Back to Journals » Journal of Inflammation Research » Volume 17
Caffeine in Hepatocellular Carcinoma: Cellular Assays, Animal Experiments, and Epidemiological Investigation
Authors Shan L, Zhao N, Wang F, Zhai D, Liu J, Lv X
Received 5 June 2023
Accepted for publication 29 February 2024
Published 11 March 2024 Volume 2024:17 Pages 1589—1605
DOI https://doi.org/10.2147/JIR.S424384
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Liang Shan,1– 4,* Ning Zhao,2– 4,* Fengling Wang,1 Dandan Zhai,1 Jianjun Liu,1 Xiongwen Lv2– 4
1Department of Pharmacy, the Second People’s Hospital of Hefei, Hefei Hospital Affiliated to Anhui Medical University, Hefei, Anhui, 230011, People’s Republic of China; 2Anhui Province Key Laboratory of Major Autoimmune Diseases, Anhui Medical University, Hefei, 230032, People’s Republic of China; 3Inflammation and Immune Mediated Diseases Laboratory of Anhui Province, Hefei, 230032, People’s Republic of China; 4The Key Laboratory of Major Autoimmune Diseases, Hefei, Anhui Province, 230032, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianjun Liu, Department of Pharmacy, the Second People’s Hospital of Hefei, Hefei Hospital Affiliated to Anhui Medical University, Hefei, Anhui, 230011, People’s Republic of China, Tel +86 551 62965335, Fax +86 551 62965335, Email [email protected] Xiongwen Lv, Anhui Province Key Laboratory of Major Autoimmune Diseases, Anhui Medical University, Hefei, 230032, People’s Republic of China, Tel +86 55165161000, Fax +86 551 65161000, Email [email protected]
Abstract: The use of caffeine in treating various liver diseases has made substantial progress in the past decade owing to advances in science, technology, and medicine. However, whether caffeine has a preventive effect on hepatocellular carcinoma (HCC) and its mechanism are still worth further investigation. In this review, we summarize and analyze the efficacy and safety of caffeine in the prevention of HCC. We conducted a review of articles published in PubMed and Web of Science in the past 2 decades until December 6, 2023, which were searched for using the terms “Caffeine” and “Hepatocellular Carcinoma.” Studies have found that coffee intake is negatively correlated with HCC risk, especially caffeinated coffee. Recent studies have found that caffeine has beneficial effects on liver health, decreasing levels of enzymes responsible for liver damaging and slowing the progression of hepatic fibrosis and cirrhosis. Caffeine also acts against liver fibrosis through adenosine receptors (ARs), which promote tissue remodeling by inducing fibrin and collagen production. Additionally, new studies have found that moderate consumption of caffeinated beverages can decrease various the levels of various collagens in patients with chronic hepatitis C. Furthermore, polyphenolic compounds in coffee can improve fat homeostasis, reduce oxidative stress, and prevent liver steatosis and fibrosis. Moreover, many in vitro studies have shown that caffeine can protect liver cells and inhibit the activation and proliferation of hepatic stellate cells. Taken together, we describe the benefits of caffeine for liver health and highlight its potential values as a drug to prevent various hepatic diseases. As a protective agent of liver inflammation, non-selective AR inhibitor caffeine can inhibit the growth of HCC cells by inhibiting adenosine and AR binding to initiate immune response, providing a basis for the future development of caffeine as an adjuvant drug against HCC.
Keywords: coffee, caffeine, hepatocellular carcinoma, adenosine, adenosine receptor
Introduction
Caffeine, with the chemical formula C8H10N4O2, is a bitter white crystal belonging to the xanthine alkaloid compound family. Thus, it is chemically similar to the adenine and guanine present in deoxyribonucleic acid (DNA).1 Consuming caffeinated beverages can relieve or prevent drowsiness and increase energy. The part of the coffee plant that contains caffeine is extracted to make beverages.2 These drinks are popular in North America, where 90% of adults consume caffeine every day. Caffeine is a central nervous stimulant that temporarily dispels drowsiness and restores energy. Additionally, caffeine is the most widely used legal and unregulated psychoactive drug in the world because it is widely used clinically in coma resuscitation, as well as in caffeinated coffee, tea, soft drinks, and energy drinks.3 Most prominently, caffeine is a nonselective adenosine receptor (AR) antagonist that reversibly blocks the action of adenosine on the receptor, thereby blocking adenosine and AR binding-induced sleepiness in the central nervous system. Recently, emerging data suggest that coffee consumption is inversely associated with the progression of liver fibrosis to cirrhosis and even hepatocellular carcinoma (HCC), although there is no evidence of a causal relationship.1,4
In the last decade, studies have found that caffeine has pharmacological effects, including against Alzheimer’s disease, in which it excites the cardiovascular system and cerebrovascular system, as well as against diabetes, liver cancer, and liver fibrosis.5–7 New epidemiological reports HCC is the sixth most commonly diagnosed cancer and the third most common cause of cancer-related death globally, with 900,000 new cases and 830,000 deaths in 2020.4 Previous studies have explored the effects of caffeine on HCC and have attracted attention from researchers worldwide.8–10 Our previous studies have shown that caffeine has certain preventive and therapeutic effects on alcoholic liver fibrosis in rats, and that the cAMP-PKA-CREB-signaling pathway may play a role in this process. Additionally, acetaldehyde has been used to stimulate hepatic stellate cell-T6 (HSC-T6) in rats to establish an in vitro model of alcoholic liver fibrosis, in which the PLC-DAG-PKC-signaling pathway is thought to play a role.11,12 Overall, caffeine appears to benefit the liver; however, limiting caffeine consumption is important to avoid adverse effects. And the latest prospective analysis also confirmed that coffee drinking this lifestyle factors and the incidence of HCC is closely related to the lower risk.13
The last review on this field was published by Kennedy’s research team in 2017.14 We decided to update the data in this field and synthesize information that includes the pathophysiological mechanisms, results of experimental and clinical studies, mechanisms of drug resistance and ways to overcome them, adverse effects of caffeine, recent patents in this field, and future developments. Thus, we present this review from articles published in PubMed and Web of Science in the past decades before December 6, 2023, which were obtained by searching the terms “Caffeine” and “Hepatocellular Carcinoma.” We found that majority of literatures reported a reduced risk of HCC in people with regulated coffee consumption. Secondly, caffeine, the main active ingredient in coffee, inhibits the binding of adenosine to AGs, which may be the main reason for its effect. Third, the anti-HCC effect of caffeine at physiological dose is weak. At present, it has been reported that caffeine can assist other anticancer drugs in the treatment of HCC in clinical practice. The main problems are the lack of targeting of caffeine and the low drug concentration at the local lesion site. And future studies will focus on addressing these 2 issues.
Inhibitors of the Adenosine Pathway Alleviating HCC
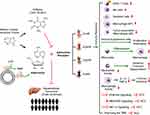
Previous studies have shown that adenosine is produced by the hydrolysis of adenosine triphosphate (ATP) by the ectonucleotidases CD39 and CD73 and acts as an anti-inflammatory regulator of immune responses in peripheral tissues.15 Interestingly, A2aAR blockade with istradefylline (KW6002) has been found to inhibit tumor growth, APCP and KW6002 co-targeting CD73 and A2aAR respectively have synergistic inhibitory effects on the growth of HCC cells.15 Recent studies have shown that the immune response induced by adenosine and AR binding is central to the prevention and treatment of HCC.16 An increased extracellular adenosine concentration induces an immune-tolerant tumor microenvironment (TME) and reduces the response of HCC cells to immune checkpoint inhibitors.17 Caffeine is structurally similar to adenosine and is a natural non-selective AR inhibitor that competently inhibits ARs in vitro and in vivo.18 In inflammatory and damaged tissues (such as cancer), extracellular adenosine concentrations can increase up to 100 times and accumulate, inducing an immune response that promotes tumor progression. Inhibiting adenosine and AR binding in inflammatory or cancer tissues has been shown to increase the proportions of immune cells, including dendritic cells (DCs), macrophages, natural killer (NK) cells, and CD8+ T cells.19 Extracellular adenosine concentrations can also decrease the proliferation and polarization of immunosuppressive cells, thus inhibiting tumor progression. Furthermore, inhibition of ARs can reduce differentiation of monocytes into macrophages, and decrease immune tolerance by inhibiting macrophage activity and reducing macrophage production of interleukin (IL)-12 and tumor necrosis factor-alpha (TNF-α) in patients with HCC.16,20 However, inhibition of adenosine increases the activity of neutrophils, thereby increasing their adhesion, transport, and degranulation.16,21 Therefore, targeted inhibition of adenosine and AR binding in tumor tissue is a method of anti-tumor immunity. Several drugs that target the inhibition of adenosine or ARs, are effective in preclinical studies and in animal tumor models.22 New antibodies and small molecules that target inhibition of the adenosine pathway are currently being investigated for early-stage clinical trials in patients with advanced cancer.23 The AR family contains four types of AR subtypes (A1AR, A2aAR, A2bAR, and A3AR), of which A2aAR has been studied extensively.8,24 Taken together, injury-induced release of ATP into extracellular regions is a dangerous alarm signal for recruitment of immune cells, which express CD39 and CD73 to clear extracellular ATP and produce immunosuppressive adenosine. The mechanism by which caffeine initiates the immune response against HCC by inhibiting adenosine and AR binding is shown in the figure (Figure 1).
