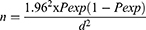Back to Journals » Infection and Drug Resistance » Volume 14
Carriage, Risk Factors, and Antimicrobial Resistance Patterns of Salmonella Isolates from Raw Beef in Jimma, Southwestern Ethiopia
Received 9 April 2021
Accepted for publication 4 June 2021
Published 24 June 2021 Volume 2021:14 Pages 2349—2360
DOI https://doi.org/10.2147/IDR.S313485
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Minda Asfaw Geresu,1 Wondimu Zeleke Desta2
1Department of Veterinary Science, College of Agriculture and Environmental Science, Arsi University, Asella, Ethiopia; 2School of Veterinary Medicine, College of Agriculture and Veterinary Medicine, Jimma University, Jimma, Ethiopia
Correspondence: Minda Asfaw Geresu
Department of Veterinary Science, College of Agriculture and Environmental Science, Arsi University, PO Box 193, Asella, Ethiopia
Tel +251910431505
Email [email protected]
Purpose: Consumption of raw beef infected with multidrug-resistant Salmonella is pertinent to the world public health risk of antimicrobial resistance. Henceforth, this study aimed to investigate the carriage, antimicrobial resistance (AR) patterns, and the revealing risk factors of Salmonella-contaminating beef in abattoirs and butcher shops in Jimma town.
Methods: A cross-sectional study was conducted to investigate the carriage and AR patterns and to reveal the risk factors of beef contaminated by Salmonella spp. Three hundred and forty-eight swab samples were collected from abattoirs (n=210) and butcher shops (n=138) and the conventional cultural methods were employed for identification of Salmonella. Isolates were subjected to 12 antimicrobials using the Kirby–Bauer disk diffusion method for AR patterns.
Results: The occurrence of Salmonella isolates from the abattoir samples was 11.4%, whereas about 6.52% of isolates were recovered from butcher shops. Educational status (abattoir: odds ratio (OR)=8.40, confidence interval (CI)=1.186– 59.493; butcher shops: OR=9.17, CI=1.15– 73.239), job related training (abattoir: OR=5.50, CI=1.065– 28.416), contamination risk perception (abattoir: OR=5.31, CI=1.256– 22.489), neatness of knives (abattoir: OR=7.6, CI=0.892– 65.376), source of contamination (abattoir: OR=8.44, CI=1.682– 42.39), wearing of protective cloth (butcher shops: OR=8.44, CI=1.682– 42.39), manner of hand washing (butcher shops: OR=7.25, CI=1.210– 43.442), and money handling (butcher shops: OR= 9.69, CI=1.578– 59.474) were among the potential risk factors significantly associated with Salmonella carcass contamination in the abattoir and butcher shops. Of the 33 Salmonella isolates, 14 (58.3%) and six (66.7%) of the abattoir and butcher shops isolates, correspondingly, were resistant to two or more antibiotics.
Conclusion: The finding of this investigation exhibited extensive multidrug-resistant Salmonella isolates in the study setting. Hence, establishing standard meat safety requirements and provision of training for meat handlers and prudent use of antimicrobials are recommended.
Keywords: abattoir, antimicrobial resistance, beef, butcher shop, Jimma, Salmonella
Introduction
Salmonella is one of the foremost causes of foodborne disease epidemics worldwide.1,2 Salmonella spp. are the prominent bacterial pathogens amid other food-borne pathogens and are responsible for instigating gastroenteritis in humans.3 Infections caused by Salmonella spp. in farm animals has been documented as the leading cause of considerable economic losses worldwide.4,5 Globally, about 93.8 million cases and 155,000 deaths are associated with gastroenteritis due to Salmonella species every year.6 Reports revealed that about 85.6% were estimated to be food borne, and infection was associated with many different food types, including beef and beef products.7
Studies revealed that contaminated feeds, transportation of animals to the abattoir, the slaughtering operation, and edible organs being contaminated by fecal storage, distribution, and preparation for consumption may contribute to Salmonella contamination of raw meat.8 Uncleanness of equipment, utensils, and personal hygiene of food handlers aid in the spread of Salmonella.9
Due to the habit of consuming raw animal origin food, the infection rate of food-borne Salmonella has been augmented intensely in Ethiopia during the past few years. Though the extent of food-borne illness and its severity has been known much more, effort has not been made to overcome the problem.8 Investigations carried out in different corners of the country have demonstrated the occurrence of Salmonella in diverse food animals and food products.6,8,10,11
Though the above studies revealed that Salmonella spp. are the leading food-borne pathogens that extremely overwhelm the economic growth of the country, there are only a few small-scale reports on the bacteriological quality, sanitary conditions, and practices in abattoirs and retailer (butcher) shops of beef meat in Jimma Zone.
Therefore, the absence of a comprehensive recent study on the carriage and antimicrobial resistance patterns of Salmonella isolates from raw beef in Jimma municipal abattoir and meat retailer (butcher) shops multifaceted with increased consumption of raw/minced meat (locally known as “kitfo” and “Kurt”) by the community at large entails isolation, identification, antimicrobial profile characterization, hygienic practices of the abattoir and butcher shop workers/consumers, and concomitant putative risk factors exposing to Salmonella food borne infections in the study setting.
Materials and Methods
Study Site
This study was carried out in Jimma municipal abattoir and meat retailers (butcher) shops from September 2016 to July 2017. One municipal abattoir and 74 meat retailer (butcher) shops were located in Jimma town, 352 km southwest of Addis Ababa, which directly receive a slaughter service from the abattoir.6 Yet, almost all of the residents practiced illegitimate backyard slaughtering of animals in the zone. Daily about 30–50 cattle, 10–25 sheep, and 5–10 goats which originated from different areas of the zone were slaughtered in the abattoir. In the town municipal abattoir, ante-mortem and post-mortem inspection was carried out by veterinarians, whereas the rest of the activities were performed by technicians.
Population
All cattle in the abattoir ready for slaughtering were the source of the population. The target study population were comprised of all ages and sexes of beef cattle slaughtered in Jimma town municipal abattoir and those meat cutters in the butcher houses of the town, and beef meat consumers.
