Back to Journals » International Journal of Women's Health » Volume 16
Case Report: A Rare Case of Primary Angiosarcoma of the Cervix with a Literature Review
Authors Song Y, Li R, Wang L , Wang H
Received 11 September 2023
Accepted for publication 22 January 2024
Published 12 February 2024 Volume 2024:16 Pages 265—271
DOI https://doi.org/10.2147/IJWH.S439583
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Yuelin Song,1,2,* Ruizhe Li,1,2,* Lifei Wang,3 Hongjing Wang1,2
1Department of Obstetrics and Gynecology, West China Second University Hospital of Sichuan University, Chengdu, People’s Republic of China; 2Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, People’s Republic of China; 3Department of Obstetrics and Gynecology, The Fourth Affiliated Hospital Zhejiang University School of Medicine, Yiwu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hongjing Wang, West China Second University Hospital of Sichuan University, No. 20, Section 3, Renmin South Road, Wuhou District, Chengdu, Sichuan Province, 610041, People’s Republic of China, Tel +86 181 8060 9060, Email [email protected]
Abstract: Primary angiosarcomas are a rare type of soft-tissue sarcomas that originate from endothelial cells. These sarcomas can develop in any part of the body and have a poor prognosis. However, they are commonly found in the skin of elderly white men, particularly on the scalp and head region. Primary angiosarcoma of the cervix is exceptionally rare. To date, only two cases of this disease have been reported worldwide. The diagnosis of the disease is difficult microscopically, requiring immunohistochemistry and genetic testing to distinguish. We report a recent case, in which the lesion was preoperatively considered a high-grade endometrial stromal sarcoma. A 35-year-old woman presented with vaginal bleeding and cervical erosions. A high-grade endometrial stromal sarcoma involving the cervix was considered and a modified radical hysterectomy was performed with bilateral salpingo-oophorectomy and sentinel lymph nodes resection. The gene diagnosis performed by fluorescence in situ hybridization for YWHAE translocation fusion was negative excluding a YWHAE-translocated high-grade endometrial stromal sarcoma. A primary angiosarcoma of the cervix was finally diagnosed. Primary angiosarcoma of the cervix is rare, and gynecologic pathologists do not know it well, so it is easy to be wrongly considered. Immunohistochemistry and genetic testing help confirm the diagnosis.
Keywords: angiosarcoma, case report, cervix, genetic testing, immunohistochemistry
Introduction
Angiosarcoma is a rare type of malignant soft tissue neoplasm that is highly aggressive and endothelial originated, accounting for less than 1% of all soft tissue sarcomas.1 Radiation exposure, chronic lymphedema, exotoxin, and family cancer syndrome are generally recognized as the most important risk factors associated with angiosarcoma.1 It typically occurs in older white men, mainly affects the skin of the head and neck region, and commonly presents as a bruise or papule. Soft tissues, breast, liver, spleen, and bone may also be affected. The diagnosis of angiosarcoma is difficult since its pathological features vary greatly. Immunohistochemical (IHC) analyses and genetic testing may help in confirming the diagnosis. Surgery with wide resection margins is commonly considered the first choice of treatment. Adjuvant radiotherapy with large dose and wide field is also recommended to reduce the recurrent risks.2,3 Nevertheless, the rates of local recurrence and metastasis of angiosarcoma are still high, with a generally poor prognosis and a median survival of 7 months.4
Naturally originated from vascular endothelial cells, angiosarcoma may occur in any tissue or organ theoretically. However, primary angiosarcoma originating from the uterine cervix is rare. We utilized PubMed, Google Scholar, Embase, and Web of Science as primary search engines, and included MESH terms such as “Cervix Neoplasms”, “Angiosarcoma”, and “Primary Neoplasms” in combination with relevant keywords to enhance the search. Only two cases of primary angiosarcoma of the cervix have been reported in previous literature, as far as we know.5,6 Here we report another case, in which the lesion was considered a high-grade endometrial stromal sarcoma preoperatively.
Case Presentation
A 35-year-old woman presented with vaginal bleeding for 6 months and erosions on the vaginal surface of the cervix. No other symptoms or signs complained. She had a history of abortion, and her mother suffered from ovarian cancer. She had no history of childbirth, exposure to radiations or chemicals, malignancy, usage of intrauterine devices or other surgeries. The outpatient pap test and human papillomavirus (HPV) test were negative. Colposcopy showed a type 2 transformation zone and an ulcer on the posterior lip, suspected to be a low-grade squamous intraepithelial lesion.