Caffeine in HCC
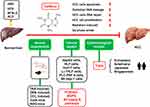
At present, HCC is the sixth most common cancer worldwide and the second deadliest tumor after pancreatic cancer.25,26 The widespread use of serum α-fetoprotein (AFP) for clinical biomarker detection of HCC has many controversies and limitations. Newly-developed cell-free DNA, based on its genomic and epigenetic alterations, is expected to be a hopeful biomarker for early HCC diagnosis in the future.26 Chronic hepatitis caused by hepatitis B virus (HBV) is considered the primary cause of HCC. The ingestion of food contaminated with chemical carcinogens (eg, aflatoxins), and excessive alcohol consumption may lead to the eventual development of HCC in HBV carriers.25,26 The prognosis of HCC is poor and recurrence and metastasis rates are high. Currently, in addition to sorafenib, second-generation targeted drugs, including regorafenib and ramvaritinib, can be used as anti-tumor angiogenesis targeted drugs for advanced HCC.27,28 The effectiveness of the three current artificial preparations is unsatisfactory, with side effects including body rash, diarrhea, hypertension, hair loss, itching, nausea, vomiting, loss of appetite, and, most commonly, hand and foot syndrome.27 Many epidemiological studies have reported a protective effect of coffee consumption on various cancer types, including colorectal, prostate, liver, breast, and endometrial cancers, but HCC has been the most studied and reported.29–31 A large cohort of prostate cancer cases enrolled 5727 men with prostate cancer from seven US, Australian, and European studies confirmed that high coffee intake was in relation to overall survival and prostate cancer–specific survival, especially in that men with clinically localized disease and fast caffeine metabolism genotype, future studies are needed to assess coffee and/or caffeine intake in men after a prostate cancer diagnosis.29 A recent study found that tumor-derived adenosine promotes macrophage proliferation in human HCC, suggesting the possibility of inhibiting HCC by targeting tumor-derived adenosine to regulate the TME. However, whether caffeine plays a role through inhibiting adenosine in this process remains to be elucidated.32 But most current knowledge regarding targeting tumor-derived adenosine to regulate the TME has been based on murine models, future studies could pay more attention to human HCC.32,33 The antioxidant effect of caffeine and the inhibition of phosphoinositide 3-kinase (PI3K)/Akt pathway may be other explanations for its inhibitory effect on HCC.33 Small molecule adenosine is a potent immunosuppressive metabolite of ATP, which is often elevated in TME, so inhibiting adenosine binding to its receptors may be beneficial for tumor therapy by activating the immune system.33 In some cancers, the protective effect of caffeine remains controversial. For example, Song et al reported that coffee consumption was not associated with stomach cancer risk.34 And a Mendelian randomization study provided strong support for a causal association of coffee consumption with esophageal cancer, but not for most other cancer types.30 Therefore, more extensive and in-depth research is needed. New research has shown that coffee and caffeine can affect the physiological function of several organs and/or tissues.35 Meta-analysis which was performed recently found eligible studies including 21 involving 2,492,625 participants and 5980 cases of HCC reporting coffee intake, which provides a substantial basis for studying coffee intake and reduced risk of HCC.36 The effects of coffee/caffeine on liver cancer in animal experiments, cell experiments, and epidemiological studies are summarized in the figure (Figure 2).
Cellular Assays
Previously published studies found that caffeine inhibits DNA repair and suggested that it increased the anti-tumor effect of cisplatin.37–40 Caffeine administration (0.5 mM) increased the anti-tumor effect of cisplatin on the proliferation of various HCC cell types, decreased the half maximal inhibitory concentration (IC50) of single cisplatin administration in up to four types of human HCC cell lines (Li-7, HLF, Huh-7, and HepG2).27,37 Additionally, non-selective AR antagonist caffeine and its analog CGS 15943 (molecular weight: 285.689, CAS: 104615-18-1) inhibited the growth of human HCC cells (including HepG2, HLF, PLC-PRF-5, and SK-Hep-1) via targeting the PI3K/Akt signal pathway.41,42 Furthermore, another in vitro study demonstrated that a physiologically applicable concentration of caffeine (<412 μM) restrained the progression of HCC by inhibiting the activation, proliferation, migration, and invasion of HepG2 and Huh-7 through the Akt signal pathway, with a % inhibition invasion of 412 μM.39 The above studies suggest that a physiological dose of caffeine (<412 μM) inhibited the activation and proliferation of various human HCC cells. Chlorogenic acid (CGA) is an active component of coffee with well-known antioxidant property, Yin et al described that incubated various HCC cell lines including Huh7, Hep3B, LO2, and Hep1-6 with a series of concentrations of CGA (1, 10, and 20 μM) for 24 h could attenuate the therapeutic effect of radiotherapy through inhibiting radiation-induced apoptosis and DNA damage by scavenging excessive reactive oxygen species and activating NF-E2-related factor 2 antioxidant system.43 Kahweol is also a natural diterpene which extracted from coffee, Seo et al found that treatment with 40 µM kahweol for 24 h induced apoptosis effectively in human HCC cell lines including Hep3B, SNU182, and SNU423 through inhibiting Src/mechanistic target of rapamycin (mTOR)/signal transducer and activator of transcription 3 (STAT3) signaling pathway and kahweol was a potential inhibitor of HCC cell activation and proliferation.44 Many recent studies have investigated the in vitro effects of caffeine and other ingredients in coffee on the activation and proliferation in various HCC cells. IC50 values of caffeine and other anti-cancer drugs in different HCC cell lines are summarized in table (Table 1). However, the results of these cell assays still need to be further confirmed by animal experiments, future studies will focus on whether the concentration of these components in vivo can effectively inhibit the growth of HCC cells and targeting characteristics.
 |
Table 1 IC50 Values of Caffeine and Other Anti-Cancer Drugs in Different HCC Cell Lines |
New studies have found that caffeine inhibits the progression of various cancers by inhibiting adenosine and AR binding-mediated activation of various immune factors in the TME, including HCC.45–47 Fagundes et al found that incubation with 39.3 µM (IC50) caffeine for 24 h enhanced the cytotoxic effect of dacarbazine on B16F10 mouse melanoma cells in vitro; the mechanism of which was associated with the ability of caffeine to enhance dacarbazine-induced cytotoxicity by increasing dacarbazine-induced biotransformation, cancer cell apoptosis, DNA damage, and the malondialdehyde level.45 Caffeine has an anti-proliferation effect on HCC cell lines (HepG2, HLF, Huh7, and PLC/PRF5), the mechanism is that caffeine inhibit the phosphorylation of extracellular signal-regulated kinase (ERK)1/2, while another possible mechanism is oxidative stress as malondialdehyde and superoxide dismutase levels are increased in cell assays.45 Plasma caffeine concentrations in adults are usually reported to be approximately 10–50 μM, depending on the amount of coffee consumed daily, while toxic effects are observed at plasma caffeine concentrations >200 μM.46 The study also confirmed that 50 µM caffeine increases apolipoprotein A-1 and paraoxonase-1 protein, while 200 µM of caffeine obviously improves the paraoxonase-1 protein level in HepG2 cell lines, suggesting that caffeine may play a role in HCC progression through multiple pathways.46 A recent study further confirmed the high antioxidant and anti-inflammatory activity of CGA and the high anti-inflammatory activity of caffeine, which mitigated lipopolysaccharide-stimulated macrophage activation by inhibiting NO, superoxide anion, and the pro-inflammatory cytokines TNF-α and IL-6.47 Moreover, the results of in vitro assays showed that caffeine (100 and 500 µM) has no significant effect on the protein production of sex hormone-binding globulin (SHBG) in HepG2 cells, while in vivo experiments found that humanized SHBG transgenic mice administered oral caffeine (0.1 mg/mL) for 12 days clearly improved hepatic SHBG production, induced an increase in adiponectin, and upregulated the level of hepatocyte nuclear factor 4-alpha in human SHBG transgenic mice, suggesting that caffeine mediates the progression of different liver diseases through multiple pathways.48 Caffeine is well known to have several mechanisms of action, the most prominent of which is to bind reversibly to ARs, blocking their action. However, it is currently reported that caffeine has antioxidant and pro-oxidation properties, so more in-depth research is needed.46 Growing evidences are piling up to prove that caffeine and its derivatives are promising drugs for the treatment of cancer or infectious diseases.49,50 A recent study found that the newly synthesized Cu (II)-Caffeine Complex has potential superoxide dismutase-like activity and acts as a good antioxidant. Moreover, in vitro studies have shown its anti-cancer activity against colorectal adenocarcinoma (Caco-2) and breast cancer (Mcf-7) cell lines, demonstrating that complex A has great potential as an effective cytotoxic agent against colorectal and breast cancers.49 The study of the anticancer properties of the Metal-Caffeine Complex could help us design other related metal-drug complexes that would be more effective against drug resistance as well as chemotherapy. In vitro studies have shown that caffeine at concentrations ranging from 0.4 mM to 20 mM can inhibit the activation, proliferation, migration, and invasion of various HCC cell lines.42 This concentration of caffeine can also promote the apoptosis of various liver cancer cells. Pharmacokinetic studies have been reported on the enhancement of caffeine concentration in HCC tissue sites by targeting the drug delivery system.50 Yang et al reported that the inhibition rate of HepG2 cell proliferation by caffeine alone was 24.36%, whereas the inhibition rate through the combination of caffeine and lysozyme at the same concentration was 47.21%, the mechanism of action was further explained by molecular spectroscopic experiments, indicating that the caffeine-lysozyme complex could inhibit the proliferation and induce apoptosis of HepG2 cells.51 The use of new caffeine formulations as adjunctive therapy for cancer is being investigated, and caffeine has potential value as an adjunct to HCC. Therefore, combined use of caffeine and other anti-HCC drugs is the development direction of the future.