Study Design
A cross-sectional study supported by questionnaire survey was carried out to investigate the carriage of Salmonella isolates and their antimicrobial resistance patterns and to assess the knowledge gaps of abattoir workers and meat handlers. Carcass samples from municipal abattoir and retailer (butcher) shops were selected by simple random sampling. Microbiological sample analysis was undertaken to isolate and characterize Salmonella spp. as per standards set in ISO-17604.12
Information pertaining to demographic data (educational status and sex), predisposing factors for salmonellosis such as hygiene (hand washing, knives, and equipment), knowledge and attitude of abattoir workers, retailers and consumer of raw beef were collected by using a pretested, structured, and self-administered questionnaire.
Sample Size Determination
A single population proportion formula developed by Thrusfield13 was used to determine the sample size to be collected from the abattoir and retailer (butcher) shops.
where 1.962 is the z statistic for level of confidence, n is the required sample size, Pexp is the expected prevalence, and d is the desired absolute precision.
Sample size calculation was based on the pooled expected prevalence of 4.53% beef carcass from the abattoir in different parts of the country.14 Hence, by using 4.53% as expected prevalence, the minimum calculated sample size was 67.
However, in order to increase the precision of the estimate, the sample size was inflated by 5% so 70 samples (carcass, workers hand, and knife samples) were collected from the abattoir.
The sample size to be collected from the retailer (butcher) shops was 118 using pooled expected prevalence of 8.34%.14 Accordingly, the sample size was inflated by 5% so as to increase the precision of the estimate, the sample size for retailer (butcher) shops was 124. Nevertheless, there are only 74 retailer (butcher) shops in Jimma town which were lower than the calculated sample size for the study. In order to solve this inconsistency, finite population correction factor (FPC factor) formula was used to adjust the sample size to the population size. Since the original required sample size was 124, adjustment of the sample size to the population size was made as per the correction formula and the sample size was calculated as follows.
Adjusted sample size for retailer (butcher) shops was 46, where  is the adjusted sample size,
is the adjusted sample size, 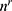 is the original required sample size, and N is the population size.15
is the original required sample size, and N is the population size.15
Sampling Strategy and Salmonella Isolation
A total of 210 municipal abattoir swab samples (carcass, n=70; workers hand, n=70; and knife, n=70) and 138 retailer (butcher) shops swab samples (carcass (meat for sale), n=46; workers hand, n=46; and chopping board, n=46) were collected for conventional bacteriological culturing.
About 100 cm2 of beef carcass surface around the neck, brisket, fore rib, flank, and rump was swabbed by wiping the cotton swabs on each sampling site, over the delineated area horizontally and then vertically several times, whereas meat cutting equipment (knives) and workers hand in contact with the meat in the abattoir were swabbed from a 15–20 m2 surface of the knives and workers hand using a sterile surgical glove. Then, the samples were kept in separate sterile plastic bags (Seward, England) to prevent spilling and cross-contamination.
Butcher (retailer) shops swab samples were collected from the surface of the carcass, meat chopping boards, and hands of the meat cutter. The swab cotton was moistened with sterile buffered peptone water (BPW) (Oxoid) and rubbed firmly across the exposed area of the sample source several times in all directions and introduced into a test tube containing 10 mL diluents of BPW and shaken vigorously.16 Finally, all samples were labeled for identification, kept in an ice-box at 4°C and 1 hour after collection, transported to the Veterinary Microbiology Laboratory of School of Veterinary Medicine of Jimma University. Detection and phenotypic characterization of Salmonella isolates from raw beef sample was performed according to the standard culture technique (ISO-6579).12 And then, briefly, the following isolation activities were carried out.
Pre-Enrichment and Selective Enrichment
For isolation and identification of Salmonella spp. the swab samples were pre-enriched in an appropriate amount of BPW and incubated at 37°C for 24 hours. One hundred microliters of the pre‐enriched culture was transferred to 10 mL of Rappaport‐Vassiliadis Enrichment Broth (RVB), (Oxoid) and incubated for 24 hours at 42°C. Another1 mL of the suspension was also transferred to 10 mL of Selenite F broth (SFB) (Oxoid) and incubated for 24 hours at 37°C.
Plating Out and Identification
A loop full of inoculums from each RVB and SFB cultures were plated onto Xylose lysine desoxycholate (XLD) (Oxoid) and Brilliant green (BGA) (Oxoid) agar plates and incubated at 37°C for 24 hours for identification. Characteristic red colonies with black centers on XLD and pink colonies on BGA plates were examined and considered as presumptive Salmonella colonies. Five typical colonies from both XLD and BGA were streaked onto the surface of pre-dried nutrient agar plates (Oxoid) and incubated at 37°C for 24 hours.
Then, typical colonies from nutrient agar were inoculated into the following biochemical tests for further identification as per ISO-6579 guidelines12 and incubated for 24 hours at 37°C. The biochemical tests conducted include triple sugar iron (TSI) agar (Hi Media), lysine iron agar (Hi Media), Simmon’s citrate agar (Hi Media), urea broth (Hi Media), and indole reaction (motility indole ornithine, MIO) medium (Pronadisa)
Antimicrobial Resistance Pattern Testing
Salmonella isolates susceptibility to a panel of antimicrobials were performed according to the Clinical and Laboratory Standards Institute (CSLI) guidelines17 using Kirby–Bauer disk diffusion method on Muller–Hinton agar plates (Oxoid). Antimicrobial disks used in this study were all from Sensi‐Discs, Becton, Dickinson and Company and they were the following,with their disc potencies (μg): ampicillin (10), cefoxitin (30), chloramphenicol (30), ciprofloxacin (5), gentamicin (10), kanamycin (30), nalidixic acid (30), neomycin (30), norfloxacillin (10), streptomycin (10), trimethoprim (5), and tetracycline (30). The quality control organism used to carry out the antimicrobial susceptibility test was Escherichia coli ATCC 25922 and the interpretation of the susceptibility test result was based on the CLSI guidelines.18
Data Analysis
Microbiological and questionnaire survey data were analyzed using SPSS version 20 software. The prevalence of Salmonella isolates in raw beef of abattoir and retailer (butcher) shops was calculated by dividing the frequency of positive samples by the total number of samples examined. A Chi-square Fisher exact test was used to compare the occurrence of Salmonella in both abattoir and retailer (butcher) shops of the study setting. Data from the questionnaire were used to assess the association of the risk factors with Salmonella identification. A univariate analysis (Chi-square Fisher exact test) was conducted using the Salmonella status of the abattoir and butcher (retailer) shops as the outcome variable. A multivariable analysis was performed to relate the likely risk factors to Salmonella outcomes (presence or absence of the isolate) in samples and abattoir or butcher shops. Finally, associations were reported as being statistically significant whenever the p‐value was <0.05.
