department, the patient’s cerebrospinal fluid was collected and sent for examination. The cerebrospinal fluid cytology showed a white blood cell count of 22/mm3, with 49% lymphocytes, 11% monocytes, and 25% neutrophils. The tryptophan test was positive (Figure 2). Additionally, the cerebrospinal fluid biochemistry revealed high protein levels of 941mg/L and low chloride levels of 106.5mmol/L, while glucose levels remained normal. Despite negative results from ink staining, Gram staining, cryptococcal capsular antigen testing, modified acid-fast staining, and Wright’s staining, and negative findings from cerebrospinal fluid PCR testing for HSV-1, HSV-2, VZV, EBV, CMV, HHV-6, HHV-7, Orientia tsutsugamushi,11–13 no pathogens were cultivated during the process. the possibility of intracranial infection could not be ruled out. Considering the patient’s medical history and laboratory results, and observing the clinical response to antibacterial and antiviral treatment, the possibility of common bacterial, viral, fungal, and tuberculosis infections was eliminated. After discussions among the neurology department, the possibility of a rare pathogen was considered. With the consent of the patient and family, the cerebrospinal fluid sample was sent for mNGS testing, which detected 2 reads of specific sequences of Orientia tsutsugamushi (Figure 3), which resulted in a diagnosis of Orientia tsutsugamushi encephalitis. Although the patient did not exhibit typical eschar symptoms, the cerebrospinal fluid PCR test showed negative results for Orientia tsutsugamushi, and the read count in mNGS was low. However, no other specific pathogens were reported in the results. After calibration, as the genome coverage was 0.4978%, we decided to adopt a suspected diagnosis plan.14 The final diagnosis was confirmed as Orientia tsutsugamushi encephalitis. Following the confirmed diagnosis, in conjunction with the latest clinical trial reports by Varghese et al15 and relevant diagnostic and treatment guidelines, the current antibiotics were immediately discontinued, and doxycycline was administered at a dosage of 200 mg/day. Within 2 days of the change in treatment, the patient’s body temperature did not exceed 37°C, and symptoms such as headache, dizziness, and nausea did not reappear. The patient’s mental state improved, and a subsequent neurological examination showed clear consciousness, coherent conversation, and no significant abnormalities in memory, calculation, spatial orientation, and time recognition. There were no notable abnormalities in the cranial nerves, muscle strength was graded as level V, muscle tone was normal, and deep and superficial reflexes were unremarkable. The patient’s coordination of movement also showed no abnormalities. Due to financial reasons, the patient did not undergo further imaging such as cranial MRI. The progress of patients during hospitalization was summarized in Figure (Figure 4).
 |
Figure 4 Patient’s visit timeline. |
During the patient’s hospitalization, they expressed satisfaction with the treatment plan of the department. Following discharge, the patient adhered to medical advice and took doxycycline 100mg q12h as prescribed. After one week of taking medication, the patient’s symptoms improved and the medication was discontinued. One month later, they were readmitted for reassessment. The cerebrospinal fluid cytology showed normal findings. Biochemical analysis of the cerebrospinal fluid revealed protein levels of 350mg/L and chloride levels of 124mmol/L. Staining tests for ink, Gram staining, cryptococcal capsular antigen, modified acid-fast staining, and Wright’s staining were negative. Considering the economic burden and respecting the patient’s wishes, no further tests were performed. However, based on the re-examination of relevant blood and cerebrospinal fluid results, and the absence of significant clinical symptoms, it can be inferred that the patient’s prognosis is good. The patient expressed satisfaction with the treatment effectiveness of the department.