Using macrophage-specific CD39 knockout mice, researchers recently found that cell-specific CD39 expression in macrophages and CD73 expression in HCC cells synergically activate the eATP-adenosine pathway and produce more adenosine, which damages CD8+ T cell function and promotes anti-programmed cell death protein 1 (PD1) resistance, which is a novel mode of anti-PD1 resistance in HCC.21 CircTMEM181, an HCC-derived exosome, contributes to immunosuppression and anti-PD1 resistance by enhancing CD39 expression, while inhibition of the ATP-adenosine pathway by targeting CD39 in macrophages can reverse anti-PD1 resistance in HCC.21 Thus, CD39 on the surface of macrophages co-activates ATP-adenosine pathway with CD73 of HCC cells to weaken anti-tumor immunity, targeting macrophage CD39 is reverse potential treatment strategy of resistance to PD1 resistance of HCC. Liver inflammation is triggered and maintained by cytokines and chemokines secreted by innate immune cells. Indeed, the disruption of cytokine and chemokine production leads to severe hepatocyte damage and the development of many acute and chronic liver diseases.52 Cytokine-dependent lymphocyte infiltration inhibits tumor growth and enhances the efficacy of immune checkpoint blockade.52 Whether liver innate immune cells or blood-derived immune cells mediate the liver innate immune response through adenosine signaling pathway needs to be further investigated at the single-cell level. Adenosine is an important mediator that is central to the progression of HCC. New studies have shown that in vivo knockout of ribose nucleic acid (RNA) N6-adenosine methyltransferase can significantly inhibit the occurrence of HCC and lung metastasis.53 However, overexpression of RNA N6-adenosine methyltransferase has been found to significantly promote the growth of HCC, both in vitro and in vivo, through the CRISPR/dCas9-VP64 activation system.53 Currently, the diversity and reversible chemical modification of RNA have become the focus of research on the pathogenesis of HCC.
New research has identified anti-HCC effects of glycogen synthase kinase-3β inhibition by activating AMP-activated protein kinase (AMPK)/mTOR signaling, which negatively affects glycolysis and HCC cell proliferation.54 A recent study demonstrated that N6 methyladenosine regulation of phosphatase and tensin homolog by HBV affected innate immunity by inhibiting interferon regulatory factor 3 nuclear import and HCC progress via activating the PI3K/Akt signaling pathway.55 A distinguishing feature of cancer cells is the high level of glycolysis even in the presence of oxygen, which is known as the Warburg effect, or metabolic reprogramming. Metabolic reprogramming is considered to be the key to tumor cell growth and proliferation.56 Cellular energy imbalance is considered to be a hallmark of human cancer, and the main source of energy in cells is ATP. Therefore, regulating ATP metabolism in HCC cells is currently an attractive anticancer method.55,56 Therefore, different adenosine derivatives can exert anti-HCC effects through different pathways.
CD73 is an AMP hydrolytic enzyme that regulates the conversion of extracellular ATP to adenosine and acts as a powerful immunosuppressant, which is used to maintain tissue homeostasis and prevent immune responses during inflammation.20 New studies have found that CD73 expression is positively correlated with HCC metastasis and is an independent prognostic indicator of HCC.20 Additionally, CD73 promotes HCC progression and epithelial–mesenchymal transition by activating the PI3K/Akt signaling pathway in many cancer cell lines. Both in vivo and in vitro studies found that the combination of CD73 and A2AR inhibitors provided a more synergistic tumor inhibition in HCC than either regimen alone.20 New small molecule antibodies and inhibitors targeting ATP-adenosine signaling pathway have entered the clinical research stage. The liver has an important anti-tumor monitoring role, as evidenced by its unique immune profile, containing many cytotoxic CD8+ T cells and a significant innate lymphocyte population, including NK cells, γδ T cells, mucosal-associated invariant T cells, and invariant natural killer T (NKT) cells.57 HCC is a relatively common result of chronic liver infection or inflammation; blocking adenosine signaling to reverse immunosuppressive effects represents a potential treatment option when used in combination with other immunotherapies.57 Anti-HCC drugs that target the immune system are now at the forefront of cancer drug development and trial design. In HCC, CD39 and CD73 promote tumor cell survival by creating adenosine-rich TME that allow immune cells to escape immune surveillance, in a process that is regulated by hypoxia inducible factor (HIF-1).58 Chiu et al showed HIF-1 promoted the accumulation of myeloid-derived suppressor cells via CD39 and CD73 in HCC.58 Interestingly, another new study has also confirmed that plasmacytoid DCs recruited by HIF-1α/extracellular adenosine/A1AR signaling induce immunosuppression in HCC, while targeted inhibition of HIF-1α alleviates HCC.23 TME is formed when cancer cells invade and change the structure of surrounding tissues. The TME is composed of tumor cells, immune cells, and stromal cells, and is a unique ecosystem that provides protection for tumor cell growth. Blocking adenosine signaling in TME to reverse immunosuppression is a research hotspot. Caffeine activates the immune response by inhibiting the binding of extracellular adenosine and ARs, activating various immune cells, reducing immune escape, and improving the TME to inhibit HCC progression.