Results
Identification of Salmonella by Conventional Culture
In the current study, out of 210 and 138 swab samples from the abattoir and retailer (butcher) shops, correspondingly, the overall prevalence of Salmonella positive swab samples from the abattoir was 11.43% (24/210), whilst it was 6.52% (9/138) from butcher shops. Of the abattoir samples, the highest were obtained from carcasses compared to the isolates obtained from knives (11.43%, 8/70) and workers hands (8.57%, 6/70), whereas out of the nine isolates from retailer (butcher) shops, 8.7% (4/46), 6.52% (3/46), and 4.35% (2/46) were recovered from the carcass, chopping boards, and workers hands, respectively, as depicted in Table 1.
 |
Table 1 The Overall Prevalence of Salmonella Isolated from Abattoir and Butcher Shops in the Study Setting |
Risk Factors Revealing Beef Contamination by Salmonella in Municipal Abattoir
A binary logistic regression analysis revealed that educational status (OR=8.4, 95% CI=1.186–59.493), job related training (OR=5.5, 95% CI=1.065–28.416), contamination risk perception (OR=5.31, 95% CI=1.256–22.489), neatness of knives (OR=7.6, 95% CI=0.892–65.376), and source of contamination (OR=8.44, 95% CI=1.682–42.39) were among the potential risk factors associated with Salmonella occurrence in the abattoir, as illustrated in Table 2.
 |
Table 2 Putative Exposing Risk Factors to Beef Contamination by Salmonella in Jimma Abattoir Workers |
Putative Risk Factors Related with Beef Contamination by Salmonella in Butcher Shops
Of the risk factors considered for the butcher shops, educational status (OR=9.17; 95% CI=1.15–73.239), wearing of protective cloth (OR=11.42; 95% CI=1.829–71.419), manner of hand washing (OR=7.25; 95% CI=1.210–43.442), and money handling (OR=9.69; 95% CI=1.578–59.474) were among the putative risk factors significantly associated with the occurrence of Salmonella in a contaminated carcass, as designated in Table 3.
 |
Table 3 Putative Risk Factors Associated with the Incidence of Salmonella in Butcher Shop Workers |
Antimicrobial Resistance Patterns
Mono Antimicrobial Resistance Patterns of Salmonella Isolates
In the present study, antimicrobial resistance patterns of 33 Salmonella isolates recovered from raw beef were tested against 12 antimicrobials so as to evaluate their resistance level (Table 4). Out of 24 isolates from abattoir raw beef, 14 (58.3%), 14 (58.3%), and 16 (66.7%) of them were resilient to tetracycline, ampicillin, and streptomycin, correspondingly, whereas noisolate was resistant to norfloxacilin, gentamicin, and ciprofloxacin. Besides, of the nine Salmonella isolates from retailer (butcher) shops, 5 (55.6%), 5 (55.6%), and 6 (66.7%) of them were resistant to tetracycline, ampicillin, and streptomycin, respectively, while no isolate was resistant to norfloxacilin, gentamicin, and ciprofloxacin. A higher intermediate resistance of the isolates to streptomycin (66.7%) was observed compared to the rest of the antibiotics, as designated in Table 4.
 |
Table 4 Mono-Antimicrobial Resistant Patterns of Salmonella Isolates from Raw Beef in Jimma |
Multi-Drug Resistance Patterns of Salmonella Isolates
Out of 24 isolates obtained from the abattoir, 14 (58.3%) were resistant to two or more antibiotics. Among the 14 multi-drug resistant isolates, five (35.7%) were resistant to tetracycline, ampicillin, and streptomycin, whereas two were resistant to tetracycline, streptomycin, chloramphenicol, kanamycin, ampicillin, and neomycin. Moreover, only two isolates from retailer (butcher) shops were resistant to five different antibiotics, as depicted in Table 5.
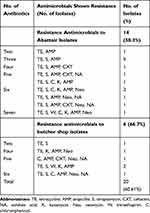 |
Table 5 Multiple Antimicrobial Resistance Profile of Salmonella Isolates from Raw Beef in Jimma |
Discussion
In the current study, the carriage and antimicrobial resistance pattern of Salmonella isolates from raw beef in Jimma town abattoir and retailer (butcher) shops were estimated. Consequently, Salmonella isolates were recovered from 24 (20.2%) and nine (6.52%) swab samples of abattoir and retailer (butcher) shops, respectively. The frequency of Salmonella isolated from the abattoir is consistent with the report of Sibhat et al,19 who reported 10.9% from a commercial Slaughterhouse in Debre Zeit, whereas Beyene et al20 reported a slightly comparable result from Asella abattoir (8.5%). The current finding is higher than the report of Shilangale et al,21 who reported a 0.85% incidence of Salmonella from the three beef export abattoirs in Namibia and the 1.5% isolation rate reported by Ibrahim22 from cattle carcasses at Kano abattoir in Nigeria. Contrarily, this finding is lower than the reports of Wassie et al,23 Hiko et al,24 and Amenu25 from Gonder (20.8%), Addis Ababa (26.6%), and Arbaminch (31.5%) municipal abattoirs, respectively.
In our observation, the abattoir was extremely poor in sanitation due to the absence of water and blood drainage, and the accumulation of waste materials which were disposed of near to the slaughtering house. Therefore, the difference in the frequency of Salmonella isolation rate in the present study from different authors work could be attributed to variation in sampling strategy, detection procedures, target populations, topographical origins of the animals, numbers of animals sampled, study design, season, hygienic status of the abattoir and retailer shops, and antimicrobial treatment warranted during the process. This might be due to the poor hygiene to the abattoir, which is consistent with the report of Kusumaningrum et al,26 who stated that poor hygienic conditions are the source of carcass contamination in the abattoir.
