Back to Journals » Nature and Science of Sleep » Volume 14
Calcium, Magnesium, Potassium, and Sodium Oxybates Oral Solution: A Lower-Sodium Alternative for Cataplexy or Excessive Daytime Sleepiness Associated with Narcolepsy
Authors Dauvilliers Y, Bogan RK , Šonka K , Partinen M, Foldvary-Schaefer N, Thorpy MJ
Received 19 November 2021
Accepted for publication 8 March 2022
Published 29 March 2022 Volume 2022:14 Pages 531—546
DOI https://doi.org/10.2147/NSS.S279345
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Ahmed BaHammam
Yves Dauvilliers,1,2 Richard K Bogan,3 Karel Šonka,4 Markku Partinen,5 Nancy Foldvary-Schaefer,6 Michael J Thorpy7
1Sleep and Wake Disorders Centre, Department of Neurology, Gui de Chauliac Hospital, Montpellier, France; 2University of Montpellier, INSERM Institute Neuroscience Montpellier (INM), Montpellier, France; 3University of South Carolina School of Medicine, Columbia, SC, USA; 4Department of Neurology, First Faculty of Medicine, Charles University and General University Hospital, Prague, Czech Republic; 5Helsinki Sleep Clinic, Terveystalo Healthcare, and Department of Clinical Neurosciences, University of Helsinki, Helsinki, Finland; 6Sleep Disorders Center, Department of Neurology, Cleveland Clinic, Cleveland, OH, USA; 7Department of Neurology, Albert Einstein College of Medicine, Bronx, NY, USA
Correspondence: Yves Dauvilliers, 80 Avenue Augustin Fliche, Montpellier, 34295, France, Tel +334 67 33 72 77, Email [email protected]
Abstract: Lower-sodium oxybate (LXB) is an oxybate medication approved to treat cataplexy or excessive daytime sleepiness (EDS) in patients with narcolepsy 7 years of age and older in the United States. LXB was developed as an alternative to sodium oxybate (SXB), because the incidence of cardiovascular comorbidities is higher in patients with narcolepsy and there is an elevated cardiovascular risk associated with high sodium consumption. LXB has a unique formulation of calcium, magnesium, potassium, and sodium ions, containing 92% less sodium than SXB. Whereas the active oxybate moiety is the same for LXB and SXB, their pharmacokinetic profiles are not bioequivalent; therefore, a phase 3 trial in participants with narcolepsy was conducted for LXB. This review summarizes the background on oxybate as a therapeutic agent and its potential mechanism of action on the gamma-aminobutyric acid type B (GABAB) receptor at noradrenergic and dopaminergic neurons, as well as at thalamocortical neurons. The rationale leading to the development of LXB as a lower-sodium alternative to SXB and the key efficacy and safety data supporting its approval for both adult and pediatric patients with narcolepsy are also discussed. LXB was approved in August 2021 in the United States for the treatment of idiopathic hypersomnia in adults. Potential future developments in the field of oxybate medications may include novel formulations and expanded indications for other diseases.
Keywords: narcolepsy, therapeutics, drug development, cataplexy, excessive daytime sleepiness, idiopathic hypersomnia
Graphical Abstract:
Plain Language Summary
Lower-sodium oxybate (Xywav™) is a medication for people with narcolepsy 7 years of age and older. Xywav treats excessive daytime sleepiness (EDS) and cataplexy (attacks of muscle weakness triggered by emotion). Xywav was developed as an alternative to the first such medication, sodium oxybate (Xyrem®), as patients with narcolepsy have a higher rate of cardiovascular disease, and lowering the amount of sodium they consume could help to reduce their cardiovascular risk. Researchers combined calcium, magnesium, and potassium ions with a small amount of sodium to make Xywav, containing 92% less sodium than Xyrem. Strict controls are in place that have successfully restricted how they are distributed due to the potential for abuse of Xyrem and Xywav.
Xywav and Xyrem have the same amount of oxybate, which is the part that affects narcolepsy symptoms. How oxybate affects EDS and cataplexy is not well understood, but it may act on a specific receptor (gamma-aminobutyric acid type B [GABAB]) present in parts of the brain controlling sleep and wake. Although the body absorbs and processes Xywav slightly differently than Xyrem, both medications bring about improvements in EDS and cataplexy symptoms in narcolepsy. Adverse side effects with Xywav are like those seen in previous studies with Xyrem. Xywav is also approved to treat idiopathic hypersomnia, another sleep disorder, in adults.
Introduction
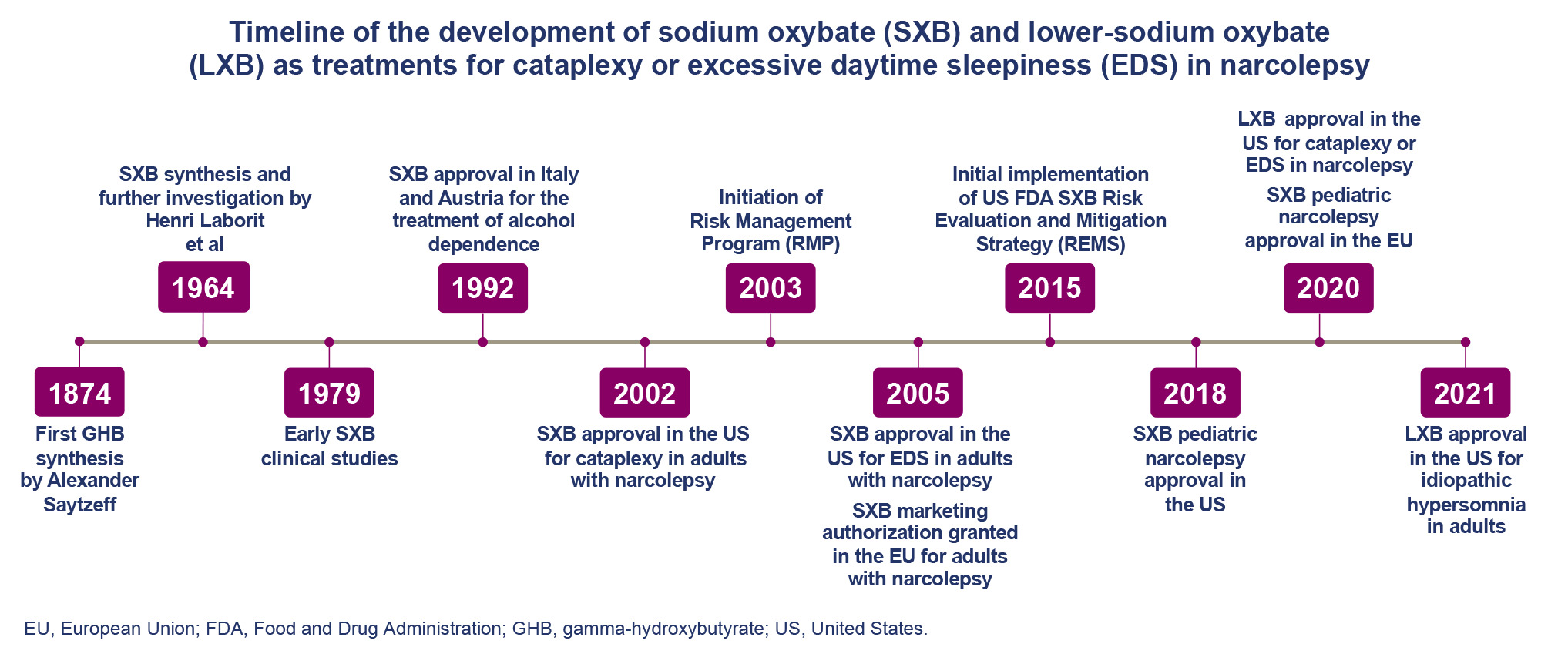
Sodium oxybate (SXB), first developed in the 1960s, is a central nervous system depressant recommended by the American Academy of Sleep Medicine, European guidelines, and expert statements for the treatment of excessive daytime sleepiness (EDS) and cataplexy in narcolepsy.1,2 SXB was first approved to treat cataplexy in patients with narcolepsy in the United States (2002) and in Europe (2006).3–6 SXB is currently approved in the United States for the treatment of cataplexy or EDS in patients 7 years of age and older with narcolepsy and in Europe for the treatment of narcolepsy with cataplexy in patients 7 years of age and older.4,7 The initial approvals of SXB for use in adult patients with narcolepsy in the early 2000s were based on a number of short- and long-term clinical trials evaluating its safety and efficacy in improving symptoms of narcolepsy, including EDS, cataplexy, and disrupted nighttime sleep.8–12 The mechanism of action for the therapeutic effect of SXB is incompletely understood but is hypothesized to be driven primarily by activity of its active oxybate moiety on neurons with gamma-aminobutyric acid (GABA) type B (GABAB) receptors in regions controlling sleep-wake homeostasis.4 Although illicit use of the active drug oxybate, also known as gamma-hydroxybutyrate (GHB), was reported around the time of its approval, monitoring for instances of abuse through a strictly controlled access program has shown low diversion of prescription SXB.6,13 GHB is at times used to refer to gamma-hydroxybutyrate (as above), and other times to its sodium salt (sodium gamma-hydroxybutyrate or sodium 4-hydroxybutyrate14); SXB is the international nonproprietary name for sodium gamma-hydroxybutyrate.
