Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Can Adipokine FAM19A5 Be a Biomarker of Metabolic Disorders?
Authors Wesołek-Leszczyńska A , Pastusiak K , Bogdański P, Szulińska M
Received 18 January 2024
Accepted for publication 19 March 2024
Published 10 April 2024 Volume 2024:17 Pages 1651—1666
DOI https://doi.org/10.2147/DMSO.S460226
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Agnieszka Wesołek-Leszczyńska,1,2 Katarzyna Pastusiak,1 Paweł Bogdański,1,* Monika Szulińska1,*
1Department of Treatment of Obesity, Metabolic Disorders and Clinical Dietetics, Poznan University of Medical Sciences, Poznań, Poland; 2Doctoral School, Poznan University Of Medical Sciences, Poznań, Poland
*These authors contributed equally to this work
Correspondence: Agnieszka Wesołek-Leszczyńska, Department of Treatment of Obesity, Metabolic Disorders and Clinical Dietetics, Poznan University of Medical Sciences, 82/84 Szamarzewskiego Street, Poznań, 60-569, Poland, Tel +48 721 947 738, Email [email protected]; [email protected]
Aim: One of the most critical functions of adipose tissue is the production of adipokines, ie, numerous active substances that regulate metabolism. One is the newly discovered FAM19A5, whose older name is TAFA-5.
Purpose: The study aimed to review the literature on the FAM19A5 protein.
Methods: The review was conducted in December 2023 using the PubMed (Medline) search engine. Sixty-four papers were included in the review.
Results: This protein exhibits the characteristics of an adipokine with positive features for maintaining homeostasis. The results showed that FAM19A5 was highly expressed in adipose tissue, with mild to moderate expression in the brain and ovary. FAM19A5 may also inhibit vascular smooth muscle cell proliferation and migration through the perivascular adipose tissue paracrine pathway. Serum levels of FAM19A5 were decreased in obese children compared with healthy controls. There are negative correlations between FAM19A5, body mass index, and fasting insulin. Serum FAM19A5 level is correlated with type 2 diabetes, waist circumference, waist-to-hip ratio, glutamic pyruvic transferase, fasting plasma glucose, HbA1c, and mean shoulder pulse wave velocity. FAM19A5 expression was reduced in mice with obesity. However, the data available needs to be clarified or contradictory.
Conclusion: Considering today’s knowledge about FAM19A5, we cannot consider this protein as a biomarker of the metabolic syndrome. According to current knowledge, FAM19A5 cannot be considered a marker of metabolic disorders because the results of studies conducted in this area are unclear.
Keywords: fat tissue, metabolic syndrome, adipokines, adipocytes, obesity
Visceral Adipose Tissue
Adipose tissue, in addition to its energy storage function, is a vital endocrine organ. It also protects against heat loss and mechanical injuries. One of the most critical functions of adipose tissue is the production of adipokines, ie, numerous active substances that regulate metabolism. Both excess and deficiency of adipose tissue negatively affect health. Overall mortality, cardiovascular diseases, metabolic diseases, and obesity are related to body fat content. Visceral fat tissue is particularly metabolically active.1
The essential component of adipose tissue is adipocytes, scattered within the skeleton formed by collagen fibers. There is a subpopulation in adipose tissue stem cells called stromal vascular fraction cells (SVF) and preadipocytes, fibroblasts, leukocytes, macrophages, and endothelial cells. All these elements influence the metabolic function of adipose tissue, regardless of its morphological type. White adipose tissue (WAT) is the largest body store of energy, accumulated in triglycerides (TG), and is the largest endocrine gland—brown adipose tissue (BAT) functions to generate thermal energy, mainly in children. Pink adipose tissue is involved in milk production in women during pregnancy and lactation. Depending on the location of fat tissue in the body, it is divided into subcutaneous and visceral. Subcutaneous adipocytes are large and less metabolically active, while visceral adipocytes are small and more active. Visceral tissue is more metabolically active and secretes more hormone-like substances. Its increased content in obesity significantly increases the risk of cardiovascular diseases.2,3 The summary of fat tissue’s function is presented in Figure 1.
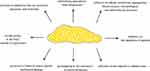 |
Figure 1 Function of fat tissue in the human body.8 |
Malnutrition and, consequently, too low body fat content may result in improper functioning of organs and, in children, growth retardation.5 Obesity is a significant problem for the public health worldwide, because it has become an epidemic. According to the World Health Organization (WHO), more than 1 billion people worldwide have obesity — 650 million adults, 340 million adolescents, and 39 million children.6,7
Assessing the concentration of body fat makes it possible to assess a person’s nutritional status and plan an appropriate nutrition plan, and in the case of obesity - to plan appropriate treatment. The simplest method of estimating overweight and obesity is to measure waist circumference (WC) and calculate the waist-hip ratio (WHR). These indicators are more effective in estimating the risk of cardiovascular diseases than assessing body weight based on body mass index (BMI). The most popular method of estimating body fat content is to perform a body composition analysis using the bioelectrical impedance method. The result is given in kilograms and %. The correct body fat percentage in women is 20–30%, while in men, it is 10–20% of the total body weight. Values exceeding 32% and 26% in women and men indicate obesity. More accurate methods for assessing body fat are computed tomography (CT), magnetic resonance imaging (MRI), and double-beam X-ray absorptiometry (DXA).1,8
Improper nutrition, lack of physical activity, increased stress, certain medications, and psychological and social factors may increase the risk of obesity. Obesity treatment includes permanent changes in eating habits and lifestyle, increasing physical activity, and health and nutrition education. Pharmacological treatment should be tailored to the patient’s capabilities and comorbidities. Available substances approved for treating obesity include tetrahydrolipstatin, bupropion hydrochloride, naltrexone hydrochloride, and liraglutide. Obesity treatment can also be supported by individual physiotherapy and psychological support.9–11
Obesity causes several health consequences. It increases the risk of, among others, stroke, cataracts, coronary heart disease, diabetes, dyslipidemia, hypertension, pancreatitis, non-alcoholic fatty liver disease, liver cirrhosis, infertility, osteoarthritis, and cancer.12 All of this conditions are mainly related to chronic inflammation induced by excess fat tissue.
Inflammation is a protective response to tissue damage or destruction. Short-term treatment destroys or dilutes both the damaging agent and the damaged tissues. However, long-term inflammation has a destructive effect on human health. This condition occurs in people with obesity. It is characterized by lymphocytes and macrophages as well as proliferation of blood vessels and connective tissue. Chronic inflammation is caused by excess fat tissue in these people. The induction and continuation of inflammation are influenced by the secretion of pro-inflammatory adipokines by adipose tissue. These include interleukin-6 (IL-6) and tumor necrosis factor α (TNF-α). IL-6 stimulates hepatocytes to synthesize and secrete c-reactive protein (CRP). Moreover, the accumulation of free fatty acids in obesity activates pro-inflammatory serine kinase cascades. These include IκB kinase (IKK) and c-Jun N-terminal kinase (JNKs), which stimulate adipose tissue to release IL-6. Elevated CRP may be an independent risk factor for cardiovascular diseases, including acute myocardial infarction. Increasing the concentration of IL-6 and TNF-α inhibits and reduces the synthesis and secretion of adiponectin. Reducing the adiponectin concentration increases the risk of cardiovascular diseases. Persistent inflammation is a decisive risk factor for developing metabolic syndrome, diabetes, and cancer. Increased adipose tissue content regulates the secretion of adipokines.13–15
New adipokines that may be the best biomarkers in metabolic disorders are still being sought.