The current study finding depicted that about 6.52% of Salmonella spp. was isolated from butcher shops in the study setting. This finding is consistent with the report of Mengistu et al27 from Haramaya University, eastern Ethiopia. Contrarily, a relatively higher prevalence of Salmonella isolates recorded in the current investigation was higher than the report of Mengistu et al27 from Dire Dawa retailer shops (1%), revealing that the course of evisceration might be the basis for carcass uncleanness besides carrier state. However, this result was lower than the incidence of 12% from raw meat28 and 17.3% from minced meat29 in Gondar town. This difference might be due to poor personnel hygiene in the butcher houses and equipment(viz. knives, table, and balance) (Personal observation).
The proportion of Salmonella isolates, in the current study, recovered from swabs of carcasses of abattoir origin was 14.29%. This finding corroborates with the findings of Takele et al6 and Dabassa and Bacha30 who reported 11.3% and 13.3% from beef carcasses in Jimma municipal abattoir, respectively, and Hiko et al24 who reported 11.8% from Addis Ababa Abattoirs Enterprise whereas this prevalence is lower than other reports from Tigray region (16.4%),31 Dire Dawa abattoir (17.7%),32 and Senegal (42.8%).33 Counter to this, the current finding is higher than the report of El-Gamal and EL-Bahi,34 Renatus et al,35 Thongsay et al,36 Kalambhe et al,37 Bahnass et al,38 Sefinew and Bayleyegn,39 and Gizachew and Mulugeta40 from Egypt (0.0%), Namibia (0.50%), Thailand (4.5%), Central India (6%), Saudi Arabia (8.5%), and Bahir Dar (4.8, 7.6%), respectively. This might be due to variation in the nature of samples, sampling strategy, and procedures origin of animals, contamination from intestinal tract breakage and fecal leakage during evisceration, and from lairage due to lack of care in the study setting.
Salmonella isolates detected from abattoir workers hand swab (8.57%) of the present study setting is higher than the reports of Bahnass et al38 and Nyeleti et al41 in Najran region (2.4%) and Addis Ababa (6.0%), respectively. Personal sanitation disparity of the food handlers could help to elucidate this inconsistency since the majority of workers in the present study setting handled rumen content and gastrointestinal tracts without washing their hands.
In the current investigation, the prevalence of Salmonella isolates detected on the cutting knife (11.4%) was slightly higher than the reports of Teklu and Nigussie,42 and Garedew et al29 from Mojo (7.4%) and Gondar (9.1%) towns, respectively. This variation is attributed to a high frequency of Salmonella in raw beef samples which might act as a cause of contamination for the knives, and due to the unhygienic condition of knives, viz. abattoir workers in the study setting put knives on the floor and then use them without washing or disinfecting.
The occurrence of Salmonella on the butcher shops cutting (chopping) boards was 6.5%. This finding is almost comparable to the 5.6% and 5.7% prevalence rates which were reported by Garedew et al29 and Wassie et al23 from chopping boards of butcher shops and an abattoir in Gonder town, respectively. A comparatively lower incidence rate of Salmonella isolates from cutting boards than that of carcasses could be ascribed to the hygienic condition of the chopping board. Reports revealed that bacteria such as Salmonella were not isolated from wooden surfaces soon after they were applied, unless huge numbers were used. Studies revealed that wood is intrinsically porous, which permits food juices and bacteria to enter the body of the wood unless a highly hydrophobic filtrate covers the surface.43
Of the risk factors considered in the current study, the educational level of both abattoir and butcher shop workers was positively associated with the occurrence of Salmonella. The probability of carcass contamination by uneducated workers was 8- and 9-times more likely than educated workers in the abattoir and retailer shops, respectively. This finding is consistent with the report of Ntanga et al44 in Tanzania which revealing that the educational level of workers was the risk factor for positive results. The educational level and training of food handlers are important for basic perception and necessities of personal hygiene and its environment play important roles in preserving the quality of food products to consumers.45 Best commencement of hygiene practices has been attributed to those employees with a basic level (at least primary) of education, while bad practices are attributed to those who were illiterate.46
Salmonella infection was more prevalent in untrained abattoir workers (37.93%) compared to the trained ones (10%). This study revealed that job related training was significantly associated with Salmonella carriage (p<0.05). Untrained personnel working in the abattoir were 6-times more likely to contaminate the carcasses with Salmonella than trained personnel.
The current finding corroborates the report of Niyonzima et al,47 who reported that job related training is found to be positively associated (p<0.05) with a reduced risk of Salmonella incidence in retailed meat in Rwanda. Job-related training of food handlers regarding basic concepts and requirements of personal hygiene play an integral part in ensuring safe products to the consumers.48 Our result indicated there was a poor knowledge on meat hygiene practice in both abattoir and retailer shop workers of the town. Hence, there is a need for more operative training in both individual and general hygiene practices for these workers. Pertaining to contamination risk perception, a higher frequency rate of Salmonella was isolated from personnel (54.55%) who had a lack of perception on contamination of carcasses in the slaughtering process as a risk. This result revealed that personnel who had a lack of knowledge on contamination in the slaughtering process were significantly associated (p<0.05) with Salmonella isolation. This in turn corroborates the report of Haileselassie et al48 from north-central Nigeria.
Lack of wearing personal protective cloth during the handling of meat was insignificantly associated with Salmonella occurrence in the abattoir workers of the current study. Though insignificantly associated, a higher prevalence of Salmonella isolates was obtained from personnel who did not use clean personal protective clothing.
This finding agrees with that of Alhaji and Baiwa,49 who reported that a substantial proportion of the workers did not follow the recommended personal protective practices in Niger state of north-central Nigeria. Though many studies identified bare hands, dirty clothes, and workers hair could be the sources of microbial contaminating the meat,50–52 in our current work, abattoir workers had no awareness about the importance of wearing clean protective cloths which may prevent the transmission of microorganisms from handlers to meat.