Discussion
Scrub typhus, also known as bush typhus, is a natural focal acute infectious disease caused by Orientia tsutsugamushi and primarily hosted by rodents. It is transmitted by the bites of chiggers. The causative agent of this disease, Orientia tsutsugamushi (belonging to the genus Orientia of the Rickettsiaceae family), is a Gram-negative obligate intracellular bacterium.16 Under electron microscopy, the pathogen typically appears circular, oval, short-rod-shaped, or dumbbell-shaped, with a size range of (0.3–0.5) × (1.2–3.0) μm. Its characteristics in Gram staining, Giemsa staining, Macchiavello staining, and structural features under electron microscopy are generally similar to other Rickettsia.17 The general features of the disease include sustained high fever, toxemia, rash, and lymphadenopathy as typical signs of infection. Severe cases can involve multiple organs such as the liver, lungs, and nervous system, potentially leading to fatality. The presence of an ulcer or eschar at the site of the bite is a characteristic presentation of scrub typhus. When combined with the patient’s recent outdoor activities in rural or forested areas, diagnosing scrub typhus can be relatively straightforward. However, for atypical cases without skin eschars, early diagnosis of scrub typhus can be quite challenging.18,19
Serological testing remains the primary laboratory diagnostic method for scrub typhus. The commonly used Weil-Felix test is cost-effective and easy to perform, serving as a preliminary diagnostic method. However, some febrile patients may exhibit cross-reactions in their serum, leading to false positives with the Weil-Felix test. Additionally, early use of antibiotics or compromised immune function in the host can result in a lack of effective immune response, resulting in false negatives. Consequently, the sensitivity and specificity of the Weil-Felix test are both relatively low.20,21 Therefore, accurate pathogen diagnosis in patient samples is crucial for early diagnosis of scrub typhus, especially for atypical cases. Indirect immunofluorescence assay (IFA) is the gold standard for diagnosing scrub typhus, but it requires duplicate samples, has serum type limitations, and high instrument demands. PCR exhibits higher sensitivity and specificity, but it requires specific technical expertise, equipment support, and is susceptible to antibiotic interference. However, currently, including our hospital, many hospitals do not have this type of testing available.
Recently, metagenomic next-generation sequencing (mNGS) has emerged as a novel diagnostic technology with the qualities of being efficient and accurate, and has been applied in the diagnosis of clinical rare, novel, and atypical infectious diseases, particularly for the diagnosis of complex infectious diseases.22 mNGS has the capability to detect all possible pathogens in clinical samples, without bias. In comparison to conventional diagnostic methods, mNGS has a shorter turnaround time (typically completed within 24 hours) and can simultaneously identify non-targeted sequences of millions of base pairs.23 Since the emergence of mNGS technology, it has been widely used in various diseases such as complex and refractory pneumonia,24,25 meningitis and encephalitis,26,27 bloodstream infections, which have increased the diagnostic rate of infectious diseases and provided strong support for precision treatment. The mNGS technology has also been successfully applied in the diagnosis of scrub typhus. In recent years, this technology has gained wide attention and application.28,29 Our findings in this study further support the clinical application of mNGS in the detection of Rickettsia.
20% of scrub typhus infections are accompanied by neurological features, potentially affecting the central or peripheral nervous system, and sometimes both simultaneously.30 This infection can lead to various diseases such as encephalitis, meningitis, immune-mediated brain diseases, and cerebrovascular diseases. The main neurological manifestations include headache, vomiting, sensory changes, seizures, abnormal reflexes, and focal motor deficits.31 In our case, the initial difficulty in diagnosis was due to the patient’s lack of insect bite history and the early antibiotic treatment masked other symptoms. The neurological manifestations were not as evident as respiratory inflammation, making diagnosis challenging through clinical examination. The pathogen could not be definitively identified until the completion of cerebrospinal fluid mNGS testing, providing a new approach for the clinical diagnosis of undifferentiated febrile neurological diseases. Early completion of mNGS testing enables early detection, diagnosis, and treatment, which is beneficial for improving the prognosis of patients’ diseases.