Lower-sodium oxybate (LXB; Xywav™; previously known as JZP-258), which preserves the active oxybate moiety, was developed as a lower-sodium alternative to SXB to reduce the cardiovascular risk associated with high sodium intake.15–18 In line with recent efforts from the US Food and Drug Administration (FDA) to reduce dietary sodium intake in the general population,19 LXB was designated as clinically superior to SXB in patients with narcolepsy by means of greater safety due to its greatly reduced chronic sodium burden; the FDA expects that
“The differences in the sodium content of the two products at the recommended doses will be clinically meaningful in reducing cardiovascular morbidity in a substantial proportion of patients for whom the drug is indicated.”18
Although the pharmacokinetic (PK) profile of LXB is not bioequivalent to SXB, its efficacy and safety are comparable.20–22 Similar to SXB, LXB has been approved in the United States for the treatment of cataplexy or EDS in patients 7 years of age and older with narcolepsy.4,23
The objective of this review is to describe the history leading to the development of LXB and its place in the treatment of patients with narcolepsy.
Historical Perspectives on Oxybate Development
Early Studies of Oxybate
Oxybate (as GHB) is both an endogenous metabolite and a precursor of the inhibitory neurotransmitter GABA; thus, GHB and GABA may be interconverted by endogenous metabolic processes.24 GHB was first synthesized in the late 1800s by adding a hydroxyl group on the 4th carbon of butyric acid, whereas the sodium salt of oxybate, SXB (also known as sodium 4-hydroxybutyrate), was developed in the early 1960s.25,26 This modification was made following the observation that butyric acid had a sedative effect but was mostly rendered inactive via β-oxidation, limiting its therapeutic potential, and ultimately excreted in urine.27,28 In their characterization of SXB, Laborit et al noted an inhibitory effect on synaptic firing and muscle responses in rat and cat models as well as a hypnotic effect when SXB was used in patients with psychiatric disorders such as depression and psychosis.29,30 Following this, SXB (then called GHB) was studied in patients with narcolepsy with cataplexy in the 1970s under the rationale that it could normalize the disrupted nighttime sleep seen in these patients. In this early trial, SXB was found to improve nighttime sleep quality and reduce symptoms of both EDS and cataplexy in patients with narcolepsy.31 SXB has also been used as a general anesthetic when administered intravenously, but does not produce complete surgical anesthesia when administered alone in adults.26
Clinical Trials and Approval
The first approval for SXB was granted in Italy and Austria in 1992 for the treatment of alcohol dependence.3 SXB has been shown to suppress symptoms of alcohol withdrawal syndrome in humans by mimicking the effects of ethanol within the central nervous system and leading to indirect activation of GABA type A (GABAA) receptors, converting oxybate to GABA. However, a study published in 2021 failed to find a significant difference between SXB and placebo for the primary efficacy endpoint of percentage of days abstinent from alcohol.32 In 2002, SXB was granted its initial approval (as Xyrem®) by the FDA for the treatment of cataplexy in adults with narcolepsy.4 In line with its demonstrated efficacy for reducing EDS as well as improving overall disease severity in participants with narcolepsy during a set of randomized-controlled trials (Table 1),8–12,21,33,34 SXB was granted an additional FDA approval for the treatment of EDS in adult patients with narcolepsy in 2005.6 In the same year, SXB also received approval for the treatment of cataplexy in patients with narcolepsy by Health Canada’s Therapeutic Products Directorate and marketing authorization for the treatment of patients with narcolepsy with cataplexy from the European Medicines Agency. SXB has also demonstrated efficacy for the treatment of cataplexy and EDS in pediatric patients with narcolepsy,33 and its approval has since been extended to patients 7 years of age and older.4,23
 |
Table 1 Key Findings from Historical Studies of Sodium Oxybate in Narcolepsy and a Recent Study of Lower-Sodium Oxybate in Idiopathic Hypersomnia8–12,21,33,34 |
Oxybate Safety, Abuse, and Misuse
Although SXB has been approved as a pharmacotherapy after demonstrating clinical efficacy and safety in multiple trials, and is approved in some countries to treat alcohol withdrawal syndrome, there is also potential for illicit use. Although not a common adverse effect of SXB or LXB when taken therapeutically, seizures have been noted in people who misuse GHB, likely via its effect on GABAB receptors.35 In the United States, illicit GHB is designated a Schedule I controlled substance under the Controlled Substances Act; however, FDA-approved products containing SXB are controlled under Schedule III.36 Schedule I includes drugs with no currently accepted medical use and high potential for abuse.37 Schedule III includes drugs with moderate to low potential for physical and psychological dependence. In the year following SXB’s approval by the FDA, the Xyrem® Risk Management Program (RMP) was initiated to control distribution of the drug, thereby minimizing the potential for diversion and promoting safe use.38 This program involved the utilization of a highly secure, centralized pharmacy as well as the implementation of both a physician and patient education program to ensure compliance with guidelines for SXB prescription and use, and the collection of post-marketing data around safety and incidences of misuse and abuse for consideration by the FDA.
A review of post-marketing safety data collected in the 5 years following approval found that, among approximately 26,000 patients prescribed SXB, there were 14 cases of SXB abuse reported worldwide.6 Cases of potential abuse included inappropriate daytime use, solicitation of a higher dose, intentional overdose, and providing the drug to others; 10 patients (0.039%) met the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria for substance abuse. Of the approximately 600,000 bottles of SXB distributed during the study timeframe, 5 (0.0009%) were diverted and used by someone other than the intended patient.6
The Xyrem® Risk Evaluation and Mitigation Strategy (REMS) program began in 2015, succeeding the prior RMP. Following the approval of LXB, this program was expanded to include Xywav. Data collected from 17,037 patients in the REMS over 2016 and 2017 evidenced that controlled access to SXB was maintained, with no patients shipped SXB under more than 1 name or under overlapping active prescriptions.13 Thirteen out of 146,426 (0.009%) shipments and 26 out of 375,173 (0.007%) bottles of SXB were lost and unrecovered. Additionally, few notifications regarding abuse (n = 31), misuse (n = 343), or diversion (n = 22) were discussed by the central pharmacy with providers.
These data illustrate that the restricted distribution and patient and physician education programs employed to prevent SXB misuse and diversion have been effective, and that the abuse potential for prescribed oxybate medications is low.
