Back to Journals » Clinical, Cosmetic and Investigational Dentistry » Volume 15
Botulinum Toxin Injection for Masseteric Hypertrophy Using 6 Point Injection Technique – A Case Report. Proposal of a Clinical Technique to Quantify Prognosis
Received 5 December 2022
Accepted for publication 9 March 2023
Published 21 March 2023 Volume 2023:15 Pages 45—49
DOI https://doi.org/10.2147/CCIDE.S396057
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Christopher E. Okunseri
Neha N Rathod, Rubin S John
Department of Oral and Maxillofacial Surgery, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences (SIMATS), Saveetha University, Chennai, Tamil Nadu, 600077, India
Correspondence: Rubin S John, Department of Oral and Maxillofacial Surgery, Saveetha Dental College and hospitals, Saveetha Institute of Medical and Technical Sciences (SIMATS), Saveetha University, Chennai, Tamil Nadu, 600077, India, Email [email protected]
Introduction: Masseter hypertrophy presents as unilateral or bilateral swellings over the ramus and angle of the mandible. It is caused by malocclusion, clenching, TMJ disorders, etc and alters facial symmetry, leading to discomfort and negative cosmetic impact in many patients, making this a popular request for aesthetic and functional correction.
Materials and Methods: This case report involves injecting Botulinum toxin into 6 equidistant bulging points on the masseter. Standardized photography and clinical parameters were used to assess facial contour and masseter muscle thickness at baseline and successive follow ups.
Results and Discussion: Significant masseteric bulk reduction was observed in subsequent follow ups.
Conclusion: The 6-point technique was found to be an effective treatment modality for Botox injection in masseteric hypertrophy. The clinical method to quantify prognosis was easy and economical.
Keywords: botulinum, hypertrophy, injection, masseter, toxin
Introduction
The masseter muscle is one of the chief muscles of mastication. The width and volume of this muscle is set particularly by the biting force, chewing habits, type of food, diet, or para-functional habits like bruxism and other temporo-mandibular joint disorders. Any of these can lead to masseter muscle hypertrophy (MMH), which provides a squarish appearance to the face or to the jawline.1 It is an idiopathic, benign, uncommon condition characterized by unilateral or bilateral enlargement of the masseter muscle and perhaps accompanied by pain, which is often confused with parotid swelling. Hence this swelling is a concern for facial disfigurement. There are two treatment approaches: surgical and non-surgical. Surgical options include surgical reduction of the angle of the mandible and liposuction of the subcutaneous fat. Nonsurgical management includes use of anti-anxiety drugs, muscle relaxants, occlusal equilibration by splints/night guards and injecting botulinum toxin-A (Botox) locally into the hypertrophied muscle. Treatment approach varies from case to case and additionally from the type of prominence of mandibular angle. Type I is when the enlargement is mainly due to muscle hypertrophy and can be appreciated when viewed from the front but appears normal when viewed from the lateral aspect. In such cases, Botox is recommended rather than a surgical method. Type II is when the swelling owes mainly to prominence of angle and not much to hypertrophy of masseter muscles. Botox can be used for re-contouring the face in such cases. Type III is due to both thick mandibular angle and hypertrophy of masseter muscles and it can be appreciated from the frontal and lateral view as well. Both muscle and bone being the cause, it can be resolved by surgery in conjunction with Botox injection.2 Botox (botulinum toxin) is a neurotoxin produced by the microorganism Clostridium botulinum. It causes flaccid paralysis induced by inhibiting acetylcholine release.3 This therapeutic effect continues for 3–6 months; within that period, it corrects the patterns of muscle exercises, decreases the squarish appearance of the jaw and alleviates pain by changing the patient’s lifestyle.4 Complications of injecting botulinum toxin include pain, edema, hematoma, chewing weakness, asymmetry, headache, paralysis of facial muscles due to accidental injection to major nerves, neuropraxia due to injury to marginal mandibular nerve, etc.5 The effectiveness can be evaluated using photographic evaluation, clinical measurements as well as advanced aids like ultrasound, electromyography, CBCT and MRI.
Case Report
A 26-year-old male reported to the clinic seeking cosmetic treatment because he was not happy with the appearance of his face. The patient confirmed a history of clenching and waking up everyday with a headache. Upon inspection, he had a square-shaped lower face with prominence at mandibular angle. When the patient was asked to clench his teeth, the prominence was increased. Upon palpation, abnormal thickening of masseter muscle (triple bulging Type IV, according to the classification given by Xie et al 2014) was observed. He was diagnosed with masseteric hypertrophy Type II, as classified by Kwon et al 2019.6 He wanted to opt for a non-surgical and minimally invasive therapeutic approach, hence injecting Botox into the masseter muscle was planned to correct the hypertrophied muscle. Ethical approval was obtained from the Institution Ethical Committee (IHEC/SDC/UG-1891/22/OMFS/563) of Saveetha University. Written informed consent for publication of the details and images was obtained from the patient.