The patient received a cervical biopsy and a loop electrosurgical excision procedure (LEEP) of the cervix at the outpatient. Pathology indicated the lesions were spindle cell malignancies, which tended to be high-grade endometrial stromal sarcoma (HG-ESS). Pelvic magnetic resonance imaging (MRI) showed an abnormal signal in the left anterior wall of the uterus. A fluorodeoxyglucose F18 systematic positron emission tomography/computed tomography (18F-FDG PET/CT) scanning revealed a 1.6cm×1.1cm×0.9cm nodule-like area with high radioactive intake in the posterior wall of the uterine cervix and a 1.0cm×0.8cm piece-like area with high radioactive intake in the left anterior wall of the uterus, indicating neoplastic lesions. Both adnexa were normal, and no abnormal lymph node was noticed.
Combined with clinical features, pathologic findings, and imaging characteristics of the patient, it was considered that the lesions might be an endometrial stromal sarcoma involving the uterine cervix. The patient was then admitted. The rest laboratory and imaging examinations of the patient were all normal, including the serum tumor markers. No additional abnormality was found in the physical examination.
Based on the above conditions, our preoperative diagnosis was a malignant spindle cell tumor of the cervix and an endometrial stromal sarcoma could not be excluded. Thus, a modified radical hysterectomy was done with bilateral salpingo-oophorectomy and sentinel lymph nodes resection. The intraoperative rapid frozen section indicated that no metastatic tumor cell was observed in the resected lymph nodes. Surprisingly, integrated with IHC analyses and genetic testing, the postoperative pathological examination reported a primary angiosarcoma of the cervix with leiomyoma of the uterus. This unexpected postoperative pathological diagnosis triggered our profound reflection.
The initial cervical biopsy was performed on the posterior lip (6 to 8 o`clock position), the anterior lip (12 o’clock position) and the cervical canal. The specimen of the posterior lip showed spindle cell malignancies. IHC positive staining was detected for FH, cyclinD1 P53, P16, CD10, Vim and Ki67 proliferation index was 25%. Staining for the other markers including SMA, calponin, Des, CK-P, EMA and caldesmon were all negative. The specimens of the anterior lip and the cervical canal found no tumor cells.
The cervical specimen after LEEP showed spindle cell malignancies with necrosis and ulceration. IHC analyses showed CD10, cyclinD1, SMA, Des, calponin and BRg1 were all positive. Ki67 proliferation index was 40–50%. Caldesmon, BCOR, TRK, ALK, CD117, CD34, β-catenin and S-100 were all negative.
The uterus was dissected after the hysterectomy. Grossly, the uterine cervix was enlarged with surgical scars. A mass 1cm in diameter could be seen at the left anterior wall of the uterus. The endometrium and the cervical canal seemed normal (Figure 1). No gross abnormality was seen in the vagina or adnexa. Microscopically, the tumor is limited to the uterine cervix. Spindle cell malignancy of the uterine cervix (Figure 2A, B). IHC analyses showed hormone receptors were both negative. Myogenin, CAM5.2, E-cadherin and D2-40 were negative. CD34, CD31, ERG, CK7 and Fil-1 were positive.
 |
Figure 1 Grossly, the uterine presented a spongy appearance with and necrosis and ulceration. |
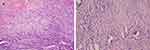 |
Figure 2 The tumor cells show vascular differentiation containing erythrocytes and mitosis (A)×40, (B)×100). |
Combining all immunohistochemical staining outcomes together, the negative staining with hormone receptors, the negative staining of Caldesmon and BCOR plus the focally positive staining of CD10 and cyclin D1 could be helpful to exclude the possibility of leiomyosarcoma and HG-ESS. The gene diagnosis outcome by fluorescence in situ hybridization (FISH) showed YWHAE translocation, always detected in HG-ESS, was negative (Figure 3). The FOS/FOSB fusion also was negative excluded the possible diagnosis of pseudomyogenic hemangioendothelioma (PMH).
 |
Figure 3 FISH showed YWHAE translocation proportion was 7 percent (≥30% is defined as positive). |
Taking all these results together, the final diagnosis of the patient was a FIGO (The International Federation of Gynecology and Obstetrics) stage IB1 primary angiosarcoma of the cervix (2018 FIGO staging classification for cervical cancer). The patient was then referred to oncologists for consideration of adjuvant radiotherapy. She suffered from postoperative urinary retention, and the urethral catheter was successfully removed 10 days after surgery. We followed up with the patient for 3 months. Her general condition was fine without obvious abnormality in laboratory and imaging examinations. Further treatment and monitoring were ongoing.