The Symptomatology of Narcolepsy
Narcolepsy is a commonly used name of 2 central disorders of hypersomnolence (type 1 [NT1] and type 2 [NT2]), with an estimated overall prevalence of 30.6 to 56.3 per 100,000 persons in the United States and 1 per 2000 persons globally.39–42 The core symptom of narcolepsy is EDS (cataplexy, which is sudden loss of muscle tone, is observed only in NT1); patients also often experience hypnagogic/hypnopompic hallucinations, sleep paralysis, and disrupted nighttime sleep.41–44 The symptomatology associated with narcolepsy is hypothesized to result in part from a combination of genetic predisposition and environmental triggers that lead to improper or reduced functioning of the hypocretin-producing neurons.45 In terms of cataplexy, the exact neural mechanisms are unknown, but it has been hypothesized to result from the inappropriate occurrence of REM sleep paralysis during periods of wakefulness.46 During wakefulness in healthy individuals, hypocretin-producing neurons stimulate locus coeruleus neurons, which suppress REM sleep and provide excitatory input to motor neurons to maintain muscle tone.46,47 When there is a loss of hypocretin-producing neurons, this pathway can become unstable, weakening the suppression of REM sleep and allowing episodes of motor neuron inhibition (manifested as episodes of cataplexy) to occur more easily during wakefulness.47 Importantly, however, deficiency of hypocretin (also called orexin) does not always lead to narcolepsy, and not all patients with narcolepsy have impaired hypocretin circuitry.45
The Mechanism of Action for Oxybate’s Therapeutic Effect
Despite its long history of study and demonstrated efficacy for reducing symptoms of narcolepsy, SXB’s mechanism of action remains largely unknown. The physiological regulation of sleep is coordinated across multiple systems of wake-promoting and sleep-promoting neuron groups.48 Although a pathological loss of the neuropeptide hypocretin underlies NT1,45,49 SXB’s therapeutic effect is not thought to depend on modulating the hypocretin system. Instead, SXB’s therapeutic effects on cataplexy and EDS are hypothesized to be driven through the GABAB receptor at noradrenergic and dopaminergic neurons, as well as at thalamocortical neurons (Figure 1).4,50–59
 |
Figure 1 Neurobiological effects of oxybate. Data from 4,50–59 Abbreviations: GABA, gamma-aminobutyric acid; GHB, gamma-hydroxybutyrate; mRNA, messenger ribonucleic acid; SXB, sodium oxybate. |
GABA
GABA is the primary inhibitory neurotransmitter in the central nervous system, and is active at 3 receptors, GABAA, GABAB, and GABAC.60 GABAergic neurons in the sleep-promoting ventrolateral preoptic nucleus and median preoptic nucleus strongly innervate and inhibit wake-promoting dopaminergic neuron groups in the ventral tegmental area and noradrenergic neuron groups in the locus coeruleus, suppressing their activity and thereby allowing sleep to occur.61 SXB, when metabolized to GHB, binds to both the GHB-specific receptor and the GABAB receptor, but is thought to primarily exert its therapeutic effects through the GABAB receptor. Several lines of evidence support this, including the findings that most actions of exogenous GHB have been shown to be blocked by GABAB receptor antagonists and that GHB has minimal behavioral or physiological effects in mice lacking functional GABAB receptors.51
Dopamine
Dopamine is a monoaminergic neurotransmitter that is involved in the regulation of sleep-wake states through its actions in the striatum and ventral tegmental area and is involved in the therapeutic response to many wake-promoting therapeutics.62 Such agents primarily increase extracellular dopamine levels either by blocking its reuptake (eg, modafinil, methylphenidate) or by enhancing its release from presynaptic terminals (eg, amphetamine).63,64 The ventral tegmental area contains a high concentration of endogenous GHB, indicating that GHB has the potential to modulate the activity of dopamine neurons.24
In hypocretin knockout mice, specific activation of dopamine-1 receptors decreased sleep attacks, whereas specific blockade of the same receptors increased sleep attacks. Specific activation and blockade of dopamine-2 receptors increased and decreased cataplexy, respectively, indicating that dopamine could differentially impact on symptoms of narcolepsy in a receptor-specific manner.65 SXB has been shown to inhibit dopaminergic neuron activity,57,66,67 which is later followed by enhanced release of dopamine.56 This mechanism is hypothesized to acutely promote sleep during the night by suppressing dopamine concentrations when oxybate levels are high and to enhance wakefulness during the day as the accumulated dopamine is subsequently released. Chronic treatment with SXB increases dopamine-1 and dopamine-2 receptor mRNA expression,59 possibly contributing to the therapeutic effect on cataplexy, which takes several weeks to manifest.65
Norepinephrine
Norepinephrine (also called noradrenaline) is another monoaminergic neurotransmitter implicated in the control of muscle tone and sleep.68 Noradrenergic neurons are prominent in the locus coeruleus where they exhibit sleep/wake-dependent activity, with their highest activity during wakefulness and inactivity during rapid-eye movement (REM) sleep. Norepinephrine re-uptake inhibitors reduce narcoleptic episodes in hypocretin-deficient mice.69 No prominent differences were observed for cerebrospinal fluid levels of monoamines, their metabolites, and trace amine levels, and few associations were found between those molecules and important clinical or neurophysiological parameters in NT1, narcolepsy type 2 (NT2), idiopathic hypersomnia, and participants without objective sleepiness.70
Because the therapeutic effect of oxybate is not thought to be dependent on the hypocretin system, the precise mechanism via which oxybate may improve cataplexy is still unclear. In rats, sustained oxybate administration attenuated the evoked burst firing of norepinephrine neurons in the locus coeruleus,58 which are known to be inhibited in a GABAB-dependent manner.71 Similar to what has been hypothesized for dopamine, SXB is therefore hypothesized to improve symptoms of narcolepsy by inhibiting noradrenergic signaling at night to promote improved sleep and allow for the accumulation of synaptic norepinephrine, which may subsequently increase wakefulness and reduce cataplexy during the day.
Thalamocortical Neurons
Experimental evidence has also demonstrated that SXB specifically inhibits thalamocortical neurons through a GABAB-dependent mechanism, which may increase slow-wave sleep and improve sleep consolidation.51,72–75 This improvement in nighttime sleep quality is likely to be beneficial for daytime alertness. The therapeutic effect of oxybate medications on the diverse symptomatology of narcolepsy is therefore likely to involve a multifaceted modulation of multiple neuronal groups across several brain areas regulating sleep and muscle tone.
Rationale for the Development of Lower-Sodium Oxybate
Patients with narcolepsy have a high comorbidity burden, including both medical and psychiatric diseases.76,77 Of particular relevance to SXB, patients with narcolepsy are more likely to be obese and have higher odds for developing multiple cardiovascular diseases (CVDs), including hypertension, stroke, myocardial infarction, and heart failure, especially when treated with wake-promoting agents.78–81 Hypocretin is thought to regulate autonomic processes in sync with sleep/wake cycles, and hypocretin deficiency in NT1 is associated with disrupted nighttime blood pressure regulation, resulting in attenuated nocturnal blood pressure dipping (an independent risk factor for the development of CVD).82–85 Furthermore, consumption of dietary sodium and sodium-containing medications is associated with increased cardiovascular risk in the general population,15–17 and the FDA recently published guidelines seeking to reduce dietary sodium intake.19
In 2019, the National Academy of Sciences, Engineering, and Medicine established 2300 mg/day as the Chronic Disease Risk Reduction (CDRR) intake at which sodium reduction is expected to reduce chronic disease risk within an apparently healthy, adult population; thresholds are as low as 1200 mg/day in children.86 At the recommended dosages in adults with narcolepsy (6–9 g/night), SXB contributes 1100–1640 mg to daily sodium intake. For pediatric doses of 1–9 g/night, SXB contributes 182–1640 mg sodium per day.4 In consideration of the high sodium content of SXB and the cardiovascular risk in patients with narcolepsy, LXB was developed as an alternative to SXB. As previously noted, LXB contains the same active moiety as SXB, but has a unique composition of calcium, magnesium, potassium, and sodium cations resulting in 92% less sodium (87–131 mg in the dose range of 6–9 g/night).21 By preserving the active oxybate moiety but reducing the total sodium content, LXB is thought likely to reduce CVD risk for patients with narcolepsy relative to treatment with SXB.18
Early Development of LXB and Pharmacokinetics Data
In an effort to reduce the sodium content of oxybate-containing medications, the first patent application for a mixed-cation oxybate formulation was filed by Jazz Pharmaceuticals in 2012 and granted in 2013.87 Key considerations in developing a new formulation included reduced sodium across all dosages, cation concentrations well within dietary reference intake guidelines, and the management of cation levels per dose to minimize the potential for adverse effects. It was also deemed important to maintain the concentration of oxybate at the same concentration found in SXB (0.413 g/mL; 3.96 M) and to preserve the same total oxybate salts concentration as in SXB (0.5 g/mL) to simplify dosing and transitioning from SXB. A multiple-cation formulation was therefore pursued in order to avoid exceeding recommended intake levels for any individual cation.