Using an uncleaned knife for splitting of carcasses was also significant with the infection of meat by Salmonella in the abattoir (p<0.05). The chance of contamination of the carcass by Salmonella was 8-times more likely when using dirty knives than clean knives. Our finding corroborates with the report of Muluneh and Kibret40 in Bahir Dar town, who reported washing the knife before beginning slaughtering reduces the risk of contamination by Salmonella. A higher bacterial load detected on raw beef from the slaughterhouses could be the possibility of cross-contamination associated to the use of uncleaned knives.47
Feces as a source of contamination was positively associated with the Salmonella isolation rate from the carcass (p<0.05) in the current study. Fecal meat contamination of Salmonella was 8-times more likely to contribute to the contamination than floor and water contamination to the carcass. On top of this, handling of carcasses with dirty equipment and hand had one more chance of contaminating of carcasses than water and floor at Jimma municipal abattoir. This result is inconsistent with the previous reports from Sudan by Abdalla et al.53
Microbial contaminations of meat carcasses by hides and intestinal contents are occurred mainly during hide removal and the evisceration process.19 In the current study setting, the esophagus and rectum were not ligated during the evisceration process, which could be attributed to high microbial contamination of raw beef.
Lack of wearing personal protective cloth during handling of meat in the current investigation was also significantly correlated to Salmonella incidence (p<0.05) in the butcher shop workers. This outcome agrees with the work of Chepkemoi et al,54 which revealed that butcher shop workers who did not wear protective clothes had a risk factor for carcass contamination. Besides this, the current study observed that only about 5.88% of butcher shop workers wore clean protective clothes, which corroborates with the report of Garedew et al.29 This could be attributed to a lack of knowledge about good sanitary practices as none of the butchers had taken formal training in food safety in the study site.
In this study, hand washing before handling meat by the butcher men respondents was statistically associated with Salmonella occurrence (p<0.05). Workers washing their hands with water only were contaminating the carcass and equipment 7-times more than those washing their hands with detergent and water. This result agrees with the report of Ntanga et al44 from Tanzania. In the current study, we observed that butcher shop workers did not wash their hands mostly due to lack of hand-washing facilities, washing basin, soap, etc., as reported by Todd et al.55
Meat cutters in butcher shops handling money with their hands while selling meat was significantly contributing to the contamination of Salmonella (p<0.05). The isolation rate of Salmonella in the current work revealed that butcher men collecting money with theit hands during selling were 10-times more likely contaminating carcasses than those collecting money using a cashier. This finding closely corroborates with the report of Garedew et al,29 who reported almost all shops workers collecting money with bare hands while serving meat. The unhygienic conditions and habits of handling money in circulation usually subjects the money to contamination with a variety of microorganisms. The money can thereafter act as a vehicle for contaminating the hands of the food seller/handler, and thus cross-contamination of food.56
Our current study finding revealed that all Salmonella isolates recovered from both abattoir and butcher shops were sensitive to norfloxacilin, gentamicin, and ciprofloxacin, which is comparable to a study conducted in Jimma town.57 This might be because they are not widely used in Ethiopia for animal treatment, and those resistance drugs can easily available for both treatment of animal.
The resistance level of the abattoir and butcher shop Salmonella isolates to streptomycin in the current study was higher than the resistance reports of Wolde and Bacha57 in Jimma town (50.8%). Contrarily, a highest resistance (100%) level of Salmonella isolates to streptomycin was reported by Kebede et al58 in slaughtered bovines and ovines at Addis Ababa abattoir Enterprise. Detection of AR Salmonella could be attributed to recurrent usage of antibiotics in both livestock and public health sectors as these antimicrobials are comparatively cheaper and frequently available.
The higher number of tetracycline resistant Salmonella isolates (58.3%) recorded in the present work is closely comparable with the finding of Madoroba et al,59 who reported 51.9% resistance in a South African abattoir, whereas about 55.6% tetracycline resistant isolates were obtained from butcher shops in this finding, which agrees with the finding of Eguale et al60 in Addis Ababa (58.3%). Contrary to this, a higher resistance (75%) to tetracycline was reported by Worku et al61 in food handlers in Jimma town. This higher resistance profile of Salmonella isolates to tetracycline might be attributed to the high level of utilization of this drug in veterinary medicines by the local community in the current study setting.
Salmonella isolates resistance to ampicillin (58.3%), from the abattoir, recorded in the current study is similar with the report of Beyene et al20 in Assella Abattoir (58.3%), whereas the prevalence of resistant isolates from the butcher shops (55.6%) in our work is higher than the report of Garedew et al29 in retailer shops in Gondar town (88.7%). The differences could be due to frequent use and easy accessibility of the drug everywhere in the country including the current research site.
The occurrence of multidrug-resistant Salmonella isolates in the abattoir and butcher shops sample was observed in this study and a higher frequency of the isolates were recovered from both abattoir (58.3%) and butcher shops (66.7%), of which were resistant to two or more of the antimicrobials. This result was lower than studies carried out in Addis Ababa and Gondar towns where 83% and 75.5%, respectively, of isolates were resistant to two or more antimicrobials.29,62
Nevertheless, the current finding is relatively higher than the report of Beyene et al20 from dairy farms, abattoir, and in contact humans of Asella town, in which about 50% of Salmonella isolates were resistant to two or more antimicrobials. A higher level of resistance could be attributed to irrational use of antibiotics in our study setting, as previously reported.63–65 In general, these findings call for constant monitoring and evaluation of antimicrobials stewardship.
Conclusion
The isolation proportion of Salmonella from Jimma town abattoir and butcher shops was 11.43% and 6.52%, respectively. Educational status, job related training, perception of contamination risk, neatness of knife, wearing protective cloth, manner of hand washing, handling of money, and fecal contamination were amid the risk factors contributing to Salmonella raw beef contamination in the current study. A higher mono antimicrobial resistance rate of Salmonella isolates was observed against tetracycline, ampicillin, and streptomycin amid the antibiotics considered in the study. Besides, multi-drug resistance (two or more antibiotics) was registered for 14 (58.3%) and six (66.7%) isolates of the abattoir and retailer (butcher) shops, respectively. These drugs are popular and usually used in the veterinary and human medicines in Ethiopia. This could attribute to the limit of therapeutic choice to prevent and control salmonellosis and other bacterial diseases in livestock and human health. Lack of public awareness about Salmonella contamination of beef meat in abattoir and butcher shops, and its transmission to humans, was found be high in the study area.