Discussion
Angiosarcoma is a rare type of cancer that forms in the lining of the blood vessels and lymph vessels, which may occur at any place in the body.1 It generally occurs in the skin of the head and neck. It rarely forms in the skin of other parts of the body, such as the breast, or in deeper tissues, such as the liver, the spleen, and the heart.1 Patients with angiosarcoma originating from female reproductive organs are extremely rare, of whom most had a history of radiotherapy for the treatment of gynecologic tumors. To our knowledge, only 80 cases of primary angiosarcoma of female internal reproductive organs have been reported to date. Among them, only two cases reported primary angiosarcoma of the uterine cervix, in which the tumor arises from the vascular linings of the cervix without risk factors including radiotherapy.5,7
Since the literature is limited, we have little knowledge of the primary angiosarcoma of the cervix. Previous cases are summarized in Table 1. By reviewing the case reports, we found that the clinical characteristics of primary angiosarcoma of the cervix varied. Combined with our experience, all the patients presented with vaginal bleeding or discharge as the main symptom, which was similar to the common types of cervical cancer. Besides, the cervical smear is insensitive to discovering endothelial-originated tumor cells. Imaging examinations are also nonspecific. The diagnosis requires cervical biopsies.
 |
Table 1 Summary of Cases with Primary Angiosarcoma of the Uterine Cervix |
Angiosarcoma grows infiltratively and lacks a capsule or a distinct boundary separating malignant tissues from normal tissues. Under light microscopy, angiosarcoma could be recognized as abnormal, pleomorphic, and malignant endothelium cells that could be spherical, polygonal, fusiform, or even epithelioid in shape.1 It could grow along with pre-existing vascular channels, and sinusoidal or cavernous areas.8 In high-grade lesions, the aberrant cells grow multi-layered and create papillary-like projections into the arterial lumen rather than forming a single endothelial-cell lining. They seem to have high mitotic and atypical cell rates, which is difficult to tell from melanoma.1
Immunohistochemically, angiosarcoma arises from vascular endothelial cells and expresses endothelial markers such as von Willebrand factor, CD31, and CD34.9 In addition, Ulex europaeus agglutinin 1, and vascular endothelial growth factor (VEGF) may also be expressed.1,10–12 The case reported by Shah et al showed a malignant vascular tumor made up of spindled and epithelioid cells, which formed vascular channels during the tumor’s development. Additionally, tumor cells showed mitotically active and had necrotic foci. Immunohistochemistry showed diffuse cytoplasmic positivity for CD31 and CD34 in tumor cells.5 In our case, we also performed CD31 and CD34 and IHC staining outcomes were positive in tumor cells.
HG-ESS is a necessary differential diagnosis. In our case, the preoperative diagnosis of the patient was an HG-ESS involving the uterine cervix. HG-ESS and cervical angiosarcoma have similar clinical signs and symptoms. Patients of HG-ESS usually present with abnormal vaginal bleeding, enlarged uterus, or pelvic mass.13 Immunohistochemical phenotype and molecular features of HG-ESS can give clinicians and pathologists some hints. Microscopically, HG-ESS mainly consists of high-grade round cells occasionally with a low-grade spindle cell component and mitotic active (10 per 10 HPF).13 Usually, necrosis is evident.14 HG-ESS shows CD10, estrogen receptor, and progesterone receptor negative but strong diffuse cyclin D1 immunoreactivity (>70% nuclei). Strong and diffuse BCOR IHC expression also is highly suggestive of HGESS.15 HG-ESS typically contains YWHAE-NUTM2 fusion as a result of t(10; 17) (q22; p13) and it can help the differential diagnosis to other gynecologic sarcomas.13 PMH always contains FOS/FOSB fusion and it can help to exclude the possible diagnosis of pseudomyogenic hemangioendothelioma (PMH).16
The treatment of primary cervical angiosarcoma lacks evidence-based guidelines. Any combination of surgery, chemotherapy and radiotherapy might be used to treat the disease. For localized angiosarcoma, radical surgery with complete resection is the primary treatment of choice. Since angiosarcoma presents aggressive and multifocal, negative margins are hard to achieve and wide margins are recommended.3,17,18 Thus, a radical hysterectomy, which cut margins wide enough, might be the first choice to treat primary cervical angiosarcoma. Since previous cases reported the disease’s lymph node metastasis, a pelvic lymphadenectomy might be necessary for comprehensive evaluation.19 In our case, a modified radical hysterectomy with bilateral salpingo-oophorectomy and sentinel lymph nodes resection was performed. We noticed a potentially inadequate surgical margin since the lesion was considered a high-grade endometrial stromal sarcoma preoperatively. Postoperative adjuvant radiotherapy was thus suggested for the patient, partially to compensate for the effect of the potentially inadequate surgical area. According to previous studies, radiotherapy for patients with narrow margins or concerning pathological features may improve long-term survival. Some studies recommended that postoperative radiation with large doses (>50 Gy) and wide treatment fields could be applied routinely.2,3 As for adjuvant chemotherapy, anthracyclines, ifosfamide, and taxanes have been used as adjuvant chemotherapy regimens to treat angiosarcoma and seemed beneficial.17,20–26 The patient in the previous case received weekly paclitaxel without radiotherapy.5 However, evaluated by oncologists, adjuvant chemotherapy was not applied to our patient.