A set of preclinical studies was therefore carried out to identify the best candidate formulation with which to move forward.87 One such preclinical study found that oxybate permeability was similar for individual cations and mixed-cation formulations in vitro and that there was no difference in gastrointestinal motility in mice when comparing SXB with individual-cation oxybates (potassium, calcium, or magnesium oxybate) or with comparing SXB with 2 mixed-cation oxybate formulations. A PK study comparing individual- and mixed-cation formulations in rats found that plasma concentration-time (area under the curve [AUC]) values were greatest for SXB and lowest for magnesium oxybate and mixed-cation formulations. Finally, a bioavailability study in dogs comparing SXB and 2 doses of a mixed-cation oxybate demonstrated bioequivalence between both formulations based on the maximum plasma concentration (Cmax) and AUC. Based on these studies, 2 lead mixed-cation candidates containing different formulations of cations were identified for further exploration, JZP-507 and LXB, with 50% and 92% less sodium than SXB, respectively. The amounts of calcium, magnesium, potassium, and sodium in LXB are all within recommended intake levels, even at the highest recommended dose, and are thus unlikely to be associated with adverse effects.87
In Phase 1 PK studies, JZP-507 demonstrated bioequivalence (similar AUC and Cmax) compared with SXB, whereas LXB compared with SXB had a similar AUC but a lower Cmax and delayed time to Cmax (tmax).20 Since absorption of oxybate is primarily mediated by active transport and dependent on sodium concentration, this delayed absorption is hypothesized to be due to the reduced sodium content in LXB.88 Though LXB was not bioequivalent to SXB and thus required a phase 3 clinical trial for regulatory approval, a decision was made to pursue this formulation over JZP-507 given its greater reduction in sodium content.
Key Data from the Phase 3 Study of LXB for Adult Patients with Narcolepsy
In 2017, the first pivotal study of LXB was begun; the clinical trial had a placebo-controlled, double-blind, randomized withdrawal design and was conducted in adult participants with narcolepsy with cataplexy (15–006; NCT03030599; Table 1).21 Participants could enter this study taking either SXB alone, SXB plus other anticataplectics, or other anticataplectics alone, or they could be anticataplectic treatment naive. Enrolled participants tapered and eventually stopped anticataplectics during a 12-week open-label optimized treatment and titration period, during which all participants were titrated to their individually optimized dose of LXB with a maximal increase of 1.5 g/night/week (up to a maximum total dose of 9 g/night). The titration rate in the clinical study was the same as is recommended in the labels for LXB and SXB.4,7,23 Following a 2-week stable-dose period (SDP), participants were randomized 1:1 to either continue LXB treatment or switch to placebo during a 2-week, double-blind, randomized withdrawal period (DBRWP). Participants then completed a 2-week safety follow-up period with optional enrollment into a 24-week open-label extension study.
LXB demonstrated efficacy for the treatment of both cataplexy and EDS in narcolepsy. Changes in the frequency of cataplexy attacks and cataplexy-free days during open-label LXB treatment in the OLOTTP and SDP depended on the type of treatment at study entry (Figure 2). Randomization to placebo treatment during the DBRWP (n = 65) was associated with a median increase of 2.35 in the weekly number of cataplexy attacks, whereas the median change was 0.00 in participants (n = 69) randomized to continued LXB treatment. Randomization to placebo treatment during the DBRWP was associated with a median increase of 2.0 in Epworth Sleepiness Scale (ESS) score from the end of SDP, whereas the median change was 0.0 in participants randomized to continued LXB treatment. Participants randomized to placebo during the DBRWP evidenced worsening on the Patient Global Impression of Change and Clinical Global Impression of Change measures, as well as poorer results on quality of life measures (36-item Short-Form Health Survey Version 2 and EuroQoL EQ-5D-5L visual analog scale) relative to those who continued LXB.21
LXB’s safety profile in this trial was similar to that previously described for SXB,4,8,10–12 though participants transitioning from SXB to LXB had a lower overall incidence of the most common treatment-emergent adverse events (TEAEs; headache, nausea, and dizziness). In a post hoc analysis, it was reported that the majority of TEAEs with LXB occurred early in the study and were generally of short duration (except for decreased appetite).89 This is consistent with findings on the timing of the incidence of TEAEs in clinical trials of SXB in participants with narcolepsy.90
Although there are insufficient data on the developmental risk associated with the use of either LXB or SXB in pregnant women, the prescribing information for both medicines contains a warning that they may cause fetal harm based on data in animals.4,23 Although there was no conclusive evidence of developmental toxicity in rats or rabbits administered SXB during organogenesis, oral administration to rats (during pregnancy and lactation) at clinically relevant doses led to increased stillbirths and lower offspring viability and growth. Further, it is known that GHB is excreted in human milk after oral administration of SXB, but there is a lack of evidence relating to the impact of SXB or LXB on breast milk production in mothers or any associated risk for a breastfed infant.
The hypothesis that reducing the sodium content in LXB may reduce the CVD risk for patients with narcolepsy has not been confirmed. The pivotal clinical trial of LXB in narcolepsy was designed to establish efficacy and the overall safety profile, but was not designed to assess cardiovascular risk (such as with surrogate endpoints including ambulatory blood pressure monitoring or measures of endothelial functioning81). Additional research comparing cardiovascular outcomes between patients with narcolepsy taking different oxybate formulations would be of interest; however, such trials would be difficult or impossible to conduct, given that narcolepsy is a rare disease and the studies would require long-term follow-up of large populations. Nonetheless, prior research has demonstrated that CVD risk and cardiovascular risk factors are elevated in patients with narcolepsy compared with controls.78–81 Furthermore, the FDA continues to stress the importance of reducing total sodium intake in the general population, given the well-established role of high sodium intake as a cardiovascular risk factor.19,91,92 Finally, the FDA has recognized the clinical superiority of LXB as compared with SXB in patients with narcolepsy, specifically because of the greatly reduced chronic sodium burden and expected clinically meaningful reductions in cardiovascular morbidity in a substantial proportion of patients for whom the drug is indicated.18
Approval of LXB for Pediatric Patients with Narcolepsy
Together with its approval for adult patients with narcolepsy, LXB was also granted FDA approval for pediatric patients with narcolepsy. The FDA approved LXB for use in this population in the absence of a separate registration trial.22 Instead, pediatric approval was granted based on the established efficacy and safety of SXB in this population, the equivalent efficacy and safety of SXB and LXB in adults, and population PK studies.
A clinical study of SXB in pediatric patients with narcolepsy93 showed that SXB had comparable efficacy in pediatric and adult participants, while clinical studies of LXB21 and SXB8,10 demonstrated comparable efficacy across both formulations in adults. It was therefore hypothesized that LXB and SXB were likely to have similar efficacy in pediatric patients. As discussed previously, a PK study of SXB and LXB in healthy adult participants found that LXB had a later tmax, and a lower Cmax, but a similar AUC compared with SXB.20 The PK properties of SXB also were determined to be similar in both adults and children with narcolepsy based on 2 additional PK studies.94,95 A population PK model previously developed to support the SXB pediatric regulatory filing was then adapted to include data from LXB95; this model identified that weight was the major contributing factor to oxybate PK for both SXB and LXB, and showed that a similar dose-exposure relationship existed for SXB and LXB in both adult and pediatric participants with narcolepsy.
Based on comparable efficacy of SXB and LXB in adults with narcolepsy, the demonstrated efficacy of SXB in children and adolescents with narcolepsy, and similar modeled adult and pediatric population PK for SXB and LXB, regulatory approval was granted for the use of LXB to treat cataplexy or EDS in pediatric patients (7 years of age and older) with narcolepsy.23 The weight-based dosing recommendations for LXB in pediatric patients are the same as those for SXB, and prescribers may transition their SXB dose gram-for-gram to LXB.