Current research indicates that Orientia tsutsugamushi primarily causes neurological symptoms through three main pathways, with the most common path involving invasion of endothelial cells to enter the central nervous system. Endothelial cells are the primary cellular target. The subsequent activation of endothelial cells leads to leukocyte adhesion and migration, platelet aggregation, and cytokine release. In the lungs, this uncontrolled activation leads to excessive infiltration of neutrophils and monocytes, triggering acute respiratory distress syndrome (ARDS).32 In the central nervous system, vasculitis induced by endothelial cell activation leads to various complications. The second pathway involves the direct entry of the pathogen into the cerebrospinal fluid, leading to meningitis and encephalitis. The third potential mechanism of neurological involvement is immune-mediated, due to Type 2 hypersensitivity against selfantigens. This explains certain delayed manifestations, such as optic neuritis, myelitis, Guillain-Barré syndrome (GBS), and transverse myelitis.30 The most common central nervous system manifestations include meningitis, meningoencephalitis, encephalitis, encephalopathy, and seizures. Less common manifestations include stroke, cerebellar involvement, hemiplegia, myelitis, cranial nerve lesions, Parkinson’s disease, acute disseminated encephalomyelitis (ADEM), hemorrhagic encephalitis, and myelitis. Peripheral nervous system involvement is less common, with only about 30 reported cases, mainly involving nerve plexus, including two cases of brachial plexus, primarily presenting as unilateral shoulder pain, muscle weakness, acute neuritis, peripheral neuropathy, and muscle involvement.32–34 Due to the diversity of its manifestations in neurological symptoms, finding an effective diagnostic method for scrub typhus is particularly important.
The research conducted by the Parimal Samir team35 indicates a significant upregulation of the IFN signaling pathway in the brain during days 6–10 of scrub typhus infection, consistent with findings from the Min team. On the 12th day of infection, an increase in IFNβ protein was observed in the plasma of mice.36 The IFN-I pathway is a double-edged sword as its high expression can effectively control the infection. However, its neurotoxic effects may result in neuronal damage leading to neurological diseases. Additionally, it hinders the central nervous system blood vessel repair after brain injury. Furthermore, IFN-γ not only directly inhibits infection but also induces immune cells such as Th1 and cytotoxic T cells to concentrate in the central nervous system, exerting a bactericidal effect. IFN-α can enhance its induction of immune cells synergistically with IFN-γ. According to recent research, this emphasizes an alternative approach to assist in the diagnosis of scrub typhus, and provides a theoretical basis for clinical diagnostic markers related to scrub typhus.
Based on the meta-analysis of treatment options for scrub typhus, tetracycline, doxycycline, azithromycin, and rifampin are effective treatment choices for the disease. Chloramphenicol may also be considered as a treatment option, but there is currently no clear consensus. For specific outcomes, low certainty evidence suggests that tetracycline, doxycycline, and azithromycin as treatment options may have little or no difference between them. Considering the low certainty evidence for rifampin and the potential risk of inducing drug resistance in undiagnosed tuberculosis, clinicians should not consider this drug as a first-line treatment choice, but rather as a second-line treatment option for scrub typhus excluding active tuberculosis.37 Our case resulted in a significant improvement with doxycycline treatment after the diagnosis of scrub typhus and Orientia tsutsugamushi encephalitis, which supports the above treatment plan.
Conclusion
Scrub typhus, as a rare disease, is difficult to diagnose in a timely manner through traditional diagnostic methods, especially in the absence of specific eschars. Metagenomic next-generation sequencing (mNGS) is a novel diagnostic technique that can accurately and rapidly identify scrub typhus, offering the potential to become an important tool in identifying the etiology of infectious diseases of unknown origin. Additionally, it is important to seek more specific biomarkers to support diagnosis. Due to the lack of effective vaccine prevention for scrub typhus and the propensity for severe neurological complications in cases without obvious eschars, early diagnosis of related diseases through mNGS is of high clinical significance in improving patient prognosis.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the Yun Wang or Yingying Xu on reasonable request. The datasets generated and/or analyzed during the current study are available in the SRA repository (SRA: PRJNA1061116).
Ethics Statement
The studies involving human participants were reviewed and approved by The Second Hospital of Shandong University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Salje J, Kline KA. Orientia tsutsugamushi: a neglected but fascinating obligate intracellular bacterial pathogen. PLoS Pathog. 2017;13(12):e1006657. doi:10.1371/journal.ppat.1006657
2. Wongsantichon J, Jaiyen Y, Dittrich S, Salje J. Orientia tsutsugamushi. Trends Microbiol. 2020;28(9):780–781. doi:10.1016/j.tim.2020.02.014
3. World health statistics 2023: monitoring health for the SDGs, sustainable development goals. Licence: CC BY-NC-SA 3.0 IGO. Geneva: World Health Organization; 2023. Available from: https://www.who.int/publications/i/item/9789240074323.