Adipokines in Obesity
Adipokines are proteins secreted by adipose tissue. These proteins influence glucose and lipid metabolism. They regulate the feeling of hunger and satiety and maintain metabolic homeostasis. Their concentrations influence the concentrations of other substances, including hormones. They influence neuroendocrine and immune functions. WAT secretes, among others, leptin, omentin, adiponectin, resistin, visfatin, apelin, vaspin, hepcidin, chemerin, TNF-α, monocytes chemoattractant protein-1 (MCP-1), IL-6, retinol-binding protein 4 (RBP-4) and plasminogen.16 Adipsin was discovered as the first adipokine in 1987.17 However, the discovery of leptin produced by mature adipocytes in 1994 increased interest in the presence of adipokines and their functions in metabolism.18
The functions of selected adipokines in the human body are presented in Table 1.
 |
Table 1 The Functions of Selected Adipokines in the Human Body |
The role of many adipokines still needs to be clarified, or the results of studies are contradictory. Such adipokines include the newly discovered Family With Sequence Similarity 19 Member A5 protein (FAM19A5), which another, older name is TAFA-5.
Metabolic Syndrome
Metabolic syndrome (MetS) is a group of comorbidities that include obesity, hypertension, and disorders of carbohydrate and lipid metabolism. However, the latest definition of MetS from 2022 suggests that it should be diagnosed as obesity comorbid with two of the following criteria - high blood pressure, impaired glucose metabolism, and increased cholesterol concentration in lipoproteins other than high-density lipoprotein (HDL) (atherogenic dyslipidemia). Obesity can be diagnosed based on Body Mass Index (BMI) or waist circumference. The population of MetS patients is not homogeneous. This is due to two or three of the above disorders accompanying obesity, which generate various combinations of pathologies occurring in each person.26
MetS is a major clinical problem, and its incidence is increasing in Poland. According to the Multi-centre National Population Health Examination Study (WOBASZ), 2014, MetS was 33% and 39% in women and men aged 20–74, respectively. This result increased by almost 3% and 9% in women and men, respectively, compared to the WOBASZ study 2003.6,27 Globally, almost 25% have MetS. Epidemiological studies highlight the problem of the increasing number of people with MetS worldwide. The increase is particularly noticeable among children and adolescents, which coincides with the increase in the number of children with obesity.28
Obesity is considered a core element of MetS. Excessive accumulation of fat tissue and the resulting activity of visceral fat tissue causes over 200 complications. MetS consequently causes impaired kidney function, metabolic-associated fatty liver disease, obstructive sleep apnea, polycystic ovary syndrome, and hyperuricemia. MetS also increases the risk of cardiovascular disease and vascular disease and increases the risk of death from cardiovascular disease. Obesity and MetS are associated with increased oxidative stress, essential in the pathogenesis of comorbidities that may develop during MetS. Furthermore, the component of the MetS that contributes most to the relationship between metabolic syndrome and oxidative stress is the component related to obesity and insulin resistance. The essential treatment for MetS is lifestyle change, increasing physical activity, stopping smoking, and changing eating habits, along with pharmacological treatment. These changes consequently cause weight loss, visceral fat tissue reduction, and reduction in the concentration of pro-inflammatory adipokines, especially from visceral adipose tissue.28
FAM19A5 Original Direction of Research
Family With Sequence Similarity 19 Member A5 protein (FAM19A5), another older name is TAFA-5, and it is one of five proteins encoded by the TAFA1-TAFA5 genes. FAM19A5 is less related to other TAFA family proteins. This protein exhibits the characteristics of an adipokine with positive features for maintaining homeostasis.29 Wang et al in 2018 showed that FAM19A5 is an adipokine. The authors analyzed the expression profile of a panel of human cDNAs from multiple tissues. The results showed that FAM19A5 was highly expressed in adipose tissue, with mild to moderate expression in the brain and ovary, and was barely detectable in other tissues. Detailed results also showed FAM19A5 expression in humans in subcutaneous, perirenal adipose tissue. FAM19A5 was also detected in the epididymal adipose tissue of mice. The secretion of this protein by fat cells of various origins was also compared in humans and mice. According to the authors, FAM19A5 was primarily secreted by rat epididymal adipocytes compared to other cell types, including the vascular fraction of adipose tissue, primary rat adventitial aortic fibroblasts, human umbilical vein endothelial cells, rat and human vascular smooth muscle cells (VSMCs), as well as primary mouse peritoneal macrophages. The same project also demonstrated the protective effect of FAM19A5. Adipose tissue-derived FAM19A5 inhibited neointimal formation after in vivo injury in mice, and circulating FAM19A5 levels negatively correlated with neointimal formation. Moreover, the authors suggest that FAM19A5 may also inhibit VSMC proliferation and migration through the perivascular adipose tissue paracrine pathway.29 FAM19A5 contributes to the wound healing process after brain injury,30 and also participates in receptor activator for nuclear factor κ B ligand (RANKL) induced differentiation of osteoclast precursor cells through interactions with the N-formyl peptide receptor 2 (FPR2).31 In humans, serum levels of FAM19A5 were decreased in obese children compared with healthy controls. Moreover, there are negative correlations between FAM19A5 and BMI and fasting insulin. These results suggest an association of the FAM19A5 protein with metabolic disorders and emphasize its suggested positive effects on the body.32
The first data on FAM19A5 genes comes from 2004. Y. Tom Tang et al then discovered a family of small secreted proteins in Homo sapiens and Mus musculus. The family consisted of five highly homologous genes. They were referred to as TAFA-1 to −5. This study used a method of identifying new gene families, which was new in 2004, encoding proteins secreted non-transmembranely. The authors showed that the TAFA genes 1 to 4 are more closely related to each other than to TAFA-5. This was demonstrated by comparing nucleotide and amino acid sequences. The study looked for the proteins encoded by these genes, but the state of knowledge still needed to be improved. TAFA-2 expression level was low. TAFA-1, TAFA-3, TAFA-4, and TAFA-5 proteins were detected in culture supernatants. The expression of these genes was also studied by quantitative real-time polymerase chain reaction (PCR). Expression has been studied in the brain, colon, heart, kidney, liver, lungs, spleen, and thymus. All TAFA genes are expressed at different levels in the brain and deficient levels in other tissues. According to the authors, genes from the TAFA family are expressed mainly in the central nervous system.33 The TAFA-5 protein discovered in 2004 will, in the future, be called the FAM19A5 protein.