Animal Experiments
Recent studies have found that a combination of coffee and different compounds alleviates the development of early fibrosis-associated hepatocarcinogenesis in rodents.59,60 Highly bioavailable coffee compounds include caffeine, trigonelline (TRI), and CGA. Ten weeks oral administration of a caffeine (50 mg/kg) + TRI (25 mg/kg) + CGA (25 mg/kg) combination decreased the incidence, quantity, activation, and proliferation (Ki-67 positive) of hepatocellular preneoplastic foci, and increased HCC cell apoptosis (cleaved caspase-3 activity elevated) in neighboring parenchyma in a diethylnitrosamine (DEN)/CCl4-induced mice model.59 Interestingly, the effect of the combination of the three components was better than that of caffeine alone; the mechanism may be related to modulating miRNA expression profile.59 The effect of caffeine, TRI or CGA on the promotion of various HCC cell lines apoptosis is rarely studied, but previous studies have shown that transfection of miR-15b-5p can increase the number of middle and advanced apoptotic cells in human cell line Hep3B by targeting and inhibiting Rab1A oncogene.59,61 Previous studies had shown that 0.02% caffeine treatment for 10 and 14 weeks was well tolerated and significantly inhibited the occurrence of DEN induced HCC in rats, in addition, 0.025% caffeine significantly reduced the expression levels of HCC markers including proliferating cell nuclear antigen and placental glutathione S-transferase in the liver of rats.62 Moreover, ordinary coffee, decaffeinated coffee, and caffeine with 37.5 mg/kg per day consumption for 8 weeks decreased serum level of alanine transaminase, collagen-volume fraction, fibrosis/inflammation scores, oxidized glutathione, and transforming growth factor-β1 protein expression in the liver of HCC rats induced by thioacetamide (TAA) (200 mg/kg, intraperitoneal) twice weekly for 8 weeks.60 Clinical studies have also reported on the effects of caffeine and/or CGA supplementation on different liver diseases.63 Interestingly, ordinary coffee (contained caffeine) and 0.1% caffeine had a greater inhibitory effect than decaffeinated coffee. To sum up, most animal studies have confirmed that caffeine has a protective effect on hepatocarcinogenesis induced by various chemical poisons including DEN, TAA, and CCl4 in rodents. However, further research is needed to assess the inner mechanisms underlying the liver protective effects of coffee drinks or their specific ingredients.60
Recent research has found that caffeine has anti-inflammatory and anti-infection properties by stimulating macrophage phagocytosis. Researchers have found that intravenous caffeine administration reduced inflammation or infection induced by intraperitoneal inoculation of Salmonella typhimurium in Swiss mice by increasing macrophage activity and reducing intracellular bacterial load. Additionally, the expression of TNF-α, IL-1β, IL-6, and inducible nitric oxide synthase was found to be down-regulated in caffeine-treated mice after infection, suggesting that caffeine can be used in combination with antibiotics for treatment of bacterial infections.64 New studies have found that prenatal exposure of 80 mg/kg caffeine can significantly reduce the average body weight of rat offspring before weaning, which may be related to the immune dysfunction in rats caused by excessive caffeine exposure; however, 100 mg/kg caffeine body weight does not cause neurobehavioral toxicity in adult mice.65 The results suggest that excessive caffeine exposure may lead to immune dysfunction and intrauterine growth restriction retardation in pregnant women, and coffee consumption before and during pregnancy is not recommended. Caffeine administration at 37.5 mg/kg per day has been shown to reduce inflammation and oxidative stress in rats with severe TAA-induced hepatic injury (intraperitoneal TAA 200 mg/kg twice a week). The dose of caffeine in rats (37.5 mg/kg/day) is equivalent to 6 mg/kg per day in human. A moderate coffee intake for an adult weighing 70 kg is approximately 400 mg caffeine per day, representing approximately 6 mg/kg. Additionally, 6 mg/kg per day of caffeine is equivalent to three cups of coffee daily in humans.60 Even low doses of caffeine may have a protective effect against liver damage; indeed caffeine at 5 and 10 mg/kg alleviate chronic liver damage in rats induced by CCl4 (0.75 mL/kg).66 Moreover, caffeine solution (0.1 mg/mL) treatment for 12 days has been shown to significantly increase SHBG production in the liver and adiponectin levels in the epididymal adipose tissue of human SHBG transgenic mice.48 Anti-fibrosis, anti-inflammatory, anti-infective, and anti-oxidant effects may explain the potential mechanism for the protective effect of caffeine on HCC induced by chemical poisons.
Using macrophage-specific CD39 knockout mice, researchers found that cell-specific CD39 expression in macrophages and CD73 expression in HCC cells synergically activate the eATP-adenosine pathway, producing more adenosine, thereby impacting CD8+ T cell function and promoting anti-PD1 resistance, suggesting that targeted inhibition of adenosine alleviated HCC.21 Animal experiments found that macrophage-specific knockout of CD39 in mouse HCC model induced by subcutaneous tumor xenograft significantly reversed resistance to PD1 therapy, suggesting that CD39 targeting macrophages may remodel the immune microenvironment.21 Overexpression of RNA N6-adenosine methyltransferase was found to effectively promote HCC tumor growth in a nude mouse model.53 The wide variety of gene knockout and immunodeficient mice provides a variety of options for studying the protective effects of caffeine and its derivatives against HCC. Additionally, activation of the AMPK/mTOR signaling has been found to play an anticancer role in a xenograft nude mouse model, suggesting that adenosine may affect HCC progression in vivo in different ways.54 However, the relationship between metabolic reprogramming of HCC cells and ATP in HCC animal models still needs further study. Furthermore, the newly found CRISPR-Cas9-mediated loss of 5-methylthioadenosine 2A, a critical S-adenosylmethionine producing enzyme, leads to T-cell dysfunction and inhibition of mouse HCC growth. Therefore, targeting 5-methylthioadenosine may be a viable therapeutic strategy for improving HCC immunity.17 Reprogramming of methionine metabolism may be one of the resistance mechanisms of HCC to immune checkpoint inhibitors, which will be further investigated in the future. Furthermore, downregulation of CD73 significantly inhibited the growth of orthotopic xenografts in NOD/SCID/γc(null) mice liver, and the combination of CD73 and A2AR inhibitors enhanced synergistic tumor inhibition in HCC compared to either regimen alone.20 Mechanistically, adenosine produced by CD73 binds to A2AR promotes HCC progression and epithelial-mesenchymal transition by activating PI3K/Akt signaling. Down-regulating triphosphate diphosphohydrolase-2/CD39L1 expression by inhibiting HIF-1 can inhibit the aggregation of myeloid-derived suppressor cells in HCC mice, which can prevent cancer from escaping immune monitoring.58 Inhibition of HIF-1α is known to decrease the expression of CD39 and CD73 in the liver tissue of HCC mice.23,67 These above in vivo results indicate that down-regulation of the CD39-CD73-adenosine pathway can inhibit HCC progression. The rapid development and diversification of CRISPR-Cas9 gene editing technology provides strong technical support for further research on the protective effect of caffeine on HCC. The emergence of various monoclonal mouse antibodies provides a new option to study the function and role of various immune cells in immune response.
Clinical Trials
Many epidemiological studies have reported that daily oral coffee intake is associated with a lower risk of HCC.68–71 Dietary patterns or foods with anti-inflammatory potential are associated with reduced risk of HCC and can influence HCC progression. Diets rich in fiber, vegetables and fruits intake, and Mediterranean diet are recommended.68,71 Ruiz et al reported that aflatoxin exposure was associated with a risk for HCC that is up to 30-fold higher than without aflatoxin exposure suggest that aflatoxin exposure was an significant inductor of HCC besides hepatitis viruses.71 A recent systematic review explored the dose-dependent relationship between coffee or decaffeinated coffee consumed daily and the risk of HCC. Researchers investigated 2,272,642 subjects and 2905 cases and found that two cups of coffee daily was associated with a 35% decrease in the risk of HCC (95% confidence interval (CI) = 0.59–0.72, relative risk (RR) = 0.65),14 while an additional two cups of daily caffeinated and decaffeinated coffee consumption (two and three cohort trials, respectively) was connected to decreases in the risk of HCC of 27% (95% CI = 0.63–0.85, RR = 0.73) and 14% (95% CI = 0.74–1.00, RR = 0.86), which was the strongest evidence to date of a link between decaffeinated coffee and HCC. The mechanism of action of caffeine against HCC has also been discussed in detail and may be related to the inhibition of HCC cell proliferation and amelioration of oxidative DNA damage.14 Previous studies have found that increasing daily intake by two cups of caffeinated and decaffeinated coffee was associated with 27% and 14% reduction in HCC risk, respectively.14,72,73 Coffee may have direct anti-cancer properties, with numerous research findings supporting this.36,72–77 The results of a meta-analysis conducted in 2023 by Yu and colleagues further confirm that drinking coffee or green tea may be a potentially effective way to prevent or mitigate HCC, but this needs to be confirmed by further well-designed observational studies and clinical trials.36 Additionally, most studies support a central role for caffeine, as decaffeinated coffee has a weaker association, possibly due to the different physiological effects of various components in coffee.42,51,62,75 In addition to caffeine, coffee contains many compounds such as cafestol, kahweol, and polyphenols.38,44,59,63,69 Recent studies have identified caffeic acid and its derivatives as potential modulators of carcinogenic molecular pathways, providing new hope for fighting cancer.78 The main finding is that caffeic acid is well tolerated and safe, and it can be considered as an potential alternative to synthetic chemotherapy drugs that have adverse reactions and resistance of various tumor cells.78 The anti-liver cancer effect of these auxiliary components remains to be further explored and some results remain controversial. For example, another in vitro study found that a caffeine content of less than 1 mM was not poisonous to living cells and did not lead to DNA damage of the HepG2 cell line.79 A case-control study in Hong Kong that recruited 234 chronic HBV carriers (109 cases and 125 controls) indicated that moderate daily coffee consumption decreased the risk of HCC in HBV carriers, although this trial had a small number of subjects (234 patients) and potential recall bias.77 These epidemiological studies reported that the biological mechanisms of coffee consumption and HCC reduction mainly include the anti-oxidant and anti-inflammatory effects, suppressing growth of liver tumor, improving metabolism and excretion of carcinogens, inhibiting the activity and replication of hepatitis virus, and decreasing the risk of type 2 diabetes of the main chemical components (CGA, polyphenols, diterpenes, cafestol, kahweol, and caffeine) in coffee.69 At present, computerized tomography and magnetic resonance imaging are still the most effective non-invasive methods for HCC diagnosis.71 In addition, more than 70% of HCC patients have AFP levels higher than 20 ng/mL with quite poor specificity, and liver biopsy remains the most effective invasive diagnostic basis for HCC.71 Results from meta-analyses, mostly from the Americas, Europe and Japan, further confirm the negative association between coffee intake and HCC risk.