4. Zhang L, Zhao Z, Bi Z, et al. Risk factors associated with severe scrub typhus in Shandong, northern China. Int J Infect Dis. 2014;29:203–207. doi:10.1016/j.ijid.2014.09.019
5. Xin H, Sun J, Yu J, et al. Spatiotemporal and demographic characteristics of scrub typhus in Southwest China, 2006–2017: an analysis of population-based surveillance data. Transbound Emerg Dis. 2020;67(4):1585–1594. doi:10.1111/tbed.13492
6. Devasagayam E, Dayanand D, Kundu D, Kamath MS, Kirubakaran R, Varghese GM. The burden of scrub typhus in India: a systematic review. PLoS Negl Trop Dis. 2021;15(7):e0009619. doi:10.1371/journal.pntd.0009619
7. Fisher J, Card G, Soong L, Salje J. Neuroinflammation associated with scrub typhus and spotted fever group rickettsioses. PLoS Negl Trop Dis. 2020;14(10):e0008675. doi:10.1371/journal.pntd.0008675
8. Bonell A, Lubell Y, Newton PN, Crump JA, Paris DH, Foley J. Estimating the burden of scrub typhus: a systematic review. PLoS Negl Trop Dis. 2017;11(9):e0005838. doi:10.1371/journal.pntd.0005838
9. Taylor AJ, Paris DH, Newton PN, Walker DH. A systematic review of mortality from untreated scrub typhus (orientia tsutsugamushi). PLoS Negl Trop Dis. 2015;9(8):e0003971. doi:10.1371/journal.pntd.0003971
10. Ghosh R, Mandal A, Leon-Ruiz M, et al. Rare neurological and neuropsychiatric manifestations of scrub typhus: a case series of 10 cases. Neurologia. 2022. doi:10.1016/j.nrleng.2022.07.001
11. Venkatesan A, Michael BD, Probasco JC, Geocadin RG, Solomon T. Acute encephalitis in immunocompetent adults. Lancet. 2019;393(10172):702–716. doi:10.1016/S0140-6736(18)32526-1
12. Venkatesan A, Tunkel AR, Bloch KC, et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013;57(8):1114–1128. doi:10.1093/cid/cit458
13. Tyler KL, Ropper AH. Acute viral encephalitis. N Engl J Med. 2018;379(6):557–566. doi:10.1056/NEJMra1708714
14. Suzuki T, Kawada JI, Okuno Y, et al. Comprehensive detection of viruses in pediatric patients with acute liver failure using next-generation sequencing. J Clin Virol. 2017;96:67–72. doi:10.1016/j.jcv.2017.10.001
15. Varghese GM, Dayanand D, Gunasekaran K, et al. Intravenous doxycycline, azithromycin, or both for severe scrub typhus. N Engl J Med. 2023;388(9):792–803. doi:10.1056/NEJMoa2208449
16. John R, Varghese GM. Scrub typhus: a reemerging infection. Curr Opin Infect Dis. 2020;33(5):365–371. doi:10.1097/QCO.0000000000000664
17. Luce-Fedrow A, Lehman ML, Kelly DJ, et al. A review of scrub typhus (orientia tsutsugamushi and related organisms): then, now, and tomorrow. Trop Med Infect Dis. 2018;3(1):8.