A year later, in 2005, genes from the TAFA family became the object of interest of Teresita Díaz de Ståhl1 et al. The team identified areas of loss or gain on chromosome 22, either constitutional or cancer-derived deoxyribonucleic acid (DNA), which could indicate the position of putative genes involved in glioblastoma development. A high-resolution tiling-path chromosome 22 array to a series of 50 glioblastoma samples to investigate the underlying abnormalities in both constitutional and tumor-derived DNA. Analysis of the amplified regions revealed the presence of two interesting candidate genes: TOP3B and TAFA-5. It was shown that this gene encodes a protein, which was then named TAFA-5 - like the gene’s name. According to the authors, the TAFA family proteins are distantly related and contain conserved cysteine residues at fixed positions. They belong to chemokines. According to the authors, the TOP3B and TAFA-5 genes may be involved in the genesis of glioblastoma.34
Until 2018, the topic of TAFA genes was not discussed. At that time, links between the TAFA-5 (FAM19A5) protein and disorders, including metabolic disorders, were sought.
In 2018, Anna A. Kashevarova et al checked the genetic analysis of a 4-year-old girl with a terminal deletion at 22q13. The girl showed developmental delays, hyperactivity, mood swings, and sleep disturbances. The array comparative genomic hybridization (aCGH) results were confirmed by real-time PCR in three tissues: lymphocytes, skin fibroblasts, and oral epithelium. Fluorescence in situ hybridization (FISH) analysis was used to confirm the ring form of chromosome 22 and to determine the level of cells with monosomy 22 among skin lymphocytes and fibroblasts. Real-time PCR confirmed the presence of microdeletions with primers for the FAM19A5 (TAFA-5) gene, SHANK3, and ACR genes. According to single nucleotide polymorphism (SNP) analysis, the 22q13.32-q13.33 microdeletion occurred de novo on the mother’s chromosome. The above results suggest a link between the FAM19A5 gene and the Phelan-McDermid syndrome described in girl.35
After the discovery of TAFA genes in 2004, interest in FAM19A5 (TAFA-5) functions has been most noticeable in neurology, neurological and psychiatric diseases.
FAM19A5 – Central Nervous System
Referring to a 2004 paper where only limited information was available on FAM19A5 function in the brain,33 Anu Shahapal et al wished to determine the expression pattern of FAM19A5 during embryogenesis and in the adult brain and to identify the types of brain cells that express FAM19A5 in FAM19A5-LacZ knock-in (KI) mice. In the results, FAM19A5-LacZ KI homozygous mice showed more vital X-gal staining than heterozygous mice. Moreover, global X-gal staining in embryonic stages showed dominant expression of FAM19A5 in the brain and spinal cord. In adult mice, FAM19A5 was widely expressed in multiple brain regions. Interestingly, increased FAM19A5 expression was shown in response to traumatic brain injury. The authors concluded that FAM19A5 is a crucial regulator of central nervous system (CNS) development and the brain’s response to injury.30 Already a year earlier, in 2018, Can Zheng showed that FAM19A5 protein expression levels correlate with brain development. The FAM19A5 protein has been shown to function during embryonic brain development by promoting neuronal differentiation and suppressing astrocyte differentiation. Despite this, the full function of the FAM19A5 protein in the brain needed clarification to the authors.36
Research on the effect of FAM19A5 in the CNS was continued. A case report from 2019, which presented a female fetus with schizencephaly accompanied by cerebro-occipital hernia, polymicrogyria, agenesis of the corpus callosum, facial dysmorphia, and ventricular septal defect in the myocardium, showed a deletion of chromosome 22q13.32, including the FAM19A5 gene. The authors suggested that the presence of the FAM19A5 gene could be associated with the development of schizencephaly.37
In the same year, Anna J Khalaj’s team demonstrated that FAM19A functions as cell type-specific regulators of neurexin modification in the nervous system. FAM19A1-A4 binds to the cysteine loop domain of neurotoxins, forming intermolecular disulfide bonds during transport through the secretory pathway. According to the authors, FAM19A serves as cell type-specific regulators of neurexin modification, which is associated with disorders such as autism spectrum disorder (ASD), schizophrenia, and mental retardation.38
Moreover, FAM19A5 protein deficiency in mice showed hyperactive locomotor behavior, long-term memory deficits, and failure to acquire fear. These results suggest that FAM19A5 influences the nervous system’s development and the mature brain’s proper functioning.39
It was also checked whether the concentration of FAM19A5 protein correlated with the severity of vascular dementia. The study included 136 patients with vascular dementia and 81 healthy controls. The results showed that serum FAM19A5 concentration was significantly higher than the control group (p < 0.001). Moreover, Spearman correlation analysis showed that serum FAM19A5 levels significantly correlate negatively in patients with vascular dementia (r = −0.414, <0.001). Based on the results, the authors suggest that FAM19A5 protein concentration may be a biomarker for predicting cognitive function in patients with vascular dementia.40
Hye Lim Lee et al checked whether FAM19A5 concentrations correlate with the optic neuritis spectrum disorder. The study included blood sera from 199 patients from 11 hospitals over 21 months. Patients were divided into neuromyelitis optica spectrum disorders with positive aquaporin-4 antibody (NMOSD-AQP4), other CNS demyelinating diseases, and healthy controls. In the results, serum FAM19A5 concentration was higher in patients with NMOSD-AQP4 and correlated with clinical characteristics. According to the authors, serum FAM19A5 may be a new clinical biomarker for NMOSD-AQP4.41
In 2020, it was shown for the first time that FAM19A5 concentrations are associated with depressive states in mice. Genetic deletion of FAM19A5 resulted in increased depressive behavior and impaired hippocampus-dependent spatial memory in mice. FAM19A5 levels in the plasma and hippocampus of chronic stress-treated mice were significantly reduced, whereas overexpression of human FAM19A5 selectively in the hippocampus may attenuate chronic stress-induced depressive behavior. This study provided a new mechanism for chronic stress-induced depression. It has been shown that FAM19A5 may be a new biomarker for the diagnosis and treatment strategy of depression.42 Kyu-Man Han et al in 2020 confirmed that serum FAM19A5 may be a potential biomarker of neurodegenerative changes in major depressive disorder (MDD) in humans. The study included 52 previously untreated patients with MDD and 60 healthy controls. Serum FAM19A5 levels were significantly higher in MDD patients than in the healthy controls group. According to the authors, serum FAM19A5 levels may reflect reactive astrogliosis and associated neuroinflammation in MDD.43
Another study from 2021 checked whether the concentration of FAM19A5 protein changed after administration of cilostazol (phosphodiesterase-3 inhibitor as an adjunctive to antidepressants) (CLS) in patients with MDD. Cilostazol is a phosphodiesterase type 3 (PDE3) inhibitor. Inhibition of PDE3 activity leads to an increase in cAMP concentration in blood platelets and smooth muscles of blood vessels, inhibiting platelet aggregation and dilating blood vessels. The study included 80 participants with MDD. They were given cilostazol 50 mg or placebo twice daily plus escitalopram (selective serotonin reuptake inhibitors) (ESC) 20 mg once daily for six weeks. Serum FAM19A5 concentrations were statistically significantly lower in the CLS group compared to the placebo group after the end of drug administration. According to the authors, FAM19A5 may be clinically helpful in assessing antidepressant response.44
Also, in 2021, FAM19A5 was investigated to be associated with Parkinson’s disease (PD) and Parkinson’s disease with depression (PDD). Plasma FAM19A5 levels (2.456 ± 0.517) were shown to be significantly higher in the PD group compared to the control group (2.23 ± 0.457) (P < 0.001). Importantly, plasma FAM19A5 concentration was also significantly higher in the PDD subgroup (2.577 ± 0.408) compared with the nondepressed subgroup (2.406 ± 0.549) (P = 0.045 < 0.05). The authors suggest that plasma FAM19A5 may serve as potential tools for early prediction of PD and PDD in clinical settings.45
The use of FAM19A5 in preventing, diagnosing, or treating central nervous system injuries, degenerative brain diseases, or central nervous system diseases seems promising.