Moreover, it is mandatory for the concerned authority to strictly and consistently provide training on keeping personal, meat, working equipment, and environmental hygiene requirements so as to decrease the level of contamination of raw beef by abattoir and butcher shop workers. More comprehensive training has to be given on proper antimicrobial usage for the concerned stakeholders and further studies are needed to describe all the virulence gene and serotype of pathogenic Salmonella strain for the emergence of drug resistance isolates in order to develop the best prevention and control measures.
Data Sharing Statement
The data accustomed to support the findings of this research work are offered from the first and/or corresponding author upon request.
Ethics Approval and Consent to Participate
Abattoir workers and owners of beef butcher shops, which were involved in the business of beef meat, were informed about the purpose of the study and the consent of each abattoir worker, and owners and workers of each beef butcher shops were obtained before physical examination of raw beef and collection of the sample. Only volunteer abattoir workers and owners’ and workers of the butcher shops have participated in the study. The questionnaire and methodology for this study was approved by the research and ethical review committee of Jimma University and the study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
We acknowledge Dr. Yosef Deneke and Dr. Tesfaye Sisay for their encouragement, material support, and information provision while collecting the sample from Jimma town abattoir and butcher shops. We also express our deepest gratitude and appreciation to the School of Veterinary Medicine, College of Agriculture and Veterinary Medicine, Jimma, Ethiopia for providing us valuable support and assistance during sample processing in the Veterinary Microbiology laboratory. We are also grateful to the laboratory staff members of the Veterinary Microbiology laboratory working intensively under the School of Veterinary Medicine.
Author Contributions
Both authors made a significant contribution to the work reported, either in the conception of the study, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas. Moreover, both authors took part in revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The funding source for the current study was College of Agriculture and Veterinary Medicine of Jimma University, Ethiopia.
Disclosure
The authors declare that they have no competing interests.
References
1. Sharkawy H, Tahoun A, Aega EG, et al. Epidemiological, molecular characterization and antibiotic resistance of Salmonella enterica serovars isolated from chicken farms in Egypt. Gut Pathog. 2017;9:8. doi:10.1186/s13099-017-0157-1.
2. Taddese D, Tolosa T, Deresa B, et al. Antibiograms and risk factors of Salmonella isolates from laying hens and eggs in Jimma Town, South Western Ethiopia. BMC Res Notes. 2019;12:472. doi:10.1186/s13104-019-4516-5
3. CDC. Incidence and trends of infection with pathogens transmitted commonly through food - Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 1996–2012. Weekly Report. 2013;62:283–287.
4. Bengtsson B, Greko C. Antibiotic resistance-consequences for animal health, welfare, and food production. Upsala J Med Sci. 2014;119(2):96–102. doi:10.3109/03009734.2014.901445.
5. Jajere SM. A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance. Vet World. 2019;12:504–521. doi:10.14202/vetworld.2019.504-521
6. Takele S, Woldemichael K, Gashaw M, et al. Prevalence and drug susceptibility pattern of Salmonella isolates from apparently healthy slaughter cattle and personnel working at Jimma municipal abattoir, South-West Ethiopia. Trop Dis Travel Med Vaccines. 2018;4:13. doi:10.1186/s40794-018-0072-6
7. Majowicz SE, Scallan E, Angulo FJ, et al. The global burden of non-typhoidal Salmonella gastroenteritis. Clinical Infect Dis. 2010;50(6):882–889. doi:10.1086/650733.
8. Wabeto W, Abraham Y, Anjulo AA. Detection and identification of antimicrobial-resistant Salmonella in raw beef at Wolaita Sodo municipal abattoir, Southern Ethiopia. J Health Population Nutr. 2017;6:52. doi:10.1186/s41043-017-0131-z.
9. Birhaneselassie M, Williams D. A study of Salmonella carriage among asymptomatic food-handlers in southern Ethiopia. Int J Nutr Food Sci. 2013;2(5):243–245. doi:10.11648/j.ijnfs.20130205.15
10. Ejeta G, Molla B, Alemayehu D, et al. Salmonella serotypes isolated from minced meat beef, mutton and pork in Addis Ababa, Ethiopia. Revue deMedecine Veterinaire. 2004;155(11):547–551.
11. Garedew L, Berhanu A, Mengesha D, et al. Identification of gram-negative bacteriafrom critical control points of raw and pasteurized cow milk consumed at Gondar town and its suburbs, Ethiopia. BMC Public Health. 2012;12(1):950. doi:10.1186/1471-2458-12-950
12. International Organizations for Standardization (ISO 6579). Microbiology of food and animal feeding stuffs - Horizontal method for the detection of Salmonella spp. 2002.
13. Thrusfield M. Sampling in Veterinary Epidemiology.
14. Tadesse G, Gebremedhin EZ. Prevalence of Salmonella in raw animal products in Ethiopia: a meta-analysis. BMC Res Notes. 2015;8:163. doi:10.1186/s13104-015-1127-7.
15. Rose S, Spinks N, Canhoto AI. Management Research: Applying the Principles. Routledge; 2014:440.
16. International commission for Microbiological Specifications for Foods (ICMSF). Microorganisms in Food 7: Microbiological Testing in Food Safety Management. Kluwer Academic/Plenum Publishers; 2002.
17. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; 24th informational supplement. M100– S24. Wayne, PA: Clinical and Laboratory Standards Institute; 2015.
18. Clinical and Laboratory Standards Institute (CLSI). Performance for antimicrobial disk susceptibility tests; approved the standard, CLSI document M02-A11. Wayne, Pa, USA: CLSI; 2012:1–76.