18. Wu J, Wu Y, Huang M. Metagenomic next-generation sequencing helped diagnose scrub typhus without eschar: a case report. Int J Infect Dis. 2020;90:1–4. doi:10.1016/j.ijid.2019.10.020
19. Chen J, Zheng XD, Dai QH, et al. Diagnosis of severe scrub typhus infection by next-generation sequencing: a case report. BMC Infect Dis. 2020;20(1):270. doi:10.1186/s12879-020-04991-y
20. Liu X, Zhang Y, Zhang J, Lou Z, Xia H, Lu Z. The early diagnosis of scrub typhus by metagenomic next-generation sequencing. Front Public Health. 2021;9:755228. doi:10.3389/fpubh.2021.755228
21. Kala D, Gupta S, Nagraik R, Verma V, Thakur A, Kaushal A. Diagnosis of scrub typhus: recent advancements and challenges. 3 Biotech. 2020;10(9):396. doi:10.1007/s13205-020-02389-w
22. Goldberg B, Sichtig H, Geyer C, Ledeboer N, Weinstock GM. Making the leap from research laboratory to clinic: challenges and opportunities for next-generation sequencing in infectious disease diagnostics. mBio. 2015;6(6):e01888–e01815. doi:10.1128/mBio.01888-15
23. Forbes JD, Knox NC, Ronholm J, Pagotto F, Reimer A. Metagenomics: the next culture-independent game changer. Front Microbiol. 2017;8:1069. doi:10.3389/fmicb.2017.01069
24. Zhou H, Larkin PMK, Zhao D, et al. Clinical impact of metagenomic next-generation sequencing of bronchoalveolar lavage in the diagnosis and management of pneumonia: a Multicenter Prospective Observational Study. J Mol Diagn. 2021;23(10):1259–1268. doi:10.1016/j.jmoldx.2021.06.007
25. Tang J, Tan W, Luo L, Xu H, Li N. Application of metagenomic next-generation sequencing in the diagnosis of pneumonia caused by Chlamydia psittaci. Microbiol Spectr. 2022;10(4):e0238421. doi:10.1128/spectrum.02384-21
26. Piantadosi A, Mukerji SS, Ye S, et al. Enhanced virus detection and metagenomic sequencing in patients with meningitis and encephalitis. mBio. 2021;12(4):e0114321. doi:10.1128/mBio.01143-21
27. Kanaujia R, Biswal M, Angrup A, Ray P. Diagnostic accuracy of the metagenomic next-generation sequencing (mNGS) for detection of bacterial meningoencephalitis: a systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis. 2022;41(6):881–891. doi:10.1007/s10096-022-04445-0
28. Li J, Chen C, Zou F, et al. Diagnosing scrub typhus without eschar: a case report using metagenomic next-generation sequencing (mNGS). Ann Transl Med. 2021;9(14):1190. doi:10.21037/atm-21-3015
29. Sun CB, Ma Z, Liu Z. Case report: optic neuritis as the initial presentation of Orientia tsutsugamushi infection detected by metagenomic next-generation sequencing. Front Immunol. 2023;14:1129246. doi:10.3389/fimmu.2023.1129246
30. Garg D, Manesh A. Neurological facets of scrub typhus: a comprehensive narrative review. Ann Indian Acad Neurol. 2021;24(6):849–864. doi:10.4103/aian.aian_739_21
31. Misra UK, Kalita J, Mani VE. Neurological manifestations of scrub typhus. J Neurol Neurosurg Psychiatry. 2015;86(7):761–766. doi:10.1136/jnnp-2014-308722
32. Trent B, Fisher J, Soong L. Scrub Typhus pathogenesis: innate immune response and lung injury during Orientia tsutsugamushi infection. Front Microbiol. 2019;10:2065. doi:10.3389/fmicb.2019.02065
33. Alam AM, Gillespie CS, Goodall J, et al. Neurological manifestations of scrub typhus infection: a systematic review and meta-analysis of clinical features and case fatality. PLoS Negl Trop Dis. 2022;16(11):e0010952. doi:10.1371/journal.pntd.0010952
34. Diaz FE, Abarca K, Kalergis AM. An update on host-pathogen interplay and modulation of immune responses during Orientia tsutsugamushi infection. Clin Microbiol Rev. 2018;31(2). doi:10.1128/CMR.00076-17
35. Liang Y, Aditi FN, Onyoni F, et al. Brain transcriptomics reveal the activation of neuroinflammation pathways during acute Orientia tsutsugamushi infection in mice. Front Immunol. 2023;14:1194881. doi:10.3389/fimmu.2023.1194881
36. Min CK, Kim HI, Ha NY, et al. A type I interferon and IL-10 induced by Orientia tsutsugamushi infection suppresses antigen-specific T cells and their memory responses. Front Immunol. 2018;9:2022. doi:10.3389/fimmu.2018.02022
37. El Sayed I, Liu Q, Wee I, Hine P. Antibiotics for treating scrub typhus. Cochrane Database Syst Rev. 2018;9(9):CD002150. doi:10.1002/14651858.CD002150.pub2
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.