FAM19A5 in Oncological Diseases
The discovery of FAM19A5 increased interest in the function of this protein in oncology. Tavan Janvilisri et al in 2015 tested new serum biomarkers for differentiating cholangiocarcinoma from benign biliary tract diseases. Using a proteomic approach, Tavan Janvilisri et al tested new serum biomarkers for differentiating cholangiocarcinoma (CCA) from benign biliary tract disease (BBTD). At that time, the available tumor markers, including CA19-9 and CEA, were ineffective and had limited use due to low sensitivity and specificity. Serum samples from 19 CCA and 17 BBTD patients were used. Five of the most essential proteins were found that showed the maximum fold change between CCA and BBTD. It included MAGED4B, KIAA0321, RBAK, and UPF3B, as well as the TAFA5 protein, which was first named as FAM19A5. According to the authors, these proteins, including FAM19A5, will be clinically helpful in preventing misdiagnosis between CCA and BBTD as they have similar clinical manifestations.46
In the same year, the study by Zhiqing Hu et al evaluated the clinical and prognostic significance of TAFA5 in 90 human gastric cancer (GC) samples and the in vitro activity of TAFA5 in cultured gastric cancer cells. TAFA5 was upregulated in GC compared to adjacent normal tissues. Moreover, TAFA5 overexpression in GCs was associated with poor differentiation and poorer tumor, lymph node, and metastasis stages. In addition, high TAFA5 expression was correlated with poor patient outcomes. The authors suggest that TAFA5 has a significant effect on GC progression.47
The study by Yan-Fang Wang et al from 2020 included 25 patients with mantle cell lymphoma (MCL), one of modern oncology’s significant challenges. The mean serum FAM19A5 concentration in MCL patients was significantly higher than in the control group (P=0.0461). FAM19A5 expression in serum and lymph nodes showed a significant correlation (r=0.8683, P=0.001). Moreover, the mean recurrence/death-free survival of MCL patients with high, intermediate, and low FAM19A5 expression was 17, 27, and 37.5 months, respectively, which showed a significant statistical difference (P=0.0360). The conclusions suggest that FAM19A5 expression levels are increased in MCL patients, and patients with high FAM19A5 expression are at increased risk of disease relapse or death. According to the authors, FAM19A5 may be a new prognostic marker and potential therapeutic target for MCL.48
The 2022 study by Shoujing Luan et al aimed to investigate whether FAM19A5 could be a new target in diagnostics and prognostic in patients with thyroid cancer. It was observed that FAM19A5 levels were increased in thyroid cancer. FAM19A5 was an independent predictor of poor prognosis in thyroid cancer patients. According to the authors, FAM19A5 may serve as a potential biomarker in the diagnosis and prognosis of thyroid cancer.49
Although the concentration of FAM19A5 is higher in patients with selected oncological diseases, there is still no information on the importance of this protein as a tumor marker.
FAM19 – Other Research Directions
In addition to research on the association of the FAM19A5 protein with nervous system disorders and cancer, its other functions in the pathophysiology of diseases were also searched for. In 2017, MinYoung Park et al demonstrated that FAM19A5 strongly stimulates murine bone marrow-derived macrophages (BMDM), causing chemotactic migration and inhibiting osteoclastogenesis induced by the receptor activator for nuclear factor κ B ligand (RANKL) gene. The authors suggest that FAM19A5 and its target N-formyl peptide receptor 2 (FPR2) may act as a novel endogenous ligand/receptor negatively regulating osteoclastogenesis.31
In the same year, in nephron progenitor cells, a DNA region in intron 2 of the Wnt9b FAM19A5 target gene was identified and shown to be physically related to β-catenin. Whether this element could act as a cell type-specific enhancer in mice and humans was tested. Although in multiple contexts, Myc is a target of β-catenin, authors’ characterization of a cell type-specific enhancer for the Wnt9b/β-catenin target gene Fam19a5 shows that Myc and β-catenin cooperate to activate gene expression controlled by this element.50
Moreover, Yanfang Wang et al demonstrated that the FAM19A5/S1PR1 signaling pathway regulates mantle cell lymphoma viability and proliferation.51
N Sevane et al looked for reasons for the rapid livestock adaptation to extreme climatic conditions. They compared the methylomes of two Creole cattle breeds living in tropical environments with their putative Spanish ancestors. They wanted to understand the epigenetic mechanisms underlying the rapid adaptation of a domestic species to a new and more physiologically challenging environment. Candidate genes involved in adaptation processes in tropical areas have been revealed, including genes variously hyper- or hypomethylated above 80% in Creole samples showing biological features and functions related to the immune response, including the FAM19A5 gene. These data suggested the need for further research on the influence of this gene in disease resistance and the improvement of cattle productivity and welfare.52
In 2022, it was checked whether there is a link between FAM19A5 and cerebral small vessel disease (cSVD). A total of 344 patients with recent small subcortical infarction (RSSI) and 265 healthy controls were included in the study. FAM19A5 was highly expressed in the RSSI group (P = 0.023), showing a positive correlation with cerebral infarct volume (P < 0.01). Furthermore, higher FAM19A5 levels reflect better outcomes in patients with RSSI. According to the authors, increased FAM19A5 levels are associated with cerebral small vessel disease and lead to better treatment outcomes.53
Data on the association of FAM19A5 with physiology and other disorders still need to be made more explicit. There needs to be more information. A summary of data on the importance of FAM19A5 in physiology and metabolic disorders is presented in Table 2.
 |
Table 2 A Summary of Data on the Importance of FAM19A5 in Other Disorders |
Research on the relationship between FAM19A5 concentration and CNS functions or disorders has increased interest in the influence of FAM19A5 protein and energy homeostasis and metabolic disorders.