Guidelines on Oxybate Use in Patients with Central Disorders of Hypersomnolence
In 2007, SXB was recommended by the American Academy of Sleep Medicine (AASM) as a standard of care for the treatment of cataplexy, EDS, and disrupted nighttime sleep due to narcolepsy, with an optional recommendation for its use in the treatment of hypnagogic hallucinations and sleep paralysis.96 In 2021, an update to these guidelines was published based on literature searches performed in 2017, 2018, and August 2020 that were reviewed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework. SXB was given a strong recommendation for its use in narcolepsy in adults, based on the findings that treatment with SXB led to clinically significant improvements in cataplexy, EDS, and overall disease severity.1,97 SXB was also given a conditional recommendation for the treatment of pediatric narcolepsy due to its demonstrated clinical improvements in EDS, cataplexy, and disease severity, but the quality of the evidence was downgraded in accordance with the GRADE framework, as fewer than 100 patients were analyzed in the clinical trial of SXB in pediatric participants with narcolepsy. SXB was also given a conditional recommendation for its use in other disorders of central hypersomnolence (idiopathic hypersomnia and hypersomnia secondary to alpha synucleinopathies [Parkinson’s disease]), reflecting its potential for the treatment of EDS outside of narcolepsy.
A corresponding update to the European guidelines on the management of narcolepsy in adults and children was developed by a task force nominated by the European Academy of Neurology, European Sleep Research Society, and European Narcolepsy Network. Following a systematic literature review performed from July to October 2018, and an updated literature review in July 2020, the task force strongly recommended SXB in adults for the treatment of EDS, cataplexy, and disrupted nighttime sleep, with a weak recommendation for the treatment of sleep paralysis/hypnagogic and hypnopompic hallucinations.2 In pediatric patients (>6 years of age), SXB was strongly recommended for the treatment of cataplexy and EDS, and given a weak recommendation for the treatment of disrupted nighttime sleep, sleep paralysis, and hypnagogic and hypnopompic hallucinations.
Though LXB demonstrated comparable efficacy and safety to SXB in its phase 3 trial,21 as previously discussed, these data were not considered during the development of either set of 2021 guidelines. This was not due to the quality of evaluable evidence; rather, LXB was excluded because the publication of the data on its pivotal trial (in October 2020) came after the final update to the literature searches performed by either task force.
Perspectives on Future Directions in Oxybate Development and Clinical Use
Despite a nearly 70-year history of research and clinical use, advancements in the field of oxybate-based medications are ongoing and include both the development of new formulations and the assessment of oxybate for use in patients with other disease states. Based on the current knowledge of oxybate’s mechanism of action and its utility in patients with EDS without hypocretin deficiency (such as NT2 and idiopathic hypersomnia), oxybate can improve sleepiness even without the hypocretin deficiency observed in NT1. As such, oxybate-based medications are therefore likely to be effective for multiple hypersomnolence disorders. SXB was given a conditional recommendation for the treatment of EDS in idiopathic hypersomnia in the 2021 AASM guidelines, and LXB is now approved by the FDA for the treatment of idiopathic hypersomnia in adults on the basis of a pivotal study.98 In the pivotal study in participants with idiopathic hypersomnia (Table 1), LXB met the primary efficacy endpoint (change in ESS score from the end of SDP to the end of DBRWP) and key secondary endpoints (score change in the Idiopathic Hypersomnia Severity Scale [a reliable and valid clinical tool for the quantification of idiopathic hypersomnia symptoms and consequences99] and Patient Global Impression of Change rating from the end of SDP to the end of DBRWP) and showed a similar safety profile to that in patients with narcolepsy.34
Though there are no ongoing trials examining the use of LXB in participants with hypersomnolence or muscle-related disorders currently registered with the US National Institutes of Health, active trials for SXB (as of July 2021) include studies examining its use for spasmodic dysphonia and voice tremor, nocturnal memory consolidation in major depressive disorder, and REM sleep behavior disorder.
Another progression in the field of oxybate is the development of extended-release formulations that seek to overcome the need to dose oxybate twice per night. Two candidates currently in development are Avadel’s FT218, which was examined in the REST-ON pivotal phase 3 study and is currently under FDA review for use in patients with narcolepsy,100 and Jazz Pharmaceuticals’ JZP324, which is currently in phase 1 clinical trials. Data from the REST-ON trial showed that once-nightly FT218 improved EDS (as measured by sleep latency on the Maintenance of Wakefulness Test) in patients with NT1 or NT2 and reduced the weekly number of cataplexy attacks (in patients with NT1).101 If approved, FT218 may therefore offer an alternative dosing schedule, but is not reported to have a reduction in sodium relative to SXB. In contrast, JZP324 uses a lower-sodium formulation of oxybate and, pending results of clinical studies, could potentially offer once-nightly dosing in conjunction with the hypothesized cardiovascular benefit associated with reduced sodium intake compared with SXB. In addition, a novel analog of oxybate that does not contain sodium or other cations, XWPharma’s XW10172, has also been designed to deliver oxybate in a once-nightly dosing regimen. Recent clinical studies evaluating the safety, tolerability, and PK profile of XW10172 support its progression into further clinical development studies.102
Conclusion
In conclusion, LXB, which contains a unique formulation of calcium, magnesium, potassium, and sodium ions, represents a significant advancement in the clinical use of oxybate-based medication for patients with narcolepsy, idiopathic hypersomnia and, potentially, other central disorders of hypersomnolence. Developed as an alternative to SXB in response to a need to reduce sodium intake in an already at-risk patient population, LXB offers similar efficacy and safety to SXB, with 92% less sodium, and can be directly transitioned dose-for-dose in both adult and pediatric patients with narcolepsy. LXB has recently been shown to be efficacious in reducing EDS not only in patients with narcolepsy, but also in those with idiopathic hypersomnia.
Abbreviations
AASM, American Academy of Sleep Medicine; AUC, area under the curve; CGI-c, clinician global impression of change; Cmax, maximum plasma concentration; CVD, cardiovascular disease; DBRWP, double-blind randomized-withdrawal period; EDS, excessive daytime sleepiness; ESS, Epworth Sleepiness Scale; FDA, US Food and Drug Administration; GABA, gamma-aminobutyric acid; GHB, gamma-hydroxybutyrate; GRADE, Grading of Recommendations Assessment, Development and Evaluation; LXB, lower-sodium oxybate; mRNA, messenger ribonucleic acid; NT1, narcolepsy type 1; NT2, narcolepsy type 2; PGI-c, Patient Global Impression of Change; PK, pharmacokinetic; REM, rapid-eye movement; REMS, risk evaluation and mitigation strategy; RMP, risk management program; SD, standard deviation; SDP, stable-dose period; SXB, sodium oxybate; TEAE, treatment-emergent adverse event; Tmax, time to maximum plasma concentration.
Data Sharing Statement
All relevant data are provided within the manuscript and supporting files.
Acknowledgments
Under the direction of the authors, Sean Anderson, PhD and Michael J. Theisen, PhD of Peloton Advantage, LLC, an OPEN Health company, provided medical writing assistance, which was funded by Jazz Pharmaceuticals in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). Although Jazz Pharmaceuticals reviewed the content of this manuscript, the ultimate interpretation and the decision to submit it for publication was made by the authors independently.
Author Contributions
All authors made a significant contribution to the work reported, including conception and drafting, revising or critically reviewing the article; all authors gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The research on LXB described in this publication was supported by Jazz Pharmaceuticals.
Disclosure
Yves Dauvilliers is a consultant for and has participated in advisory boards for Jazz Pharmaceuticals, UCB Pharma, Avadel, Idorsia, Takeda, Theranexus, and Bioprojet.
Richard K. Bogan has served on the speakers’ bureau and participated in advisory boards for Jazz Pharmaceuticals and Harmony Biosciences, received grants from Jazz Pharmaceutical, grants from Harmony Biosciences, grants from Takeda, grants from Avadel, during the conduct of the study; grants from Axsome, grants from Suven, grants from Vanda, grants from Merck, grants from Balance, outside the submitted work.
Karel Šonka has served on the speakers’ bureau for Sanofi, Angelini, and Stada and participated in advisory boards for UCB and in clinical trials for Jazz Pharmaceuticals, Flamel-Avadel, and Luitpold Pharmaceuticals; personal fees from AOP Orphan, personal fees from Flamel-Avadel, personal fees from Sanofi, outside the submitted work;
Markku Partinen has participated in advisory boards of AOP Orphan, Bioprojet, UCB, and Umecrine. He has participated in clinical trials for Bioprojet, Jazz Pharmaceuticals, MSD, and Umecrine.