Many recent epidemiological studies have found that people who drink coffee have a reduced risk of different types of cancer.29,80 A study published in 2022 investigated the relationship between coffee intake, caffeine metabolism genotypes, and survival in male patients with prostate cancer. The researchers examined data from 5727 patients with prostate cancer from seven studies in the United States, Australia, and Europe, and found that while the results were not statistically significant, higher coffee intake appeared to be associated with longer prostate cancer-specific survival. More research is needed to fully understand which population of men might benefit, and why.29 A large survey of patients with colorectal cancer found that coffee intake was inversely associated with cancer recurrence and mortality of stage III disease, levels of metabolites associated with coffee intake were associated with a lower risk of colorectal cancer incidence or diagnosis.29 Another epidemiological study published in 2022 investigated the association between lifetime caffeine intake and the risk of epithelial ovarian cancer in late adulthood.80 The results of a population-based case-control study of 497 cases and 904 controls in Montreal, Canada, showed no significant association between lifetime caffeine intake and ovarian cancer risk. However, the authors stressed that the findings are inconsistent with previous studies, so the link between caffeine consumption and reduced risk of ovarian cancer remains controversial and needs to be examined in the long term, larger epidemiological studies.80 Petrick et al found that consumption of caffeinated or decaffeinated coffee had null associations with intrahepatic cholangiocarcinoma.73 The majority of previous studies have reported the relationship between oral coffee and the reduction in HCC risk. A review in 2021 summarized caffeinated coffee consumption daily and various health outcomes among US citizens by conducting a meta-analysis of dose-response relationship and evaluation of avoided deaths and diseased cases. The review found that caffeinated coffee regularly decreased the incidence/mortality of different angiocardiopathies, as well as the occurrence of type 2 diabetes and HCC, and the largest risk reduction was observed for drinking 3–4 cups daily (approximately 120 mL/cup).81 In addition, the study confirmed and quantified the beneficial health effects of caffeinated coffee consumption on endometrial cancer, melanoma, and non-melanoma skin cancer in the US population.81 A recent study investigating the protective effect of coffee consumption on HCC after liver transplantation in cirrhosis found that coffee consumption was associated with a reduced risk of HCC recurrence and improved survival after orthotopic liver transplantation. The results may be related (at least in part) to the antagonistic activity of caffeine against adenosine-A2AR-mediated growth promoting effects on HCC cells.18 Interestingly, some studies still report no association between coffee consumption and HCC risk.31,34 Indeed, a prospective cohort study of half a million Chinese adults found that caffeine, the main component of tea, had no protective effect on the incidence of all types of cancer, including HCC.31 Additionally, a recent systematic review and meta-analysis of prospective cohort studies demonstrated that coffee consumption was not associated with a reduced risk of stomach cancer.34 However, as it remains difficult to increase the concentration of caffeine in HCC tissues, it is necessary to find new ways to improve the absorption of caffeine in the human body. A new double-blind randomized crossover study was conducted to evaluate the pharmacokinetics of caffeine and D9-caffeine in healthy subjects.50 The pharmacokinetic, pharmacological, and genotoxicity evaluation of deuterate caffeine is currently at the animal experimental stage, and no human studies have been reported so far.82 Modifying the negative physiological effects of caffeine and prolonging its effects is an active area of research. In addition to HCC, several large-scale observational cohort and case-control studies in humans in Europe and the Americas have investigated the association between coffee intake and the risk of gastric cancer, prostate cancer, lung cancer, colorectal cancer, skin cancer, ovarian cancer, endometrial cancer, and uterine cervix cancer, but the results have been inconsistent. Racial differences may be one of the reasons, the exact reason still need to be studied.
Interestingly, daily coffee consumption positively influences the prevention of HCC relapse after the operation orthotopic liver transplantation.18 Research evaluating human HepG2 cell lines has analyzed the proliferative and metastatic ability after co-culture with various concentrations of adenosine, while in vitro studies showed that adenosine mediated the activation and proliferation of tumor cells mainly through the mitogen-activated protein kinase (MAPK) and nuclear factor-kappa B (NF-kappa B) signaling pathways.18 A growing number of studies have found that caffeine inhibits normal growth of different types of cancer cell lines.34,80,81 On the one hand, caffeine interrupts growth cycle of various cancer cells, alters DNA repair processes, and disrupts apoptosis. On the other hand, the components of coffee, especially phenolic compounds, have antioxidant abilities that may have a protective effect against cancer. Therefore, further research is still needed. Future randomized studies are warranted to explore the effectiveness of increasing coffee intake in people at high risk of HCC, including patients with existing chronic liver disease. Additionally, future studies will focus on the pharmacokinetic studies of caffeine and its derivatives, which will address how to improve effective drug concentration and targeting specificity to tumor tissues as well as reduce its toxicity to normal tissues.
Conclusion and Future Research Perspectives
Kennedy et al reviewed the relationship between coffee and the risk of HCC before 2017, including caffeinated and decaffeinated coffee, and found that consuming two cups of coffee per day reduced the risk of HCC by one-third.14 Oral coffee intake is suggested to reduce the risk of HCC by inhibiting the activation and proliferation of HCC cells, which is induced by caffeine, the main component of coffee, and is considered the main mechanism by which coffee reduces HCC risk. Randomized trials are needed to support interventions before recommending increased coffee intake in patients at high risk of HCC. Our review mainly summarizes and analyzes animal experiments, cell experiments, and epidemiological studies on the reduction of HCC risk by coffee/caffeine in the past 2 decades until December 6, 2023. Preclinical and/or clinical studies on the other common (and abundant) coffee compounds are summarized by our team (Table 2). We report that caffeine initiates a series of immune responses by inhibiting the binding of adenosine and ARs, representing a new mechanism for the prevention of HCC. Additionally, inhibition of the adenosine signaling pathway induces changes in the TME; this is an important research direction of tumor immunity. New drugs and antibodies targeting adenosine signaling have appeared in recent years, as well as new caffeine preparations used in cancer adjuvant therapy. Additionally, inhibiting CD73 could slow down the growth of cancer and improve the efficiency and efficacy of immune checkpoint inhibitors, CD73 might be a good prognostic marker and therapeutic target for cancer patients, especially for those receiving immunotherapy.
 |
Table 2 Preclinical and/or Clinical Studies on the Other Common (and Abundant) Coffee Compounds |
Overall, cellular assays, animal experiments, and clinical research have demonstrated that coffee/caffeine has liver-protective effects and reduces the risk of HCC, suggesting that caffeine may be developed as a drug to prevent HCC in the future. The biggest challenge is how to increase the concentration of caffeine in the local HCC tissue. We found that drinking coffee may reduce the risk of HCC, with a recommended dose for adults of 2–3 cups per day, containing an average caffeine concentration of 60–85 mg per cup, was beneficial for human health.14,38,60,81 Additionally, studies have shown that if risk estimates are unbiased, coffee consumption reduce the proportion of HCC cases/deaths by 6–12%.81 The appropriate time of drinking coffee is 1 h after mealtime and 30 min before bed.8,83 Coffee is not recommended for patients with cardiovascular disease, diabetes and hypertension to avoid inducing adverse reactions such as arrhythmia, elevated blood sugar, and elevated blood pressure. It is important to educate young people and their parents about the risks of excessive consumption of caffeinated beverages. The potential health consequences of excessive consumption of coffee represent a major public health hazard.83 A recent clinical study found that high-flow continuous hemodialysis successfully treated severe caffeine intoxication,84 while a new toxicology report found that the combination of labetalol and hemodialysis can relieve near-fatal caffeine intoxication.85 Thus, it is critical to prevent caffeine overdose. Educational interventions are necessary to raise awareness among adolescents regarding the potential consequences of excessive consumption of these popular caffeinated beverages, as serum concentrations exceeding 80–100 μg/mL can have fatal implications in adults. Furthermore, it is crucial to educate individuals about the detrimental effects of excessive caffeine on overall bodily health.