FAM19A5 - Metabolic Disorders
The role of different type of adipokines in metabolic disturbances for eg MetS is in the great interest among researchers all around the word. Each passing year brings about a deepening understanding of the mechanism underlying the function of adipokines such a leptin, visfatin, resistin and apelin (Table 1). Currently the search for a cause and effect relationship between FAM expression and the occurrence of metabolic disorders is still ongoing. The functions of this protein are still unclear and have been the subject of interest in several works. Undoubtedly, the influence of the tested protein on the centers responsible for maintaining energy homeostasis in the CNS, mainly the hypothalamus, probably plays an important role. In the meantime, clinical studies began to appear looking for a connection between FAM19A5 and metabolic disorders.
For the first time this relationship in 2008, Sarah Juel Paulsen et al showed that neuropeptide TAFA5 is expressed in the hypothalamic paraventricular nucleus and is regulated by dehydration. New genes involved in the hypothalamic control of energy homeostasis in rats were sought. The distribution of TAFA5 mRNA expression was analyzed using quantitative PCR on a rat cDNA tissue library. Previous reports confirmed that TAFA5 is mainly expressed in the central nervous system. Additionally, TAFA5 mRNA is highly expressed in the hypothalamic paraventricular nucleus (PVN), which binds vasopressin and oxytocin in magno- and paracellular neurons. It was also checked whether fasting or dehydration can affect TAFA5 mRNA levels. The results showed that TAFA5 mRNA was explicitly down-regulated in the PVN following water deprivation. The authors showed the need for further research because the significance of the TAFA5 protein remained unclear and unconfirmed.54
In the same year, the team of Jana Bundzikova et al cited previous results on the TAFA5 gene and protein in a review where reports on the response of substances co-expressed in hypothalamic magnocellular neurons to osmotic challenges in normal and Brattleboro rats.60 Knowledge about the function and function of the TAFA5 gene and protein remained unclear in 2008.60
The functions of FAM19A5 were further searched. Dasol Kang et al in 2020 showed that FAM19A5 was expressed in mouse hypothalamic cells expressing proopiomelanocortin (POMC) and neuropeptide Y (NPY)/agouti-related peptide (AgRP) and in microglia. Moreover, TNF-α, which induces inflammatory responses in the disease, significantly increases FAM19A5 expression in the hypothalamus. Additionally, inhibition of FAM19A5 expression reduced TNF-α-induced anorexia, body weight loss, and TNF-α-induced inflammatory factor expression in mice. The authors concluded that FAM19A5 plays an essential role in hypothalamic inflammatory responses. This suggested the connection of the FAM19A5 protein with the feeling of hunger and satiety also.61
Hoyun Kwak et al 2020 examined FAM19A5 protein levels and their gene expression in mice’s central and peripheral nervous system and peripheral tissues. In the results, total FAM19A5 levels in the central and peripheral nervous system were higher than in any peripheral tissues. They were also higher than the levels in adipose tissue. These results also emphasize the need to understand the detailed functions of the FAM19A5 protein in the nervous system and adipose tissue.59
In 2019, B. An et al wanted to identify candidate genes for the organism measurement features of beef cattle 463 Wagyu (n = 463 animals). In addition to FAM19A5, 11 genes have been discovered. From this, the FAM19A5 gene was considered a novel candidate gene associated with body dimensional characteristics in the beef cattle studied. The FAM19A5 gene was associated with animals’ hip height, height and body length.62 This is the first time this protein has been linked to the dimensional features of the animal body.
You-Bin Lee et al in 2019 showed that serum FAM19A5 concentrations (pg/mL) were higher in patients with type 2 diabetes mellitus [median (interquartile range), 172.70 (116.19, 286.42)] compared to those without diabetes [92.09 (70.32, 147.24)] (p<0.001). The association of FAM19A5 with other metabolic parameters was also checked, as well as with anthropometric measurements. Increases in the serum FAM19A5 tertile were associated with trends in waist-to-hip ratio, fasting plasma glucose, glycated hemoglobin, and mean shoulder pulse wave velocity. The authors’ novel results showed that FAM19A5 serum was positively correlated with waist circumference, waist-to-hip ratio, alanine aminotransferase, fasting plasma glucose, glycated hemoglobin, and mean shoulder pulse wave velocity. This was the first study to link FAM19A5 to selected inflammation parameters in people with type 2 diabetes.55
Xia Lei, in 2019, showed that the expression of FAM19A5 in various areas of the mouse brain is induced or suppressed by undernutrition and nutritional states. In male mice, FAM19A5 deficiency altered food intake patterns during the light-dark cycle. The authors highlighted for the first time the potential role of the FAM19A5 protein in modulating physiology and links to appetite.56
Fatemeh Ali Yari et al wanted to test whether FAM19A5 is associated with non-alcoholic fatty liver disease (NAFLD). Sixty-nine patients were included in the study, including 37 with NAFLD and 32 from the control group. In the results, the plasma FAM19A5 concentration in NAFLD patients was significantly lower in NAFLD patients compared with the control group. Moreover, significant negative correlations were observed between plasma FAM19A5 levels and BMI, visceral fat, alanine aminotransferase (ALT), aspartate aminotransferase (AST), liver stiffness (LS), and carotid intima media thickness (cIMT). This is the first time circulating FAM19A5 levels have been reported to be associated with NAFLD. The authors suggested continuing research to link FAM19A5 with liver disorders.57
It was also checked whether the concentration of FAM19A5 protein differs in people with MetS compared to healthy people. This was our study, the aim of which was to look for a relationship between FAM19A5 and MetS exponents for the first time. The concentration of FAM19A5 was determined in 45 people with MetS (group A) and 35 healthy people without MetS (group B). There were no differences in the blood levels of FAM19A5 between the groups. The level of FAM19A5 was 163.16±55.11 pg/mL and 197.57±112.89 pg/mL (p=0.1341) in subjects with and without MetS, respectively. In group A, there was a correlation between FAM19A5 and diastolic blood pressure (DBP) (R= –0.40) and high-density lipoprotein (HDL) levels (R= –0.37). In patients with MetS, elevated FAM19A5 serum levels may indicate dyslipidemia development.58
The FAM19A5 protein has also been shown to be associated with cardiovascular diseases. In 2018, Yingbao Wang et al examined whether adipose tissue-derived FAM19A5 regulates vascular pathology following trauma in rats. It was shown that the FAM19A5 protein was abundantly expressed in adipose tissue. Interestingly, FAM19A5 expression was decreased in obese mice. FAM19A5 overexpression significantly inhibited vascular smooth muscle cell proliferation, migration, and neointima formation in injured rat carotid arteries. The information from the 2018 project by Yingbao Wang et al was a breakthrough in the reports on the importance of FAMA19A5. The FAM19A5 protein was first identified as an adipokine.29
Fei Ma et al 2022 checked whether there are clinical associations between FAM19A5 levels and the presence and severity of coronary artery disease (CAD). The study included 186 patients with CAD and 58 without CAD who underwent coronary angiography (CAG). Serum FAM19A5 levels in the CAD group were significantly lower than in the non-CAD group [1.53 (1.13–2.27) ng/mL vs 2.33 (1.69–3.51) ng/mL; P = 0.013]. Logistic regression analysis showed that reduced serum FAM19A5 concentration was a risk factor for coronary heart disease (OR: 0.563, 95% CI: 0.409–0.776, p < 0.001). According to the authors, serum FAM19A5 concentration has been negatively associated with the presence and severity of coronary artery disease and may represent a new biomarker for diagnosing and determining the severity of coronary artery disease.63
Chen Weia et al 2022 checked whether FAM19A5 is associated with acute coronary syndrome (ACS). The study included 168 consecutive hospitalized patients with suspected ACS. After adjusting for confounding factors, serum FAM19A5 levels were independently associated with ACS (p < 0.05). Moreover, serum FAM19A5 levels correlated with ACS severity. FAM19A5 is, therefore, a new potential clinical biomarker of ACS.64
The summary of data about connections between FAM19A5 and metabolic disorders is presented in Table 3.