19. Sibhat B, Molla ZB, Zerihun A, et al. Salmonella serovars and antimicrobial resistance profiles in beef cattle, slaughterhouse personnel and slaughterhouse environment in Ethiopia. Zoonoses Public Health. 2011;58(2):102–109. doi:10.1111/j.1863-2378.2009.01305.x
20. Beyene T, Yibeltie H, Chebo B, et al. Identification and antimicrobial susceptibility profile of Salmonella isolated from selected dairy farms, abattoir and humans at Asella town, Ethiopia. J Vet Sci Technol. 2016;7(3):320.
21. Shilangale RP, Kaaya GP, Chimwamurombe PM. Prevalence and characterization of Salmonella isolated from beef in Namibia. European J Nutr Food Safety. 2015;5(4):267–274. doi:10.9734/EJNFS/2015/17276
22. Ibrahim A. Bacterial load and isolation of Salmonella species from cattle carcasses at Kano Abattoir- Kano state, Ahmadu Bello University, Zaria, Nigeria. 2014.
23. Wassie B, Sisay W, Gashaw B, et al. Assessment of microbiological quality and meat handling practices in butcher shops and abattoir found in Gondar town, Ethiopia. Int J Microbiol Res. 2017;8(2):59–68.
24. Hiko A, Irsigler H, Ameni G, et al. Salmonella serovars along two beef chains in Ethiopia. J Infect Dev Ctries. 2016;10(11):1168–1176. doi:10.3855/jidc.6354
25. Amenu A. Prevalence and antibiotic resistance of Salmonella isolated from beef in Arba Minch, Southern Ethiopia. Haramaya, Ethiopia: MSc Thesis, Faculty of Natural and Computational Science, Department of Biology, School of Graduate Studies, Haramaya University; 2012.
26. Kusumaningrum HD, Van Asselt ED, Beumer RR, et al. A quantitative analysis of cross-contamination of Salmonella and Campylobacter spp. via domestic kitchen surfaces. J Food Protec. 2004;67(9):1892–1903. doi:10.4315/0362-028X-67.9.1892
27. Mengistu S, Abayneh E, Shiferaw D. E. coli O157: H7 and Salmonella Species: public health importance and microbial safety in beef at selected slaughter houses and retail shops in Eastern Ethiopia. J Vet Sci Technol. 2017;8:468. doi:10.4172/2157-7579.1000468
28. Ejo M, Garedew L, Alebachew Z, et al. Prevalence and antimicrobial resistance of Salmonella isolated from animal origin food items in Gondar, Ethiopia. Bio Med Res Int. 2016;2016:8. doi:10.1155/2016/4290506
29. Garedew L, Hagos Z, Addis Z, et al. Prevalence and antimicrobial susceptibility patterns of Salmonella isolates in association with hygienic status from butcher shops in Gondar town, Ethiopia. Antimicrob Resist Infect Control. 2015;4:21. doi:10.1186/s13756-015-0062-7
30. Dabassa A, Bacha K. The prevalence and antibiogram of Salmonella and Shigella isolated from abattoir, Jimma town, South West Ethiopia. Int J Pharm Biol Res. 2012;3(4):143–148.
31. Abebe M, Tafese B, Adane H. Antimicrobial resistance of Salmonella serovars isolated from food of bovine origin in selected Woredas of Tigray, Ethiopia. World J Med Sci. 2014;11(3):342–347.
32. Ferede B. Isolation, identification, antimicrobial susceptibility test and public awareness of Salmonella on raw goat meat at Dire Dawa Municipal Abattoir, Eastern Ethiopia. Addis Ababa: MSc thesis, Addis Ababa University; 2014.
33. Stevens A, Kaboré Y, Perrier-Gros-Claude JD, et al. Prevalence and antibiotic-resistance of Salmonella isolated from beef sampled from the slaughterhouse and from retailers in Dakar (Senegal). Int J Food Microbiol. 2006;110(2):178–186. doi:10.1016/j.ijfoodmicro.2006.04.018
34. El-Gamal AM, EL-Bahi EF. Molecular characterization of rectal carriage of E. coli O157:H7 and Salmonella spp. In feedlot animals and its effects on carcasses contamination. Alexandria J Vet Sci. 2016;48(1):42–49. doi:10.5455/ajvs.208144
35. Renatus P, Godwin P, Percy M. Prevalence and characterization of Salmonella isolated from beef in Namibia. European J Nutr Food Safety. 2015;5(4):267–274.
36. Thongsay S, Sujate C, Chaiwat P, et al. Salmonella prevalence in slaughtered buffaloes and cattle in Champasak Province, Lao people’s Democratic Republic. Kasetsart J Natural Sci. 2013;47:561–570.
37. Kalambhe D, Zade N, Chaudhari S, et al. Isolation, antibiogram and pathogenicity of Salmonella spp. recovered from slaughtered food animals in Nagpur region of Central India. Vet World. 2016;9:176–181. doi:10.14202/vetworld.2016.176-181
38. Bahnass M, Fathy A, Alamin M. Identification of human and animal Salmonella spp. isolates in Najran region and control of it. Int J Advanced Res. 2015;3(1):1014–1022.
39. Sefinew A, Bayleyegn M. Prevalence and antimicrobial resistance profiles of Salmonella enterica serovars isolated from slaughtered cattle in Bahir Dar, Ethiopia. Trop Anim Health Prod. 2012;44:595–600. doi:10.1007/s11250-011-9941-y
40. Muluneh G, Kibret M. Salmonella spp. and risk factors for the contamination of slaughtered cattle carcass from a slaughterhouse of Bahir Dar Town, Ethiopia. Asian Pacific J Trop Dis. 2015;5(2):130–135. doi:10.1016/S2222-1808(14)60640-X
41. Nyeleti C, Molla B, Hildebrandt G, et al. The prevalence and distribution of Salmonella in slaughter cattle, slaughterhouse personnel and minced beef in Addis Ababa, Ethiopia. Bulletin Anim Health Prod Afri. 2000;48:19–24.
42. Teklu A, Nigussie H. Assessments of risk factor and prevalence of Salmonella in slaughtered small ruminant and environments in an export abattoir, Modjo, Ethiopia. American- Eurasian J Agri Env. 2011;10:992–999.