Nancy Foldvary-Schaefer served on an advisory committee for Jazz Pharmaceuticals and participated in clinical trials supported by Jazz Pharmaceuticals, Suven Life Sciences, and Takeda Pharmaceuticals.
Michael J. Thorpy reports personal fees from Consultant/Advisory Board: Axsome, Balance Therapeutics, Flamel/Avadel, Harmony Biosciences, LLC, Jazz Pharmaceuticals, Suven Life Sciences Ltd., Takeda Pharmaceutical Co., Ltd, NLS Pharmaceuticals and Eisai Pharmaceuticals, during the conduct of the study.
References
1. Maski K, Trotti LM, Kotagal S, et al. Treatment of central disorders of hypersomnolence: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2021;17(9):1881–1893. doi:10.5664/jcsm.9328
2. Bassetti CLA, Kallweit U, Vignatelli L, et al. European guideline and expert statements on the management of narcolepsy in adults and children. J Sleep Res. 2021;30(6):e13387. doi:10.1111/jsr.13387
3. Caputo F, Del Re A, Brambilla R, et al. Sodium oxybate in maintaining alcohol abstinence in alcoholic patients according to Lesch typologies: a pilot study. J Psychopharmacol. 2014;28(1):23–30. doi:10.1177/0269881113504015
4. Xyrem® (sodium oxybate) oral solution, CIII [prescribing information]. Palo Alto, CA: Jazz Pharmaceuticals, Inc.; 2020.
5. Billiard M, Bassetti C, Dauvilliers Y, et al. EFNS guidelines on management of narcolepsy. Eur J Neurol. 2006;13(10):1035–1048. doi:10.1111/j.1468-1331.2006.01473.x
6. Wang YG, Swick TJ, Carter LP, Thorpy MJ, Benowitz NL. Safety overview of postmarketing and clinical experience of sodium oxybate (Xyrem): abuse, misuse, dependence, and diversion. J Clin Sleep Med. 2009;5(4):365–371. doi:10.5664/jcsm.27549
7. Xyrem [summary of product characteristics]. Brussels, Belgium: UCB Pharma; 2021.
8. U.S. Xyrem Multicenter Study Group. A randomized, double blind, placebo-controlled multicenter trial comparing the effects of three doses of orally administered sodium oxybate with placebo for the treatment of narcolepsy. Sleep. 2002;25(1):42–49.
9. U.S. Xyrem Multicenter Study Group. A 12-month, open-label, multicenter extension trial of orally administered sodium oxybate for the treatment of narcolepsy. Sleep. 2003;26(1):31–35.
10. U.S. Xyrem Multicenter Study Group. Sodium oxybate demonstrates long-term efficacy for the treatment of cataplexy in patients with narcolepsy. Sleep Med. 2004;5(2):119–123. doi:10.1016/j.sleep.2003.11.002
11. Xyrem International Study Group. A double-blind, placebo-controlled study demonstrates sodium oxybate is effective for the treatment of excessive daytime sleepiness in narcolepsy. J Clin Sleep Med. 2005;1(4):391–397. doi:10.5664/jcsm.26368
12. Black J, Houghton WC. Sodium oxybate improves excessive daytime sleepiness in narcolepsy. Sleep. 2006;29(7):939–946. doi:10.1093/sleep/29.7.939
13. Strunc MJ, Black J, Lillaney P, et al. The Xyrem(®) (sodium oxybate) Risk Evaluation and Mitigation Strategy (REMS) Program in the USA: results from 2016 to 2017. Drugs Real World Outcomes. 2021;8(1):15–28. doi:10.1007/s40801-020-00223-6
14. Carter LP, Pardi D, Gorsline J, Griffiths RR. Illicit gamma-hydroxybutyrate (GHB) and pharmaceutical sodium oxybate (Xyrem): differences in characteristics and misuse. Drug Alcohol Depend. 2009;104(1–2):1–10. doi:10.1016/j.drugalcdep.2009.04.012
15. George J, Majeed W, Mackenzie IS, Macdonald TM, Wei L. Association between cardiovascular events and sodium-containing effervescent, dispersible, and soluble drugs: nested case-control study. BMJ. 2013;347:f6954. doi:10.1136/bmj.f6954
16. Wei L, Mackenzie IS, MacDonald TM, George J. Cardiovascular risk associated with sodium-containing medicines. Expert Opin Drug Saf. 2014;13(11):1515–1523. doi:10.1517/14740338.2014.970163
17. Whelton PK, Appel LJ, Sacco RL, et al. Sodium, blood pressure, and cardiovascular disease: further evidence supporting the American Heart Association sodium reduction recommendations. Circulation. 2012;126(24):2880–2889. doi:10.1161/CIR.0b013e318279acbf
18. Clinical superiority findings; 2021. Available from: https://www.fda.gov/industry/designating-orphan-product-drugs-and-biological-products/clinical-superiority-findings.
19. Food and Drug Administration. Voluntary sodium reduction goals: target mean and upper bound concentrations for sodium in commercially processed, packaged, and prepared foods: guidance for industry; 2021. Available from: https://www.fda.gov/media/98264/download.
20. Chen C, Jenkins J, Zomorodi K, Skowronski R. Pharmacokinetics, bioavailability, and bioequivalence of lower-sodium oxybate in healthy participants in 2 open-label, randomized, crossover studies. Clin Transl Sci. 2021;14(6):2278–2287. doi:10.1111/cts.13087
21. Bogan RK, Thorpy MJ, Dauvilliers Y, et al. Efficacy and safety of calcium, magnesium, potassium, and sodium oxybates (lower-sodium oxybate [LXB]; JZP-258) in a placebo-controlled, double-blind, randomized withdrawal study in adults with narcolepsy with cataplexy. Sleep. 2021;44(3):zsaa206. doi:10.1093/sleep/zsaa206
22. Morse AM, Chen C, Wang YG, Skowronski RJ, Plazzi G. Evidence to support dose regimens for lower-sodium oxybate in pediatric patients with narcolepsy: phase 3 clinical data, pharmacokinetic (PK) data, and population PK modeling [oral presentation].
23. Xywav® (calcium, magnesium, potassium, and sodium oxybates) oral solution, CIII [prescribing information]. Palo Alto, CA: Jazz Pharmaceuticals, Inc.; 2021.
24. Kothare SV, Kaleyias J. Pharmacotherapy of narcolepsy: focus on sodium oxybate. Clin Med Insights Ther. 2010;2:37–52.
25. Saytzeff A. [On the reduction of succinyl chloride]. Justus Liebigs Ann Chem. 1874;171(2):258–290. doi:10.1002/jlac.18741710216
26. Gamma-hydroxybutyric acid (GHB) critical review report; 2012. Available from: https://www.who.int/medicines/areas/quality_safety/4.1GHBcritical_review.pdf.
27. Laborit H. Sodium 4-hydroxybutyrate. Int J Neuropharmacol. 1964;3:433–451. doi:10.1016/0028-3908(64)90074-7
28. Bertharion C, Laborit H. [Stereotactic study of the potentials evoked by sodium 4-hydroxybutyrate. Agressologie. 1962;3:489–496. French.
29. Danon-Boileau H, Lavitry S, Lab P, Levy E, Ruffiot S, Laborit H. [Utilization in psychiatry of gamma-OH]. Presse Med. 1962;70:2205–2207. French.
30. Rinaldi F, Puca FM, Mastrosimone F, Memoli G. [On the use of gamma-hydroxybutyrate of sodium in psychiatric therapy]. Acta Neurol (Napoli). 1967;22(1):21–41. Italian.