 |
Table 3 A Summary of Data on the Importance of FAM19A5 in Metabolic Disorders |
Considering that the growing obesity epidemic increases the number of diagnoses of cardiovascular diseases, it seems necessary to look for further links of the FAM19A5 protein with heart and blood vessel diseases and metabolic diseases. Some of the above results are contradictory and require further extension.
Abbreviations
MCP-1, monocytes chemoattractant protein-1; aCGH, array comparative genomic hybridization; ACS, acute coronary syndrome; AgRP, agouti-related peptide; ALT, glutamic pyruvic transferase; AST, aspartate transaminase; BAT, brown adipose tissue; BBTD, benign biliary tract disease; BMDM, bone marrow-derived macrophages; BMI, body mass index; CAD, coronary artery disease; CAG, coronary angiography; CAP-1, adenylyl cyclase-associated protein 1; CCA, cholangiocarcinoma; Cimt, carotid intima media thickness; CMKLR1, chemerin chemokine-like receptor 1; CNS, central nervous system; CRP, c-reactive protein; cSVD, cerebral small vessel disease; CT, computed tomography; DBP, diastolic blood pressure; DNA, deoxyribonucleic acid; DXA, double-beam X-ray absorptiometry; ESC, selective serotonin reuptake inhibitors; FAM19A5, TAFA Chemokine Like Family Member 5; FISH, fluorescence in situ hybridization; FPR2, N-formyl peptide receptor 2; GC, gastric cancer; HbA1c, glycosylated hemoglobin; HDL, high-density lipoprotein; IKK, IκB kinase; IL-6, interleukin-6; JNKs, c-Jun N-terminal kinase; KI, knock-in; LS, liver stiffness; MCL, mantle cell lymphoma; MDD, major depressive disorder; MetS, metabolic syndrome; MRI, magnetic resonance imaging; mRNA, messenger RNA; NAD, nicotinamide adenine dinucleotide; NAFLD, nonalcoholic fatty liver disease; NMOSD-AQP4, neuromyelitis optica spectrum disorders with positive aquaporin-4 antibody; NPY, neuropeptide Y; PCOS, polycystic ovary syndrome; PCR, polymerase chain reaction; PD, Parkinson’s disease; PDD, Parkinson’s disease with depression; POMC, proopiomelanocortin; PVN, paraventricular nucleus; RANKL, receptor activator for nuclear factor κ B ligand; RBP-4, retinol-binding protein 4; RSSI, recent small subcortical infarction; SNP, single nucleotide polymorphism; SVF, stromal vascular fraction cells; TAFA5, TAFA Chemokine Like Family Member 5; TG, triglycerides; TNF-α, tumor necrosis factor α; VSMCs, vascular smooth muscle cells; WAT, white adipose tissue; WC, waist circumference; WHO, World Health Organization; WHR, waist to hip ratio; WOBASZ, Multi-centre National Population Health Examination Study.
Summary
Serum FAM19A5 level is correlated with type 2 diabetes, NAFLD, waist circumference, waist-to-hip ratio, glutamic pyruvic transferase, fasting plasma glucose, HbA1c, and mean shoulder pulse wave velocity. However, considering today’s knowledge about FAM19A5, we cannot consider this protein a metabolic syndrome biomarker. The main reason for this answer to the question in the title is the divergent results of the studies presented above. It should be emphasized, however, that the importance of this adipocytokine in the pathogenesis of many diseases affecting various systems proves the excellent research potential of FAM19A5. It will also allow us to understand the importance of metabolic disorders in the entire body’s functioning. More information is needed on the association of the FAM19A5 protein with other substances, such as insulin. Like other adipokines, the FAM19A5 protein seems to be an essential substance in the human body.
Disclosure
Paweł Bogdański and Monika Szulińska are co-last authors for this study. The authors report no conflicts of interest in this work.
References
1. Thomas E, Fitzpatrick J, Etl Al MS, Taylor-Robinson SD, Bell JD. Whole body fat: content and distribution. Prog Nucl Magnet Res Spect. 2013;73:56–80. doi:10.1016/j.pnmrs.2013.04.001
2. Kuryszko J, Sławuta P, Sapikowski G. Secretory function of adipose tissue. Pol J Veterin Sci. 2016;19:441–446. doi:10.1515/pjvs-2016-0056
3. Colaianni G, Colucci S, Grano M. Anatomy and physiology of adipose tissue. In: Lenzi A, Migliaccio S, Donini L, editors. Multidisciplinary Approach to Obesity. Springer International Publishing; 2015:3–12.
4. Buczkowska M, Buczkowski K, Głogowska-Gruszka A, et al. Adipose tissue – the structure and its functions, with particular emphasis on the characteristics of selected adipokines and their effects on the organism. Med Og Nauk Zdr. 2019;25:162–169. doi:10.26444/monz/110429
5. Budzulak J, Majewska KA, Kędzia A. Malnutrition as the cause of growth retardation among children in developed countries. Ann Agric Environ Med. 2022;29:336–341. doi:10.26444/aaem/148010
6. Drygas W, Niklas AA, Piwońska A, et al. Multi-centre national population health examination survey (WOBASZ II study): assumptions, methods, and implementation. Kardiologia Polska (Polish Heart Journal). 2016;74:681–690. doi:10.5603/KP.a2015.0235
7. World Health Organisation. WHO EUROPEAN REGIONAL OBESITY REPORT 2022. Denmark; 2022.
8. Płaczkowska S, Pawlik-Sobiecka L, Kokot I, et al. Wykorzystanie bezpośrednich i pośrednich metod oceny profilu składu ciała u młodych osób – badanie pilotażowe. Fam Med Prim Care Rev. 2015;1:33–38.