This review summarizes the progress of coffee/caffeine in the prevention and treatment of HCC in the past decades from the aspects of cellular assays, animal experiments, and epidemiological studies. For people with middle to high coffee/caffeine consumption, the recommended dose is approximately 2 to 3 cups per day. In many research papers suggesting that caffeine has a half-life period of approximately 1 h and 5 h in rats and humans, respectively, rats would require a larger dosage of coffee/caffeine to reach a blood concentration similar to that of coffee/caffeine consumers. Thus, it can be concluded that caffeine intake in the form of drinking 2–3 cups/day of coffee has multidimensional beneficial effects beyond anti-fibrosis, including a reduced risk of HCC.
Data Sharing Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Acknowledgments
We thank LetPub for its linguistic assistance during the preparation of this manuscript.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Natural Science Foundation of China (Grant 82003849), the National Natural Science Foundation of China (Grant 81970518), Hefei Municipal Natural Science Foundation (Grant 2021012), the Special Doctoral Fund of Hefei Hospital Affiliated to Anhui Medical University (the 2nd People’s Hospital of Hefei) (Grant 2020BSZX04), the Key Scientific Research Foundation of the Education Department of the Province Anhui (Grant 2023AH052595), and the Scientific Research Projects of Health Commission of Anhui Province in 2023 (Grant AHWJ2023BAc20099).
Disclosure
The authors have no conflicts of interest in this work.
References
1. de Alcântara Almeida I, Mancebo Dorvigny B, Souza Tavares L, Nunes Santana L, Vitor Lima-Filho J. Anti-inflammatory activity of caffeine (1,3,7-trimethylxanthine) after experimental challenge with virulent Listeria monocytogenes in Swiss mice. Int Immunopharmacol. 2021;100:108090. doi:10.1016/j.intimp.2021.108090
2. Dranoff JA. Coffee Consumption and Prevention of Cirrhosis: in Support of the Caffeine Hypothesis. Gene Expr. 2018;18(1):1–3. doi:10.3727/105221617X15046391179559
3. Estari RK, Dong J, Chan WK, Park MS, Zhou Z. Time effect of rutaecarpine on caffeine pharmacokinetics in rats. Biochem Biophys Rep. 2021;28:101121. doi:10.1016/j.bbrep.2021.101121
4. Singal AG, Kanwal F, Llovet JM. Global trends in hepatocellular carcinoma epidemiology: implications for screening, prevention and therapy. Nat Rev Clin Oncol. 2023;20(12):864–884. doi:10.1038/s41571-023-00825-3
5. Helal MG, Ayoub SE, Elkashefand WF, Ibrahim TM. Caffeine affects HFD-induced hepatic steatosis by multifactorial intervention. Hum Exp Toxicol. 2018;37(9):983–990. doi:10.1177/0960327117747026
6. Tan X, Sun Y, Chen L, et al. Caffeine Ameliorates AKT-Driven Nonalcoholic Steatohepatitis by Suppressing De Novo Lipogenesis and MyD88 Palmitoylation. J Agric Food Chem. 2022;70(20):6108–6122. doi:10.1021/acs.jafc.2c01013
7. Alshabi AM, Alkahtani SA, Shaikh IA, Habeeb MS. Caffeine modulates pharmacokinetic and pharmacodynamic profiles of pioglitazone in diabetic rats: impact on therapeutics. Saudi Med J. 2021;42(2):151–160. doi:10.15537/smj.2021.2.25695
8. Jee HJ, Lee SG, Bormate KJ, Jung YS. Effect of Caffeine Consumption on the Risk for Neurological and Psychiatric Disorders: sex Differences in Human. Nutrients. 2020;12(10):3080. doi:10.3390/nu12103080
9. Godos J, Micek A, Marranzano M, Salomone F, Rio DD, Ray S. Coffee Consumption and Risk of Biliary Tract Cancers and Liver Cancer: a Dose-Response Meta-Analysis of Prospective Cohort Studies. Nutrients. 2017;9(9):950. doi:10.3390/nu9090950
10. Wang Z, Gu C, Wang X, et al. Caffeine enhances the anti-tumor effect of 5-fluorouracil via increasing the production of reactive oxygen species in hepatocellular carcinoma. Med Oncol. 2019;36(12):97. doi:10.1007/s12032-019-1323-8
11. Wang Q, Dai X, Yang W, et al. Caffeine protects against alcohol-induced liver fibrosis by dampening the cAMP/PKA/CREB pathway in rat hepatic stellate cells. Int Immunopharmacol. 2015;25(2):340–352. doi:10.1016/j.intimp.2015.02.012
12. Yang Y, Wang H, Lv X, et al. Involvement of cAMP-PKA pathway in adenosine A1 and A2A receptor-mediated regulation of acetaldehyde-induced activation of HSCs. Biochimie. 2015;115:59–70. doi:10.1016/j.biochi.2015.04.019
13. Zhou K, Lim T, Dodge JL, Terrault NA, Wilkens LR, Setiawan VW. Population-attributable risk of modifiable lifestyle factors to hepatocellular carcinoma: the multi-ethnic cohort. Aliment Pharmacol Ther. 2023;58(1):89–98. doi:10.1111/apt.17523
14. Kennedy OJ, Roderick P, Buchanan R, Fallowfield JA, Hayes PC, Parkes J. Coffee, including caffeinated and decaffeinated coffee, and the risk of hepatocellular carcinoma: a systematic review and dose-response meta-analysis. BMJ Open. 2017;7(5):e013739. doi:10.1136/bmjopen-2016-013739
15. Wang P, Jia J, Zhang D. Purinergic signalling in liver diseases: pathological functions and therapeutic opportunities. JHEP Rep. 2020;2(6):100165. doi:10.1016/j.jhepr.2020.100165
16. Giannone G, Ghisoni E, Genta S, et al. Immuno-Metabolism and Microenvironment in Cancer: key Players for Immunotherapy. Int J Mol Sci. 2020;21(12):4414. doi:10.3390/ijms21124414
17. Hung MH, Lee JS, Ma C, et al. Tumor methionine metabolism drives T-cell exhaustion in hepatocellular carcinoma. Nat Commun. 2021;12(1):1455. doi:10.1038/s41467-021-21804-1
18. Wiltberger G, Wu Y, Lange U, et al. Protective effects of coffee consumption following liver transplantation for hepatocellular carcinoma in cirrhosis. Aliment Pharmacol Ther. 2019;49(6):779–788. doi:10.1111/apt.15089
19. Hhm N, Lee RY, Goh S, et al. Immunohistochemical scoring of CD38 in the tumor microenvironment predicts responsiveness to anti-PD-1/PD-L1 immunotherapy in hepatocellular carcinoma. J Immunother Cancer. 2020;8(2):e000987. doi:10.1136/jitc-2020-000987
20. Ma XL, Shen MN, Hu B, et al. CD73 promotes hepatocellular carcinoma progression and metastasis via activating PI3K/AKT signaling by inducing Rap1-mediated membrane localization of P110β and predicts poor prognosis. J Hematol Oncol. 2019;12(1):37. doi:10.1186/s13045-019-0724-7
21. Lu JC, Zhang PF, Huang XY, et al. Amplification of spatially isolated adenosine pathway by tumor-macrophage interaction induces anti-PD1 resistance in hepatocellular carcinoma. J Hematol Oncol. 2021;14(1):200. doi:10.1186/s13045-021-01207-x
22. Jindal A, Thadi A. Hepatocellular Carcinoma: etiology and Current and Future Drugs. J Clin Exp Hepatol. 2019;9(2):221–232. doi:10.1016/j.jceh.2019.01.004
23. Pang L, Ng KT, Liu J, et al. Plasmacytoid dendritic cells recruited by HIF-1α/eADO/ADORA1 signaling induce immunosuppression in hepatocellular carcinoma. Cancer Lett. 2021;522:80–92. doi:10.1016/j.canlet.2021.09.022
24. Hu S, Liu K, Luo H, et al. Caffeine programs hepatic SIRT1-related cholesterol synthesis and hypercholesterolemia via A2AR/cAMP/PKA pathway in adult male offspring rats. Toxicology. 2019;418:11–21. doi:10.1016/j.tox.2019.02.015