43. Ak NO, Cliver DO, Kaspar CW. Cutting boards of plastic and wood contaminated experimentally with bacteria. J Food Protec. 1994;57:16–22. doi:10.4315/0362-028X-57.1.16
44. Ntanga PD, Mdegela RH, Nonga HE. Assessment of beef microbial contamination at abattoir and retail meat shops in Morogoro Municipality, Tanzania. Tanzania Vet J. 2014;29(2):53–61.
45. Nel S, Lues JFR, Buys EM, et al. The personal and general hygiene practices in the deboning room of a high throughput red meat abattoir. Food Control. 2004;15(7):571–578. doi:10.1016/j.foodcont.2003.09.004
46. Afnabi RB, Nameni RP, Kamdem SS, et al. Typology of the Cameroon traditional slaughterhouses based on hygiene practices. Adv Anim Vet Sci. 2014;2(8):477–487. doi:10.14737/journal.aavs/2014/2.8.477.487
47. Niyonzima E, Ongol MP, Brostaux Y, et al. Consumption patterns, bacteriological quality and risk factors for Salmonella contamination in meat-based meals consumed outside the home in Kigali, Rwanda. Food Control. 2017;73:546–554. doi:10.1016/j.foodcont.2016.09.004
48. Haileselassie M, Taddele H, Adhana K, et al. Food safety knowledge and practices of abattoir and butchery shops and the microbial profile of meat in Mekelle City, Ethiopia. Asian Pacific J Trop Biomed. 2013;3(5):407–412. doi:10.1016/S2221-1691(13)60085-4
49. Alhaji NB, Baiwa M. Factors affecting workers ‘delivery of good hygienic and sanitary operations in slaughterhouses in north-central Nigeria. Sokoto J Vet Sci. 2015;13(1):29–37. doi:10.4314/sokjvs.v13i1.5
50. Cardinale E, Gros-Claude JP, Tall F, et al. Risk factors for contamination of ready-to-eat street-ended poultry dishes in Dakar, Senegal. Int J Food Microbiol. 2005;103(2):157–165. doi:10.1016/j.ijfoodmicro.2004.12.023
51. Lues JF, Rasephei MR, Venter P, et al. Assessing food safety and associated food handling practices in street food vending. Int J Environ Health Res. 2006;16(5):319–328. doi:10.1080/09603120600869141
52. Heinz G, Hautzinger P. Meat processing technology for small to medium scale producers. Bangkok, Thailand: Food and Agriculture Organization of the United Nations Regional Office for Asia and the Pacific; 2007:470.
53. Abdalla MA, Suliman SE, Ahmed DE, et al. Estimation of bacterial contamination of indigenous bovine carcasses in Khartoum (Sudan). Afr J Microbiol Res. 2009;3(12):882–886.
54. Chepkemoi S, Lamuka PO, Abong GO, et al. Sanitation and hygiene meat handling practices in small and medium enterprise butcheries in Kenya-Case study of Nairobi and Isiolo Counties. Int J Food Safety. 2015;17:64–74.
55. Todd ED, Greig JD, Michaels BS, et al. Outbreaks where food workers have been implicated in the spread of foodborne disease Part 11. Use of antiseptics and sanitizers in community settings and issues of hand hygiene. Compliance in health care and food industries. J Food Protec. 2010;73(12):2306–2320. doi:10.4315/0362-028X-73.12.2306
56. Alemu A. Microbial contamination of currency notes and coins in circulation: a potential public health hazard. Biomed Biotechnol. 2014;2(3):46–53.
57. Wolde T, Bacha K. Prevalence and antibiotics resistance patterns of Salmonella isolated from kitchen sponges at Jimma town, Ethiopia. Afr J Microbiol Res. 2017;11(16):631–636. doi:10.5897/AJMR2016.8382
58. Kebede A, Kemal J, Alemayehu H, et al. Isolation, identification, and antibiotic susceptibility testing of Salmonella from slaughtered bovines and ovines in Addis Ababa Abattoir Enterprise, Ethiopia: a cross-sectional study. Int J Bacteriol. 2016;2016:8. doi:10.1155/2016/3714785
59. Madoroba E, Kapeta D, Gelaw AK. Salmonella contamination, serovars and antimicrobial resistance profiles of cattle slaughtered in South Africa. Onderstepoort J Vet Res. 2016;83(1):1–8. doi:10.4102/ojvr.v83i1.1109
60. Eguale T, Gebreyes WA, Asrat D, et al. Non-typhoidal Salmonella serotypes, antimicrobial resistance and co-infection with parasites among patients with diarrhea and other gastrointestinal complaints in Addis Ababa, Ethiopia. BMC Infect Dis. 2015;15(1):497. doi:10.1186/s12879-015-1235-y
61. Worku T, Jejaw A, Kannan S, et al. Isolation and antimicrobial sensitivity patterns of enteric bacterial pathogens from asymptomatic food handlers, Jimma, Ethiopia. American J Health Res. 2015;3(6):399–406. doi:10.11648/j.ajhr.20150306.24
62. Addis Z, Kebede N, Sisay Z, et al. Prevalence and antimicrobial resistance of Salmonella isolated from lactating cows and in contact humans in dairy farms of Addis Ababa: a cross sectional study. BMC Infect Dis. 2011;11:222. doi:10.1186/1471-2334-11-222
63. Galgallo DA, Roka ZG, Boru WG, et al. Investigation of a typhoid fever epidemic in Moyale Sub-County, Kenya, 2014–2015. J Health Population Nutr. 2018;37(1):14. doi:10.1186/s41043-018-0144-2
64. Horumpende PG, Said SH, Mazuguni FS, et al. Prevalence, determinants and knowledge of antibacterial self- medication: a cross sectional study in North-Eastern Tanzania. PLoS One. 2018;13(10):e0206623. doi:10.1371/journal.pone.0206623
65. Xu Z, Wang M, Zhou C, et al. Prevalence and antimicrobial resistance of retail-meat-borne Salmonella in southern China during the years 2009–2016: the diversity of contamination and the resistance evolution of multidrug-resistant isolates. Int J Food Microbiol. 2020;333:108790. doi:10.1016/j.ijfoodmicro.2020.108790.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.