9. Tchang BG, Saunders KH, Igel LI. Best practices in the management of overweight and obesity. Med Clin North Am. 2021;105:149–174. doi:10.1016/j.mcna.2020.08.018
10. Łuniewski M, Matyjaszek-Matuszek B, Lenart-Lipińska M. Diagnosis and non-invasive treatment of obesity in adults with type 2 diabetes mellitus: a review of guidelines. J Clin Med. 2023;12:4431. doi:10.3390/jcm12134431
11. Gaesser GA, Angadi SS. Obesity treatment: weight loss versus increasing fitness and physical activity for reducing health risks. iScience. 2021;24:102995. doi:10.1016/j.isci.2021.102995
12. Bogdański P, Filipiak KJ, Kowalska I, et al. Interdyscyplinarne stanowisko w sprawie rozpoznawania i leczenia otyłości. Forum Zaburzen Metabolicznych. 2020;11:47–54.
13. Ellulu MS, Patimah I, Khaza’ai H, et al. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;4:851–863. doi:10.5114/aoms.2016.58928
14. Khanna D, Khanna S, Khanna P, et al. Obesity: a Chronic Low-Grade Inflammation and Its Markers. Cureus. 2022;14(2):e22711. doi:10.7759/cureus.22711
15. Kolb H. Obese visceral fat tissue inflammation: from protective to detrimental? BMC Med. 2022;20:494. doi:10.1186/s12916-022-02672-y
16. Chwalba A, Machura E, Ziora K, Ziora D. The role of adipokines in the pathogenesis and course of selected respiratory diseases. Endokrynol Pol. 2019;70:504–510. doi:10.5603/EP.a2019.0051
17. Cook KS, Min HY, Johnson D, et al. Adipsin: a circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science. 1987;237(4813):402–405. doi:10.1126/science.3299705
18. Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;1(372):6505.
19. Al-Hussaniy HA, Alburghaif AH, Naji MA. Leptin hormone and its effectiveness in reproduction, metabolism, immunity, diabetes, hopes and ambitions. J Med Life. 2021;14(5):600–605. doi:10.25122/jml-2021-0153
20. Abdalla MMI. Role of visfatin in obesity-induced insulin resistance. World J Clin Cases. 2022;10:10840–10851. doi:10.12998/wjcc.v10.i30.10840
21. Borsuk A, Biernat W, Zięba D, et al. Wielokierunkowe działanie rezystyny w organizmie. Postępy Higieny i Medycyny Doświadczalnej. 2018;72:327–338. doi:10.5604/01.3001.0011.8252
22. Gandham R, Sumathi M, Dayanand C, et al. Apelin and its Receptor: an Overview. JCDR. 2019;13(6):BE01- BE06.
23. Stojek M. The role of chemerin in human disease. Postepy Hig Med Dosw. 2017;71:110–117. doi:10.5604/01.3001.0010.3795
24. Roguska J, Zubkiewicz-Kucharska A. Chemerin as an early marker of metabolic syndrome. Pediatr Endocrinol Diabetes Me. 2018;24:45–51. doi:10.18544/PEDM-24.01.0102
25. Badowska-Kozakiewicz AM. Biologiczna rola czynnika martwicy nowotworów α w fizjologii i patofizjologii. Przeglad Menopauzalny. 2013;141:12.
26. Dobrowolski P, Dudek D, Dyrbuś K,et al.; A joint position paper by the Polish Society of Hypertension, Polish Society for the Treatment of Obesity, Polish Lipid Association, Polish Association for Study of Liver, Polish Society of Family Medicine, Polish Society of Lifestyle Medicine, Division of Prevention and Epidemiology Polish Cardiac Society, “Club 30” Polish Cardiac Society, and Division of Metabolic and Bariatric Surgery Society of Polish Surgeons. Metabolic syndrome – a new definition and management guidelines. Arch Med Sci. 2022;18:1133–1156.
27. Wyrzykowski B. Epidemiologia zespołu metabolicznego w Polsce. Wyniki programu WOBASZ. Kardiologia Polska. 2005;63(6).
28. Mohamed SM, Shalaby MA, El-Shiekh RA, et al. Metabolic syndrome: risk factors, diagnosis, pathogenesis, and management with natural approaches. Food Chem Advan. 2023;3:100335. doi:10.1016/j.focha.2023.100335
29. Wang Y, Chen D, Zhang Y, et al. Novel adipokine, FAM19A5, inhibits neointima formation after injury through sphingosine-1-phosphate receptor 2. Circulation. 2018;138:48–63. doi:10.1161/CIRCULATIONAHA.117.032398
30. Shahapal A, Cho EB, Yong HJ, et al. FAM19A5 expression during embryogenesis and in the adult traumatic brain of FAM19A5-lacz knock-in mice. Front Neurosci. 2019;13:917. doi:10.3389/fnins.2019.00917
31. Park M, Kim HS, Lee M, et al. FAM19A5, a brain-specific chemokine, inhibits RANKL-induced osteoclast formation through formyl peptide receptor 2. Sci Rep. 2017;7. doi:10.1038/s41598-017-15586-0
32. Xie K, Liu L, Yin C, et al. Follistatin-Like 1 and family with sequence similarity to 19 member a5 levels are decreased in obese children and associated with glucose metabolism. Ann Nutr Metab. 2022;78:213–221. doi:10.1159/000524624
33. Tom Tang Y, Emtage P, Funk WD, et al. TAFA: a novel secreted family with conserved cysteine residues and restricted expression in the brain. Genomics. 2004;83:727–734. doi:10.1016/j.ygeno.2003.10.006
34. de Ståhl T D, Hartmann C, de Bustos C, et al. Chromosome 22 tiling-path array-CGH analysis identifies germ-line- and tumor-specific aberrations in patients with glioblastoma multiforme. Gen Chromos Can. 2005;44:161–169. doi:10.1002/gcc.20226
35. Kashevarova AA, Belyaeva EO, Nikonov AM, et al. Compound phenotype in a girl with r(22), concomitant microdeletion 22q13.32-q13.33 and mosaic monosomy 22. Mol Cytogenet. 2018;11:26. doi:10.1186/s13039-018-0375-3
36. Zheng C, Chen D, Zhang Y,et al.FAM19A1 is a new ligand for GPR1 that modulates neural stem-cell proliferation and differentiation. FASEB J. 2018;fj201800020RRR. doi:10.1096/fj.201800020RRR
37. Inan C, Sayin NC, Gurkan H, et al. Schizencephaly accompanied by occipital encephalocele and deletion of chromosome 22q13.32: a case report. Fetal Pediatr Pathol. 2019;38:496–502. doi:10.1080/15513815.2019.1604921