Conventional techniques involve injecting BOTOX into the most prominent bulge of the masseter. Various injection techniques have been described in the previous literature. This study involves injecting botulinum toxin into 6 points (anterosuperior, anteroinferior, posterosuperior, posteroinferior, mid-superior and mid-inferior), 5 units at each point on the right and 6 units at each point on the left, since more bulk was observed on the left side. The 6-point technique is aimed at effective and equal distribution of Botox over the entire width of the muscle. After this research, we expect that we would be able to set a standard technique to be followed for injection of Botox into the masseter.
Standard dilution ratio used was 2.5 mL of normal saline into 100 units of botulinum toxin. Before starting the procedure, topical anesthesia was given bilaterally at the mandibular angle area. The patient was then asked to clench and hold his teeth to mark the anterior border and most prominent bulge of the masseter. A line was drawn from the tragus to the corner of the mouth indicating the superior boundary and the inferior boundary was marked with the aim of restricting the injection site 1.5–2 cm away from the lower border of the mandible to avoid injury to the marginal mandibular nerve. Six equidistant points (anterosuperior, anteroinferior, posterosuperior, posteroinferior, mid-superior and mid-inferior) were marked (Figure 1). Botox was injected perpendicularly with a 1-cc syringe into the bulk of the muscle. This was repeated for 6 points and further on the other side. Each point was injected with 5 units and 6 units on the right and left side, respectively, a total of 30 units and 36 units, respectively, at each muscle. Ice pack is placed post-operatively to control bleeding.
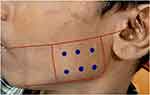 |
Figure 1 Preoperative marking of the 6 injection points. |
Standardized photography was performed to assess facial contour and masseter muscle thickness and the distance from the angle of the mandible to the most prominent point of the chin (pogonion) was compared at baseline and successive follow ups.
Results
Significant reduction in the distance from the angle of the mandible to the most prominent point of the chin (pogonion) was observed between pre-treatment (15.8 cm on right side and 17 cm on the left side) and 6 monthsfollow up (13.3 cm on the right side and 13 cm on the left side) (Figure 2).
 |
Figure 2 (A) Pre-treatment frontal view of the patient with bilateral masseter hypertrophy. (B) Post-treatment frontal view at 6-month follow up. |
Upon palpation, the thickness of muscle was significantly reduced and extraoral photography confirmed the change in facial contour and reduction in prominence at the angle of the mandible.
Discussion
Several techniques of injecting botulinum toxin-A into masseter muscle have been employed in the past. Some authors advocate multi-point injection technique since it allows a better distribution of the toxin in the muscle, while some advocate the use of the lowest injection sites possible or a single injection site. There is high heterogeneity in the injection protocol followed by previous literature as there is no standard protocol implemented for injection technique.7
The most important concept while injecting the masseter with botulinum toxin is to restrict the injection site within the boundaries of the muscle to avoid complications. Kwon et al, in their study, injected the toxin into the most prominent bulge of masseter.2 Kim et al made four injection points, 2 marked on a line from tragus to corner of mouth 1 cm apart and the other two given 1 cm above and below this reference line.8 Lindern et al injected at two sites, one at angle of mandible and the other one in the zygomatic arch.9 Bae et al employed a 3-point injection technique, one point inferior to the tragus–mouth line at the thickest point of muscle and two points 1 cm away from the anterior and posterior borders of the masseter, respectively. This helps to reduce the probability of damaging the risorius muscle and parotid gland.10 Mandel et al employed a 5-point injection technique involving the most bulging points.11 A more recent technique uses ultrasound-guided injections at 2–4 points, 1.5 cm apart within the borders of the muscle and tragus–mouth line.12 Advanced diagnostic aids, including CT,8 MRI, panoramic radiography,9 3D volumetry, serial ultrasound, electromyography,13 etc, can be performed periodically to assess the effect of botulinum toxin on masseteric hypertrophy in successive visits. Most of the studies mentioned above have evaluated the effectiveness of their technique by visual examination and few have employed advanced diagnostic aids like periodic electromyography, serial ultrasound, MRI, etc. However, the exposure of the patient to radiation is an added risk in these techniques. Xie et al, in their study, classified masseter muscle hypertrophy using a combination of clinical palpation, ultrasound and anatomical dissection and proposed a tailored botulinum toxin type A injection protocol for dosage and injection sites. Five different bulging types of the contracted masseter – minimal, mono, double, triple, and excessive – were classified and treated with different injection protocols and produced satisfactory results.14 Shome et al, in their study, compared the long-term effectiveness of botulinum toxin across groups that received subsequent injections and found that the patients who received three injections exhibited very high reduction of masseter muscle volume, around 38.72% until second follow up year, which implies that subsequent injections in maintenance phase yield higher results than the groups with lesser subsequent injections.15 In our case report, 15–25% reduction was seen in a follow-up period of 6 months, with a single injection. The patient will be followed up for subsequent injections for long-term management.