25. Salem R, Tselikas L, De Baere T. Interventional treatment of hepatocellular carcinoma. J Hepatol. 2022;77(4):1205–1206. doi:10.1016/j.jhep.2022.03.037
26. Johnson P, Zhou Q, Dao DY, Lo YMD. Circulating biomarkers in the diagnosis and management of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2022;19(10):670–681. doi:10.1038/s41575-022-00620-y
27. Rawat D, Shrivastava S, Naik RA, Chhonker SK, Mehrotra A, Koiri RK. An Overview of Natural Plant Products in the Treatment of Hepatocellular Carcinoma. Anticancer Agents Med Chem. 2018;18(13):1838–1859. doi:10.2174/1871520618666180604085612
28. Yau T, Park JW, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, Phase 3 trial. Lancet Oncol. 2022;23(1):77–90. doi:10.1016/S1470-2045(21)00604-5
29. Gregg JR, Kim J, Logothetis C, et al. Coffee Intake, Caffeine Metabolism Genotype, and Survival Among Men with Prostate Cancer. Eur Urol Oncol. 2023;6(3):282–288. doi:10.1016/j.euo.2022.07.008
30. Ellingjord-Dale M, Papadimitriou N, Katsoulis M, et al. Coffee consumption and risk of breast cancer: a Mendelian randomization study. PLoS One. 2021;16(1):e0236904. doi:10.1371/journal.pone.0236904
31. Li X, Yu C, Guo Y, et al.; China Kadoorie Biobank Collaborative Group. Association between tea consumption and risk of cancer: a prospective cohort study of 0.5 million Chinese adults. Eur J Epidemiol. 2019;34(8):753–763. doi:10.1007/s10654-019-00530-5
32. Wang J, Wang Y, Chu Y, et al. Tumor-derived adenosine promotes macrophage proliferation in human hepatocellular carcinoma. J Hepatol. 2021;74(3):627–637. doi:10.1016/j.jhep.2020.10.021
33. Lange NF, Radu P, Dufour JF. Prevention of NAFLD-associated HCC: role of lifestyle and chemoprevention. J Hepatol. 2021;75(5):1217–1227. doi:10.1016/j.jhep.2021.07.025
34. Song H, Shen X, Chu Q, Zheng X. Coffee consumption is not associated with the risk of gastric cancer: an updated systematic review and meta-analysis of prospective cohort studies. Nutr Res. 2022;102:35–44. doi:10.1016/j.nutres.2022.03.002
35. Van Dam RM, Hu FB, Willett WC. Coffee, Caffeine, and Health. N Engl J Med. 2020;383(4):369–378. doi:10.1056/NEJMra1816604
36. Yu J, Liang D, Li J, et al. Coffee, Green Tea Intake, and the Risk of Hepatocellular Carcinoma: a Systematic Review and Meta-Analysis of Observational Studies. Nutr Cancer. 2023;75(5):1295–1308. doi:10.1080/01635581.2023.2178949
37. Kawano Y, Nagata M, Kohno T, et al. Caffeine increases the antitumor effect of Cisplatin in human hepatocellular carcinoma cells. Biol Pharm Bull. 2012;35(3):400–407. doi:10.1248/bpb.35.400
38. Saab S, Mallam D, Cox GA, Tong MJ. Impact of coffee on liver diseases: a systematic review. Liver Int. 2014;34(4):495–504. doi:10.1111/liv.12304
39. Dong S, Kong J, Kong J, et al. Low Concentration of Caffeine Inhibits the Progression of the Hepatocellular Carcinoma via Akt Signaling Pathway. Anticancer Agents Med Chem. 2015;15(4):484–492. doi:10.2174/1871520615666150209110832
40. Oda Y, Hidaka M, Suzuki A. Caffeine Has a Synergistic Anticancer Effect with Cisplatin via Inhibiting Fanconi Anemia Group D2 Protein Monoubiquitination in Hepatocellular Carcinoma Cells. Biol Pharm Bull. 2017;40(11):2005–2009. doi:10.1248/bpb.b17-00457
41. Edling CE, Selvaggi F, Ghonaim R, Maffucci T, Falasca M. Caffeine and the analog CGS 15943 inhibit cancer cell growth by targeting the phosphoinositide 3-kinase/Akt pathway. Cancer Biol Ther. 2014;15(5):524–532. doi:10.4161/cbt.28018
42. Okano J, Nagahara T, Matsumoto K, Murawaki Y. Caffeine inhibits the proliferation of liver cancer cells and activates the MEK/ERK/EGFR signalling pathway. Basic Clin Pharmacol Toxicol. 2008;102(6):543–551. doi:10.1111/j.1742-7843.2008.00231.x
43. Yin X, He X, Wu L, Yan D, Yan S. Chlorogenic Acid, the Main Antioxidant in Coffee, Reduces Radiation-Induced Apoptosis and DNA Damage via NF-E2-Related Factor 2 (Nrf2) Activation in Hepatocellular Carcinoma. Oxid Med Cell Longev. 2022;2022:4566949. doi:10.1155/2022/4566949
44. Seo HY, Lee SH, Lee JH, Lee JH, Jang BK, Kim MK. Kahweol Induces Apoptosis in Hepatocellular Carcinoma Cells by Inhibiting the Src/mTOR/STAT3 Signaling Pathway. Int J Mol Sci. 2021;22(19):10509. doi:10.3390/ijms221910509
45. Fagundes TR, Madeira TB, Melo GP, et al. Caffeine improves the cytotoxic effect of dacarbazine on B16F10 murine melanoma cells. Bioorg Chem. 2022;120:105576. doi:10.1016/j.bioorg.2021.105576
46. Özgün G S, Özgün E, Tabakçıoğlu K, Süer Gökmen S, Eskiocak S, Çakır E. Caffeine Increases Apolipoprotein A-1 and Paraoxonase-1 but not Paraoxonase-3 Protein Levels in Human-Derived Liver (HepG2) Cells. Balkan Med J. 2017;34(6):534–539. doi:10.4274/balkanmedj.2016.1217
47. Lemos MF, Salustriano NA, Costa MMS, et al. Chlorogenic acid and caffeine contents and anti-inflammatory and antioxidant activities of green beans of conilon and arabica coffees harvested with different degrees of maturation. J Saudi Chem Soc. 2022:101467. doi:10.1016/j.jscs.2022.101467
48. Briansó-Llort L, Fuertes-Rioja L, Ramos-Perez L, Hernandez C, Simó R, Selva DM. Caffeine Upregulates Hepatic Sex Hormone-Binding Globulin Production by Increasing Adiponectin Through AKT/FOXO1 Pathway in White Adipose Tissue. Mol Nutr Food Res. 2020;64(17):e1901253. doi:10.1002/mnfr.201901253
49. Kisku T, Paul K, Singh B, et al. Synthesis of Cu(II)-Caffeine Complex as potential therapeutic Agent: studies on Antioxidant, anticancer and pharmacological activities. J Mol Liq. 2022;364:119897. doi:10.1016/j.molliq.2022.119897
50. Sherman MM, Tarantino PM, Morrison DN, Lin CH, Parente RM, Sippy BC. A double-blind, randomized, two-part, two-period crossover study to evaluate the pharmacokinetics of caffeine versus d9-caffeine in healthy subjects. Regul Toxicol Pharmacol. 2022;133:105194. doi:10.1016/j.yrtph.2022.105194
51. Yang H, Li J, Cui L, et al. Synergistic cytotoxicity and mechanism of caffeine and lysozyme on hepatoma cell line HepG2. Spectrochim, Acta A Mol, Biomol, Spectrosc. 2018;193:169–174. doi:10.1016/j.saa.2017.12.020
52. Chen R, Du J, Zhu H, Ling Q. The role of cGAS-STING signalling in liver diseases. JHEP Rep. 2021;3(5):100324. doi:10.1016/j.jhepr.2021.100324
53. Chen M, Wei L, Law CT, et al. RNA N6-methyladenosine methyltransferase-like 3 promotes liver cancer progression through YTHDF2-dependent posttranscriptional silencing of SOCS2. Hepatology. 2018;67(6):2254–2270. doi:10.1002/hep.29683
54. Fang G, Zhang P, Liu J, et al. Inhibition of GSK-3β activity suppresses HCC malignant phenotype by inhibiting glycolysis via activating AMPK/mTOR signaling. Cancer Lett. 2019;463:11–26. doi:10.1016/j.canlet.2019.08.003
55. Kim GW, Imam H, Khan M, et al. HBV-Induced Increased N6 Methyladenosine Modification of PTEN RNA Affects Innate Immunity and Contributes to HCC. Hepatology. 2021;73(2):533–547. doi:10.1002/hep.31313
56. Kierans SJ, Taylor CT. Regulation of glycolysis by the hypoxia-inducible factor (HIF): implications for cellular physiology. J Physiol. 2021;599(1):23–37. doi:10.1113/JP280572