38. Khalaj AJ, Sterky FH, Sclip A, et al. Deorphanizing FAM19A proteins as pan-neurexin ligands with an unusual biosynthetic binding mechanism. J Cell Biol. 2020;219:e202004164. doi:10.1083/jcb.202004164
39. Yong HJ, Ha N, Cho EB, et al. The unique expression profile of FAM19A1 in the mouse brain and its association with hyperactivity, long-term memory and fear acquisition. Sci Rep. 2020;10:3969. doi:10.1038/s41598-020-60266-1
40. Li J, Li S, Song Y, et al. Association of serum FAM19A5 with cognitive impairment in vascular dementia. Dis Markers. 2020;2020:8895900. doi:10.1155/2020/8895900
41. Lee HL, Seok HY, Ryu H-W, et al. Serum FAM19A5 in neuromyelitis optica spectrum disorders: can it be a new biomarker representing clinical status? Mult Scler. 2020;26:1700–1707. doi:10.1177/1352458519885489
42. Huang S, Zheng C, Xie G, et al. FAM19A5/TAFA5, a novel neurokine, plays a crucial role in depressive-like and spatial memory-related behaviors in mice. Mol Psychiatry. 2021;26:2363–2379. doi:10.1038/s41380-020-0720-x
43. Han KM, Tae W-S, Kim A, et al. Serum FAM19A5 levels: a novel biomarker for neuroinflammation and neurodegeneration in major depressive disorder. Brain Behav Immun. 2020;87:852–859. doi:10.1016/j.bbi.2020.03.021
44. Abdallah MS, Ramadan AN, Omara‐Reda H, et al. Double-blind, randomized, placebo-controlled pilot study of the phosphodiesterase-3 inhibitor cilostazol as an adjunctive to antidepressants in patients with major depressive disorder. CNS Neurosci Ther. 2021;27:1540–1548. doi:10.1111/cns.13731
45. Li XN, Hao D-P, Qu M-J, et al. Development and validation of a plasma FAM19A5 and MRI-based radiomics model for prediction of parkinson’s disease and parkinson’s disease with depression. Front Neurosci. 2021;15:795539. doi:10.3389/fnins.2021.795539
46. Janvilisri T, Leelawat K, Roytrakul S, et al. Novel Serum Biomarkers to Differentiate Cholangiocarcinoma from Benign Biliary Tract Diseases Using a Proteomic Approach. Dis Markers. 2015;2015:105358. doi:10.1155/2015/105358
47. Hu Z, Niu G, Ren J, et al. TAFA5 promotes proliferation and migration in gastric cancer. Mol Med Rep. 2019;20:4477–4488. doi:10.3892/mmr.2019.10724
48. Wang YF, Huang S-Y, Zhang Z-H, et al. Clinical efficacy of allo-HSCT on FLT3-ITD positive AML patients. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2020;28(4):1183–1188. doi:10.19746/j.cnki.issn.1009-2137.2020.04.017
49. Luan S, Li J, Qin S, et al. FAM19A5 is an independent prognostic biomarker in thyroid cancer. Res Square. 2022. doi:10.21203/rs.3.rs-1712708/v1
50. Pan X, Karner CM, Carroll TJ. Myc cooperates with β-catenin to drive gene expression in nephron progenitor cells. Development. 2017;144:4173–4182. doi:10.1242/dev.153700
51. Wang Y, Zhang Z, Wan W, et al. FAM19A5/S1PR1 signaling pathway regulates the viability and proliferation of mantle cell lymphoma. J Recept Signal Transduction Res. 2022;42:225–229. doi:10.1080/10799893.2021.1895220
52. Sevane N, Martínez R, Bruford MW. Genome-wide differential DNA methylation in tropically adapted Creole cattle and their Iberian ancestors. Anim Genet. 2019;50:15–26. doi:10.1111/age.12731
53. Hao Z, Yang S, Yin R, et al. Increased level of FAM19A5 is associated with cerebral small vessel disease and leads to a better outcome. PeerJ. 2022;10:e13101. doi:10.7717/peerj.13101
54. Paulsen SJ, Christensen MT, Vrang N, et al. The putative neuropeptide TAFA5 is expressed in the hypothalamic paraventricular nucleus and is regulated by dehydration. Brain Research. 2008;1199:1–9. doi:10.1016/j.brainres.2007.12.074
55. Lee YB, Hwang H-J, Kim JA, et al. Association of serum FAM19A5 with metabolic and vascular risk factors in human subjects with or without type 2 diabetes. Diab Vasc Dis Res. 2019;16:530–538. doi:10.1177/1479164119860746
56. Lei X, Liu L, Terrillion CE, et al. FAM19A1, a brain-enriched and metabolically responsive neurokine, regulates food intake patterns and mouse behaviors. THE FASEB Journal. 2019;33:14734–14747. doi:10.1096/fj.201901232RR
57. Yari FA, Shabani P, Karami S, et al. Circulating levels of FAM19A5 are inversely associated with subclinical atherosclerosis in non-alcoholic fatty liver disease. BMC Endocr Disord. 2021;21:153. doi:10.1186/s12902-021-00820-8
58. Wesolek A, Skoracka K, Skrypnik K, et al. Assessment of progranulin and FAM19A5 protein blood levels in patients with metabolic syndrome. J Physiol Pharmacol. 2022;73.
59. Kwak H, Cho EH, Lee YN, et al. Is FAM19A5 an adipokine? Peripheral FAM19A5 in wild-type, FAM19A5 knock-out, and LacZ knock-in mice. bioRxiv. 2020. doi:10.1101/2020.02.19.955351
60. Bundzikova J, Pirnik Z, Zelena D, et al. Response of substances co-expressed in hypothalamic magnocellular neurons to osmotic challenges in normal and brattleboro rats. Cell Mol Neurobiol. 2008;28:1033–1047. doi:10.1007/s10571-008-9306-x
61. Kang D, Kim HR, Kim KK, et al. Brain-specific chemokine FAM19A5 induces hypothalamic inflammation. Biochem Biophys Res Commun. 2020;523:829–834. doi:10.1016/j.bbrc.2019.12.119
62. An B, Xia J, Chang T, et al. Genome-wide association study reveals candidate genes associated with body measurement traits in Chinese wagyu beef cattle. Anim Genet. 2019;50:386–390. doi:10.1111/age.12805
63. Ma F, Hao J, Zhao J, et al. Circulating FAM19A5 level is associated with the presence and severity of coronary artery disease. Int J Cardiol. 2022;354:50–55. doi:10.1016/j.ijcard.2022.03.011
64. Wei C, Liu Y, Xing E, et al. Association between novel pro- and anti- inflammatory adipocytokines in patients with acute coronary syndrome. Clin Appl Thromb Hemost. 2022;28:10760296221128020. doi:10.1177/10760296221128021
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