In conclusion, primary angiosarcoma of the cervix is an extremely rare type of cancer that requires careful diagnosis and treatment. Vaginal bleeding is the main symptom, but a Pap test is not effective in screening for this disease. A cervical biopsy and appropriate immunohistochemical markers, such as CD31, are essential for accurate diagnosis. Differential diagnosis from other gynecologic sarcomas, such as HG-ESS and PMH, is crucial to ensure proper treatment. Surgical resection with wide margins is the primary treatment option, and adjuvant radiotherapy and chemotherapy may be considered based on individual cases. It is important for medical practitioners to be aware of this rare pathological type of cervical neoplasm and to differentiate it from other soft tissue sarcomas, particularly HG-ESS. Further research and studies are needed to establish evidence-based guidelines for the management of primary cervical angiosarcoma.
Conclusion
Based on the presented case and available literature, it is recommended that gynecologic oncologists and pathologists familiarize themselves with the clinical characteristics and diagnostic methods of primary angiosarcoma of the cervix. Improved awareness and understanding of this rare disease will facilitate early detection, accurate diagnosis, and appropriate treatment. Further research should be conducted to develop evidence-based guidelines for the management of this condition, considering the limited number of reported cases and the lack of standardized treatment approaches. Collaboration among medical professionals, including surgeons, radiation oncologists, and medical oncologists, is crucial to providing comprehensive and individualized care to patients with primary cervical angiosarcoma.
Ethics Statement
This research was reviewed and approved by the Ethics Committee for Research in Human Beings of West China Second University Hospital of Sichuan University. The patient provided her written informed consent for participation in this study and publication of the case.
Acknowledgments
This work was supported by the Chengdu Technology Innovation research and development project [grant numbers 2022-YF05-01690-SN]; and the Clinical Research Foundation of West China Second Hospital [grant number KL113].
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Young RJ, Brown NJ, Reed MW, Hughes D, Woll PJ. Angiosarcoma. Lancet Oncol. 2010;11(10):983–991. doi:10.1016/S1470-2045(10)70023-1
2. Mark RJ, Poen JC, Tran LM, Fu YS, Juillard GFA. A report of 67 patients and a review of the literature. Cancer. 1996;77(11):2400–2406. doi:10.1002/(SICI)1097-0142(19960601)77:11<2400::AID-CNCR32>3.0.CO;2-Z
3. Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98(8):1716–1726. doi:10.1002/cncr.11667
4. Yang OO, Lan T, He JL, et al. Magnetic resonance imaging and contrast-enhanced ultrasound findings of a recurrent primary breast angiosarcoma: a case report. Medicine. 2021;100(5):e24625. doi:10.1097/MD.0000000000024625
5. Shah VI, Gl R, Thompson IW, Sumathi VP, McCluggage WG. Primary angiosarcoma of the cervix: case report of a rare lesion. Int J Gynecol Pathol. 2020;39(1):97–102. doi:10.1097/PGP.0000000000000567
6. Chinczewski L, Taube ET, Feldhaus FW, et al. Angiosarcomas of primary gynecologic origin - a case series and review of the literature. Anticancer Res. 2020;40(10):5743–5750. doi:10.21873/anticanres.14590
7. Ohayi SA, Ezugwu EC, Aderibigbe AS, Udeh EI. Angiosarcoma of the cervix: a case and literature review. Niger J Med. 2013;22(4):362–364.