57. Piñeiro Fernández J, Luddy KA, Harmon C, O’Farrelly C. Hepatic Tumor Microenvironments and Effects on NK Cell Phenotype and Function. Int J Mol Sci. 2019;20(17):4131. doi:10.3390/ijms20174131
58. Chiu DK, Tse AP, Xu IM, et al. Hypoxia inducible factor HIF-1 promotes myeloid-derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma. Nat Commun. 2017;8(1):517. doi:10.1038/s41467-017-00530-7
59. Romualdo GR, Prata GB, da Silva TC, et al. The combination of coffee compounds attenuates early fibrosis-associated hepatocarcinogenesis in mice: involvement of miRNA profile modulation. J Nutr Biochem. 2020;85:108479. doi:10.1016/j.jnutbio.2020.108479
60. Amer MG, Mazen NF, Mohamed AM. Caffeine intake decreases oxidative stress and inflammatory biomarkers in experimental liver diseases induced by thioacetamide: biochemical and histological study. Int J Immunopathol Pharmacol. 2017;30(1):13–24. doi:10.1177/0394632017694898
61. Yang Y, Hou N, Wang X, et al. miR-15b-5p induces endoplasmic reticulum stress and apoptosis in human hepatocellular carcinoma, both in vitro and in vivo, by suppressing Rab1A. Oncotarget. 2015;6(18):16227–16238. doi:10.18632/oncotarget.3970
62. Fujise Y, Okano J, Nagahara T, Abe R, Imamoto R, Murawaki Y. Preventive effect of caffeine and curcumin on hepato-carcinogenesis in diethylnitrosamine-induced rats. Int J Oncol. 2012;40(6):1779–1788. doi:10.3892/ijo.2012.1343
63. Mansour A, Mohajeri-Tehrani MR, Samadi M, et al. Effects of supplementation with main coffee components including caffeine and/or chlorogenic acid on hepatic, metabolic, and inflammatory indices in patients with non-alcoholic fatty liver disease and type 2 diabetes: a randomized, double-blind, placebo-controlled, clinical trial. Nutr J. 2021;20(1):35. doi:10.1186/s12937-021-00694-5
64. Dorvigny BM, Tavares LS, de Almeida IA, et al. Antiinflammatory and antiinfective effect of caffeine in a mouse model of disseminated salmonellosis. Phytother Res. 2022;36(4):1652–1663. doi:10.1002/ptr.7349
65. Zhang WZ, Sun NN, Hu Y, Cao Y, Amber S. Caffeine Exposure Causes Immune Dysfunction and Intrauterine Growth Restriction Retardation in Rats. Biomed Environ Sci. 2022;35(2):170–173. doi:10.3967/bes2022.025
66. Cachón AU, Quintal-Novelo C, Medina-Escobedo G, Castro-Aguilar G, Moo-Puc RE. Hepatoprotective Effect of Low Doses of Caffeine on CCl4-Induced Liver Damage in Rats. J Diet Suppl. 2017;14(2):158–172. doi:10.1080/19390211.2016.1207003
67. Velázquez-Miranda E, Díaz-Muñoz M, Vázquez-Cuevas FG. Purinergic signaling in hepatic disease. Purinergic Sig. 2019;15(4):477–489. doi:10.1007/s11302-019-09680-3
68. Zheng J, Zhao L, Dong J, et al. The role of dietary factors in nonalcoholic fatty liver disease to hepatocellular carcinoma progression: a systematic review. Clin Nutr. 2022;41(10):2295–2307. doi:10.1016/j.clnu.2022.08.018
69. Deng Y, Huang J, Wong MCS. Associations between six dietary habits and risk of hepatocellular carcinoma: a Mendelian randomization study. Hepatol Commun. 2022;6(8):2147–2154. doi:10.1002/hep4.1960
70. Carrieri P, Carrat F, Di Beo V, et al. Severe liver fibrosis in the HCV cure era: major effects of social vulnerability, diabetes, and unhealthy behaviors. JHEP Rep. 2022;4(6):100481. doi:10.1016/j.jhepr.2022.100481
71. Ruiz-Margáin A, Román-Calleja BM, Moreno-Guillén P, et al. Nutritional therapy for hepatocellular carcinoma. World J Gastrointest Oncol. 2021;13(10):1440–1452. doi:10.4251/wjgo.v13.i10.1440
72. Bai K, Cai Q, Jiang Y, Lv L. Coffee consumption and risk of hepatocellular carcinoma: a meta-analysis of eleven epidemiological studies. Onco Targets Ther. 2016;9:4369–4375. doi:10.2147/OTT.S109656
73. Petrick JL, Freedman ND, Graubard BI, et al. Coffee Consumption and Risk of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma by Sex: the Liver Cancer Pooling Project. Cancer Epidemiol Biomarkers Prev. 2015;24(9):1398–1406. doi:10.1158/1055-9965
74. Bravi F, Bosetti C, Tavani A, Gallus S, La Vecchia C. Coffee reduces risk for hepatocellular carcinoma: an updated meta-analysis. Clin Gastroenterol Hepatol. 2013;11(11):1413–21.e1. doi:10.1016/j.cgh.2013.04.039
75. Bamia C, Lagiou P, Jenab M, et al. Coffee, tea and decaffeinated coffee in relation to hepatocellular carcinoma in a European population: multicentre, prospective cohort study. Int, J, Cancer. 2015;136(8):1899–1908. doi:10.1002/ijc.29214
76. Johnson S, Koh WP, Wang R, Govindarajan S, Yu MC, Yuan JM. Coffee consumption and reduced risk of hepatocellular carcinoma: findings from the Singapore Chinese Health Study. Cancer Causes Control. 2011;22(3):503–510. doi:10.1007/s10552-010-9725-0
77. Leung WW, Ho SC, Chan HL, Wong V, Yeo W, Mok TS. Moderate coffee consumption reduces the risk of hepatocellular carcinoma in hepatitis B chronic carriers: a case-control study. J Epidemiol Community Health. 2011;65(6):556–558. doi:10.1136/jech.2009.104125
78. Mirzaei S, Gholami MH, Zabolian A, et al. Caffeic acid and its derivatives as potential modulators of oncogenic molecular pathways: new hope in the fight against cancer. Pharmacol Res. 2021;171:105759. doi:10.1016/j.phrs.2021.105759
79. Leão TK, Ribeiro DL, Machado ART, Costa TR, Sampaio SV, Antunes LMG. Synephrine and caffeine combination promotes cytotoxicity, DNA damage and transcriptional modulation of apoptosis-related genes in human HepG2 cells. Mutat Res Genet Toxicol Environ Mutagen. 2021;868-9:503375. doi:10.1016/j.mrgentox.2021.503375
80. Grundy A, Sandhu S, Arseneau J, et al. Lifetime caffeine intake and the risk of epithelial ovarian cancer. Cancer Epidemiol. 2022;76:102058. doi:10.1016/j.canep.2021.102058
81. Di Maso M, Boffetta P, Negri E, La Vecchia C, Bravi F. Caffeinated Coffee Consumption and Health Outcomes in the US Population: a Dose-Response Meta-Analysis and Estimation of Disease Cases and Deaths Avoided. Adv Nutr. 2021;12(4):1160–1176. doi:10.1093/advances/nmaa177
82. Parente RM, Tarantino PM, Sippy BC, Burdock GA. Pharmacokinetic, pharmacological, and genotoxic evaluation of deuterated caffeine. Food Chem Toxicol. 2022;160:112774. doi:10.1016/j.fct.2021.112774
83. De Sanctis V, Soliman N, Soliman AT, et al. Caffeinated energy drink consumption among adolescents and potential health consequences associated with their use: a significant public health hazard. Acta Biomed. 2017;88(2):222–231. doi:10.23750/abm.v88i2.6664
84. Kobashi D, Kamijo Y, Hanazawa T, Yoshizawa T, Nakamura M. Severe caffeine poisoning successfully treated with high flow continuous hemodialysis. Am J Emerg Med. 2022;58:351.e3–351.e5. doi:10.1016/j.ajem.2022.05.019
85. Ou HC, Deng JF, Yang CC, et al. A successful experience using labetalol and hemodialysis to treat near-fatal caffeine poisoning: a case report with toxicodynamics. Am J Emerg Med. 2022;55:224.e1–224.e4. doi:10.1016/j.ajem.2021.11.049
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.