31. Broughton R, Mamelak M. The treatment of narcolepsy-cataplexy with nocturnal gamma-hydroxybutyrate. Can J Neurol Sci. 1979;6(1):1–6. doi:10.1017/S0317167100119304
32. Guiraud J, Addolorato G, Aubin HJ, et al. Treating alcohol dependence with an abuse and misuse deterrent formulation of sodium oxybate: results of a randomised, double-blind, placebo-controlled study. Eur Neuropsychopharmacol. 2021;52:18–30. doi:10.1016/j.euroneuro.2021.06.003
33. Plazzi G, Ruoff C, Lecendreux M, et al. Treatment of paediatric narcolepsy with sodium oxybate: a double-blind, placebo-controlled, randomised-withdrawal multicentre study and open-label investigation. Lancet Child Adolesc Health. 2018;2(7):483–494. doi:10.1016/S2352-4642(18)30133-0
34. Dauvilliers Y, Arnulf I, Foldvary-Schaefer N, et al. Efficacy and safety of lower-sodium oxybate in adults with idiopathic hypersomnia: a phase 3, placebo-controlled, double-blind, randomized withdrawal study. Lancet Neurol. 2022;21(1):53–65.
35. Venzi M, Di Giovanni G, Crunelli V. A critical evaluation of the gamma-hydroxybutyrate (GHB) model of absence seizures. CNS Neurosci Ther. 2015;21(2):123–140. doi:10.1111/cns.12337
36. GHB drug fact sheet; 2020. Available from: https://www.dea.gov/factsheets/ghb-gamma-hydroxybutyric-acid.
37. Drug scheduling; 2019. Available from: https://www.dea.gov/drug-scheduling.
38. Fuller DE, Hornfeldt CS, Kelloway JS, Stahl PJ, Anderson TF. The Xyrem risk management program. Drug Saf. 2004;27(5):293–306. doi:10.2165/00002018-200427050-00002
39. Silber MH, Krahn LE, Olson EJ, Pankratz VS. The epidemiology of narcolepsy in Olmsted County, Minnesota: a population-based study. Sleep. 2002;25(2):197–202. doi:10.1093/sleep/25.2.197
40. Longstreth WT
41. Vignatelli L, Antelmi E, Ceretelli I, et al. Red flags for early referral of people with symptoms suggestive of narcolepsy: a report from a national multidisciplinary panel. Neurol Sci. 2019;40(3):447–456. doi:10.1007/s10072-018-3666-x
42. Dauvilliers Y, Arnulf I, Mignot E. Narcolepsy with cataplexy. Lancet. 2007;369(9560):499–511. doi:10.1016/S0140-6736(07)60237-2
43. Narcolepsy Type 2. The International Classification of Sleep Disorders.
44. Narcolepsy Type 1. The International Classification of Sleep Disorders.
45. Bassetti CLA, Adamantidis A, Burdakov D, et al. Narcolepsy - clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat Rev Neurol. 2019;15(9):519–539. doi:10.1038/s41582-019-0226-9
46. Fraigne JJ, Torontali ZA, Snow MB, Peever JH. REM sleep at its core - circuits, neurotransmitters, and pathophysiology. Front Neurol. 2015;6:123. doi:10.3389/fneur.2015.00123
47. Scammell TE. Narcolepsy. N Engl J Med. 2015;373(27):2654–2662. doi:10.1056/NEJMra1500587
48. Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437(7063):1257–1263. doi:10.1038/nature04284
49. Kornum BR, Knudsen S, Ollila HM, et al. Narcolepsy. Nat Rev Dis Primers. 2017;3:16100. doi:10.1038/nrdp.2016.100
50. Pardi D, Black J. gamma-Hydroxybutyrate/sodium oxybate: neurobiology, and impact on sleep and wakefulness. CNS Drugs. 2006;20(12):993–1018. doi:10.2165/00023210-200620120-00004
51. Crunelli V, Emri Z, Leresche N. Unravelling the brain targets of gamma-hydroxybutyric acid. Curr Opin Pharmacol. 2006;6(1):44–52. doi:10.1016/j.coph.2005.10.001
52. Roth RH. Striatal dopamine and gamma-hydroxybutyrate. Pharmacol Ther B. 1976;2(1):71–88. doi:10.1016/0306-039x(76)90020-9
53. Spano PF, Tagliamonte A, Tagliamonte P, Gessa GL. Stimulation of brain dopamine synthesis by gamma-hydroxybutyrate. J Neurochem. 1971;18(10):1831–1836. doi:10.1111/j.1471-4159.1971.tb09588.x
54. Howard SG, Feigenbaum JJ. Effect of gamma-hydroxybutyrate on central dopamine release in vivo. A microdialysis study in awake and anesthetized animals. Biochem Pharmacol. 1997;53(1):103–110. doi:10.1016/S0006-2952(96)00664-8
55. Aghajanian GK, Roth RH. Gamma-hydroxybutyrate-induced increase in brain dopamine: localization by fluorescence microscopy. J Pharmacol Exp Ther. 1970;175(1):131–138.
56. Hechler V, Gobaille S, Bourguignon JJ, Maitre M. Extracellular events induced by gamma-hydroxybutyrate in striatum: a microdialysis study. J Neurochem. 1991;56(3):938–944. doi:10.1111/j.1471-4159.1991.tb02012.x
57. Roth RH, Walters JR, Aghajanian GK. Effect of impulse flow on the release and synthesis of dopamine in the rat striatum. In: Usdin E, Snyder SH, editors. Frontiers in Catecholamine Research. Proceedings of the Third International Catecholamine Symposium Held at the University of Strasbourg, Strasbourg, France May 20–25, 1973. New York, NY: Pergamon Press Inc.; 1973:567–574.
58. Szabo ST, Gold MS, Goldberger BA, Blier P. Effects of sustained gamma-hydroxybutyrate treatments on spontaneous and evoked firing activity of locus coeruleus norepinephrine neurons. Biol Psychiatry. 2004;55(9):934–939. doi:10.1016/j.biopsych.2003.12.013
59. Schmidt-Mutter C, Muller C, Zwiller J, Gobaille S, Maitre M. Gamma-hydroxybutyrate and cocaine administration increases mRNA expression of dopamine D1 and D2 receptors in rat brain. Neuropsychopharmacology. 1999;21(5):662–669. doi:10.1016/S0893-133X(99)00066-4
60. Gottesmann C. GABA mechanisms and sleep. Neuroscience. 2002;111(2):231–239. doi:10.1016/S0306-4522(02)00034-9
61. Scammell TE, Arrigoni E, Lipton JO. Neural circuitry of wakefulness and sleep. Neuron. 2017;93(4):747–765. doi:10.1016/j.neuron.2017.01.014
62. Wisor JP. Dopamine and wakefulness: pharmacology, genetics, and circuitry. Handb Exp Pharmacol. 2019;253:321–335.
63. Gruner JA, Marcy VR, Lin YG, Bozyczko-Coyne D, Marino MJ, Gasior M. The roles of dopamine transport inhibition and dopamine release facilitation in wake enhancement and rebound hypersomnolence induced by dopaminergic agents. Sleep. 2009;32(11):1425–1438. doi:10.1093/sleep/32.11.1425
64. Dauvilliers Y, Tafti M, Landolt HP. Catechol-O-methyltransferase, dopamine, and sleep-wake regulation. Sleep Med Rev. 2015;22:47–53. doi:10.1016/j.smrv.2014.10.006
65. Burgess CR, Tse G, Gillis L, Peever JH. Dopaminergic regulation of sleep and cataplexy in a murine model of narcolepsy. Sleep. 2010;33(10):1295–1304. doi:10.1093/sleep/33.10.1295
66. Roth RH, Doherty JD, Walters JR. Gamma-hydroxybutyrate: a role in the regulation of central dopaminergic neurons? Brain Res. 1980;189(2):556–560. doi:10.1016/0006-8993(80)90368-6
67. Engberg G, Nissbrandt H. gamma-Hydroxybutyric acid (GHBA) induces pacemaker activity and inhibition of substantia nigra dopamine neurons by activating GABAB-receptors. Naunyn Schmiedebergs Arch Pharmacol. 1993;348(5):491–497. doi:10.1007/BF00173208
68. Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92(3):1087–1187. doi:10.1152/physrev.00032.2011
69. Schmidt C, Leibiger J, Fendt M. The norepinephrine reuptake inhibitor reboxetine is more potent in treating murine narcoleptic episodes than the serotonin reuptake inhibitor escitalopram. Behav Brain Res. 2016;308:205–210. doi:10.1016/j.bbr.2016.04.033
70. Barateau L, Jaussent I, Roeser J, Ciardiello C, Kilduff TS, Dauvilliers Y. Cerebrospinal fluid monoamine levels in central disorders of hypersomnolence. Sleep. 2021;44(7):zsab012. doi:10.1093/sleep/zsab012
71. Shefner SA, Osmanovic SS. GABAA and GABAB receptors and the ionic mechanisms mediating their effects on locus coeruleus neurons. Prog Brain Res. 1991;88:187–195.