8. Gaballah AH, Jensen CT, Palmquist S, et al. Angiosarcoma: clinical and imaging features from head to toe. Br J Radiol. 2017;90(1075):20170039. doi:10.1259/bjr.20170039
9. Ohsawa M, Naka N, Tomita Y, Kawamori D, Kanno H, Aozasa K. Use of immunohistochemical procedures in diagnosing angiosarcoma. evaluation of 98 cases. Cancer. 1995;75(12):2867–2874. doi:10.1002/1097-0142(19950615)75:12<2867::aid-cncr2820751212>3.0.co;2-8
10. Itakura E, Yamamoto H, Oda Y, Tsuneyoshi M. Detection and characterization of vascular endothelial growth factors and their receptors in a series of angiosarcomas. J Surg Oncol. 2008;97(1):74–81. doi:10.1002/jso.20766
11. Hashimoto M, Ohsawa M, Ohnishi A, et al. Expression of vascular endothelial growth factor and its receptor mRNA in angiosarcoma. Lab Invest. 1995;73(6):859–863.
12. Fujimoto M, Kiyosawa T, Murata S, et al. Vascular endothelial growth factor in angiosarcoma. Anticancer Res. 1998;18(5B):3725.
13. Lee CH, Marino-Enriquez A, Ou W, et al. The clinicopathologic features of YWHAE-FAM22 endometrial stromal sarcomas: a histologically high-grade and clinically aggressive tumor. Am J Surg Pathol. 2012;36(5):641–653. doi:10.1097/PAS.0b013e31824a7b1a
14. Mbatani N, Olawaiye AB, Prat J. Uterine sarcomas. Int J Gynaecol Obstet. 2018;143(Suppl 2):51–58. doi:10.1002/ijgo.12613
15. Alkanat NE, Uner A, Usubutun A. High-grade endometrial stromal sarcoma: morphologic and clinical features, the role of immunohistochemistry and fluorescence in situ hybridization in diagnosis. Int J Surg Pathol. 2022;10668969221098087. doi:10.1177/10668969221098087
16. Hung YP, Fletcher CD, Hornick JL. FOSB is a useful diagnostic marker for pseudomyogenic hemangioendothelioma. Am J Surg Pathol. 2017;41(5):596–606. doi:10.1097/PAS.0000000000000795
17. Fury MG, Antonescu CR, Van Zee KJ, Brennan MF, Maki RG. A 14-year retrospective review of angiosarcoma: clinical characteristics, prognostic factors, and treatment outcomes with surgery and chemotherapy. Cancer J. 2005;11(3):241–247. doi:10.1097/00130404-200505000-00011
18. Abraham JA, Hornicek FJ, Kaufman AM, et al. Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol. 2007;14(6):1953–1967. doi:10.1245/s10434-006-9335-y
19. Liu H, Zhang H, Zhang C, et al. Pan-soft tissue sarcoma analysis of the incidence, survival, and metastasis: a population-based study focusing on distant metastasis and lymph node metastasis. Front Oncol. 2022;12:890040. doi:10.3389/fonc.2022.890040
20. Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data; Sarcoma meta-analysis collaboration. Lancet. 1997;350(9092):1647–1654.
21. Schlemmer M, Reichardt P, Verweij J, et al. Paclitaxel in patients with advanced angiosarcomas of soft tissue: a retrospective study of the EORTC soft tissue and bone sarcoma group. Eur J Cancer. 2008;44(16):2433–2436. doi:10.1016/j.ejca.2008.07.037
22. Budd GT. Management of angiosarcoma. Curr Oncol Rep. 2002;4(6):515–519. doi:10.1007/s11912-002-0066-3
23. Penel N, Bui BN, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol. 2008;26(32):5269–5274. doi:10.1200/JCO.2008.17.3146
24. Lahat G, Dhuka AR, Lahat S, et al. Outcome of locally recurrent and metastatic angiosarcoma. Ann Surg Oncol. 2009;16(9):2502–2509. doi:10.1245/s10434-009-0569-3
25. Asmane I, Litique V, Heymann S, et al. Adriamycin, cisplatin, ifosfamide and paclitaxel combination as front-line chemotherapy for locally advanced and metastatic angiosarcoma. Analysis of three case reports and review of the literature. Anticancer Res. 2008;28(5B):3041.
26. Skubitz KM, Haddad PA. Paclitaxel and pegylated-liposomal doxorubicin are both active in angiosarcoma. Cancer. 2005;104(2):361–366. doi:10.1002/cncr.21140
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