This case report proposes a new method to quantify prognosis by measuring important anatomical landmarks; ie, the distance from the angle of the mandible to the most prominent point of the chin (pogonion) was compared in subsequent visits to evaluate the effectiveness of botulinum toxin on masseter hypertrophy. We claim that our technique is the simplest, fastest, safest and most economical, with minimal discomfort to the patient, and the injection technique showed excellent results and patient satisfaction over a follow-up period of 6 months.
Conclusion
Botulinum toxin-A injection is a non-invasive, safe, and effective treatment for masseter muscle hypertrophy. The 6-point injection technique was found to be effective, patient satisfactory, with a good outcome over a 6-month follow-up period and we recommend its use for effective and uniform distribution of Botox into the masseter muscle. The clinical method for evaluating muscle bulk is an economical alternative to radiographic techniques.
Limitations and Future Scope
This injection technique has to implemented with more cases to prove its efficacy and safety. Comparison with other injection techniques must be done in the future to determine its superiority over conventional techniques and derive a standard protocol for injection of botulinum toxin in masseter muscle hypertrophy.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cheng J, Hsu SH, McGee JS. Botulinum toxin injections for masseter reduction in East Asians. Dermatol Surg. 2019;45:566–572. doi:10.1097/DSS.0000000000001859
2. Kwon K-H, Shin KS, Yeon SH, et al. Application of botulinum toxin in maxillofacial field: part I. Bruxism and square jaw. Maxillofac Plast Reconstr Surg. 2019;41(1):38. doi:10.1186/s40902-019-0218-0
3. Simpson LL. Identification of the characteristics that underlie botulinum toxin potency: implications for designing novel drugs. Biochimie. 2000;82:943–953. doi:10.1016/S0300-9084(00)01169-X
4. Freund B, Schwartz M, Symington JM. The use of botulinum toxin for the treatment of temporomandibular disorders: preliminary findings. J Oral Maxillofac Surg. 1999;57:
5. Yeh Y-T, Peng J-H, Peng H-LP. Literature review of the adverse events associated with botulinum toxin injection for the masseter muscle hypertrophy. J Cosmet Dermatol. 2018;17:675–687. doi:10.1111/jocd.12721
6. Kwon K-H, Shin KS, Yeon SH, et al. Application of botulinum toxin in maxillofacial field: part III. Ancillary treatment for maxillofacial surgery and summary. Maxillofac Plast Reconstr Surg. 2019;41(1):45. doi:10.1186/s40902-019-0226-0
7. Rauso R, Lo Giudice G, Tartaro G, et al. Botulinum toxin type A injections for masticatory muscles hypertrophy: a systematic review. J Craniomaxillofac Surg. 2022;50(1):7–18. doi:10.1016/j.jcms.2021.09.019
8. Kim HJ, Yum K-W, Lee S-S, et al. Effects of botulinum toxin type A on bilateral masseteric hypertrophy evaluated with computed tomographic measurement. Dermatol Surg. 2003;29:484–489. doi:10.1046/j.1524-4725.2003.29117.x
9. von Lindern JJ, Niederhagen B, Appel T, et al. Type A botulinum toxin for the treatment of hypertrophy of the masseter and temporal muscles: an alternative treatment. Plast Reconstr Surg. 2001;107:327–332. doi:10.1097/00006534-200102000-00004
10. Bae J-H, Choi D-Y, Lee J-G, et al. The risorius muscle: anatomic considerations with reference to botulinum neurotoxin injection for masseteric hypertrophy. Dermatol Surg. 2014;40(12):1334–1339. doi:10.1097/DSS.0000000000000223
11. Mandel L, Tharakan M. Treatment of unilateral masseteric hypertrophy with botulinum toxin: case report. J Oral Maxillofac Surg. 1999;57:1017–1019. doi:10.1016/S0278-2391(99)90029-0
12. Quezada-Gaon N, Wortsman X, Peñaloza O, et al. Comparison of clinical marking and ultrasound-guided injection of Botulinum type A toxin into the masseter muscles for treating bruxism and its cosmetic effects. J Cosmet Dermatol. 2016;15(3):238–244. doi:10.1111/jocd.12208
13. To EW, Ahuja AT, Ho WS, et al. A prospective study of the effect of botulinum toxin A on masseteric muscle hypertrophy with ultrasonographic and electromyographic measurement. Br J Plast Surg. 2001;54:197–200. doi:10.1054/bjps.2000.3526
14. Xie Y, Zhou J, Li H, et al. Classification of masseter hypertrophy for tailored botulinum toxin type A treatment. Plast Reconstr Surg. 2014;134(2):209e–218e. doi:10.1097/PRS.0000000000000371
15. Shome D, Vadera S, Shiva Ram M, et al. Efficacy of Incobotulinum toxin-A for the treatment of masseter muscle hypertrophy in Asian Indian patients: a 2-year follow-up study. J Cosmet Dermatol. 2020;19(8):1892–1899. doi:10.1111/jocd.13552
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