72. Williams SR, Turner JP, Crunelli V. Gamma-hydroxybutyrate promotes oscillatory activity of rat and cat thalamocortical neurons by a tonic GABAB, receptor-mediated hyperpolarization. Neuroscience. 1995;66(1):133–141. doi:10.1016/0306-4522(94)00604-4
73. Gervasi N, Monnier Z, Vincent P, et al. Pathway-specific action of gamma-hydroxybutyric acid in sensory thalamus and its relevance to absence seizures. J Neurosci. 2003;23(36):11469–11478. doi:10.1523/JNEUROSCI.23-36-11469.2003
74. Roth T, Dauvilliers Y, Mignot E, et al. Disrupted nighttime sleep in narcolepsy. J Clin Sleep Med. 2013;9(9):955–965. doi:10.5664/jcsm.3004
75. Maski K, Mignot E, Plazzi G, Dauvilliers Y. Disrupted nighttime sleep and sleep instability in narcolepsy. J Clin Sleep Med. 2022;18(1):289–304. doi:10.5664/jcsm.9638
76. Ohayon MM. Narcolepsy is complicated by high medical and psychiatric comorbidities: a comparison with the general population. Sleep Med. 2013;14(6):488–492. doi:10.1016/j.sleep.2013.03.002
77. Cohen A, Mandrekar J, St Louis EK, Silber MH, Kotagal S. Comorbidities in a community sample of narcolepsy. Sleep Med. 2018;43:14–18. doi:10.1016/j.sleep.2017.11.1125
78. Black J, Reaven NL, Funk SE, et al. Medical comorbidity in narcolepsy: findings from the Burden of Narcolepsy Disease (BOND) study. Sleep Med. 2017;33:13–18. doi:10.1016/j.sleep.2016.04.004
79. Jennum PJ, Plazzi G, Silvani A, Surkin LA, Dauvilliers Y. Cardiovascular disorders in narcolepsy: review of associations and determinants. Sleep Med Rev. 2021;58:101440. doi:10.1016/j.smrv.2021.101440
80. Dahmen N, Bierbrauer J, Kasten M. Increased prevalence of obesity in narcoleptic patients and relatives. Eur Arch Psychiatry Clin Neurosci. 2001;251(2):85–89. doi:10.1007/s004060170057
81. Bosco A, Lopez R, Barateau L, et al. Effect of psychostimulants on blood pressure profile and endothelial function in narcolepsy. Neurology. 2018;90(6):e479–e491. doi:10.1212/WNL.0000000000004911
82. Grimaldi D, Calandra-Buonaura G, Provini F, et al. Abnormal sleep-cardiovascular system interaction in narcolepsy with cataplexy: effects of hypocretin deficiency in humans. Sleep. 2012;35(4):519–528. doi:10.5665/sleep.1738
83. Vandi S, Rodolfi S, Pizza F, et al. Cardiovascular autonomic dysfunction, altered sleep architecture, and muscle overactivity during nocturnal sleep in pediatric patients with narcolepsy type 1. Sleep. 2019;42(12):zsz169. doi:10.1093/sleep/zsz169
84. Ohkubo T, Hozawa A, Yamaguchi J, et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens. 2002;20(11):2183–2189. doi:10.1097/00004872-200211000-00017
85. Dauvilliers Y, Jaussent I, Krams B, et al. Non-dipping blood pressure profile in narcolepsy with cataplexy. PLoS One. 2012;7(6):e38977. doi:10.1371/journal.pone.0038977
86. National Academies of Sciences Engineering and Medicine. Dietary Reference Intakes for Sodium and Potassium. Washington, DC: The National Academies Press; 2019.
87. Junnarkar G, Allphin C, Profant J, et al. Development of a lower-sodium oxybate formulation for the treatment of patients with narcolepsy and idiopathic hypersomnia. Expert Opin Drug Discov. 2022;17(2):109–119. doi:10.1080/17460441.2022.1999226
88. Wang Q, Lin T, Allphin C, van Osdol WW, Bolger MB, Chen C. Physiologically based pharmacokinetic modeling of oxybate: the role of counter-ions in gastrointestinal absorption of oxybate [poster].
89. Bogan RK, Foldvary-Schaefer N, Skowronski R, Chen A, Thorpy MJ. Timing and duration of treatment-emergent adverse events in a clinical trial of lower-sodium oxybate in participants with narcolepsy with cataplexy [poster 486].
90. Husain AM, Bujanover S, Ryan R, Scheckner B, Black J, Profant J. Incidence and duration of common, early-onset adverse events occurring during 2 randomized, placebo-controlled, phase 3 studies of sodium oxybate in participants with narcolepsy. J Clin Sleep Med. 2020;16(9):1469–1474. doi:10.5664/jcsm.8530
91. Gardener H, Rundek T, Wright CB, Elkind MS, Sacco RL. Dietary sodium and risk of stroke in the Northern Manhattan study. Stroke. 2012;43(5):1200–1205. doi:10.1161/STROKEAHA.111.641043
92. Strazzullo P, D’Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ. 2009;339:b4567. doi:10.1136/bmj.b4567
93. Plazzi G, Ruoff C, Hassan F, et al. Clinical and patient global impression in a study of sodium oxybate in children and adolescents with narcolepsy with cataplexy [abstract P515]. J Sleep Res. 2018;27(suppl1):326.
94. Borgen LA, Okerholm RA, Lai A, Scharf MB. The pharmacokinetics of sodium oxybate oral solution following acute and chronic administration to narcoleptic patients. J Clin Pharmacol. 2004;44(3):253–257. doi:10.1177/0091270003262795
95. Chen C, Rosen CL, Ruoff C, et al. Population and noncompartmental pharmacokinetics of sodium oxybate support weight-based dosing in children and adolescents with narcolepsy with cataplexy. Clin Transl Sci. 2020;13(5):932–940. doi:10.1111/cts.12780
96. Morgenthaler TI, Kapur VK, Brown T, et al. Practice parameters for the treatment of narcolepsy and other hypersomnias of central origin. Sleep. 2007;30(12):1705–1711. doi:10.1093/sleep/30.12.1705
97. Maski K, Trotti LM, Kotagal S, et al. Treatment of central disorders of hypersomnolence: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2021;17(9):1895–1945. doi:10.5664/jcsm.9326
98. Jazz Pharmaceuticals announces U.S. FDA approval of Xywav®(calcium, magnesium, potassium, and sodium oxybates) oral solution for idiopathic hypersomnia in adults [press release]; 2021. Available from: http://investor.jazzpharma.com/news-releases/news-release-details/jazz-pharmaceuticals-announces-us-fda-approval-xywavr-calcium.
99. Dauvilliers Y, Evangelista E, Barateau L, et al. Measurement of symptoms in idiopathic hypersomnia: the Idiopathic Hypersomnia Severity Scale. Neurology. 2019;92(15):e1754–e1762. doi:10.1212/WNL.0000000000007264
100. Avadel Pharmaceuticals announces ongoing FDA review of NDA for FT218 for patients with narcolepsy [press release]; 2021. Available from: https://www.globenewswire.com/news-release/2021/10/15/2315210/0/en/Avadel-Pharmaceuticals-Announces-Ongoing-FDA-Review-of-NDA-for-FT218-for-Patients-with-Narcolepsy.html.
101. Kushida CA, Shapiro CM, Roth T, et al. Once-nightly sodium oxybate (FT218) demonstrated improvement of symptoms in a phase 3 randomized clinical trial in patients with narcolepsy. Sleep. 2021. doi:10.1093/sleep/zsab200
102. Xiang W, Xiang J-N, Canafax D. Clinical pharmacokinetics of XW10172 for once-nightly therapy in patients with narcolepsy or sleep disorders in patients with neurodegenerative diseases [poster].
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

