Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
Bone Autophagy: A Potential Way of Exercise-Mediated Meg3/P62/Runx2 Pathway to Regulate Bone Formation in T2DM Mice
Authors Chen X, Yang K , Jin X, Meng Z, Liu B, Yu H, Lu P , Wang K, Fan Z, Tang Z, Zhang F, Liu C
Received 30 December 2020
Accepted for publication 29 April 2021
Published 17 June 2021 Volume 2021:14 Pages 2753—2764
DOI https://doi.org/10.2147/DMSO.S299744
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Xianghe Chen,1,* Kang Yang,2,* Xing Jin,2 Zhaoxiang Meng,2 Bo Liu,1 Huilin Yu,1 Pengcheng Lu,1 Kui Wang,2 Zhangling Fan,2 Ziang Tang,2 Feng Zhang,2 Chengye Liu2
1College of Physical Education, Yangzhou University, Yangzhou City, Jiangsu Province, People’s Republic of China; 2Rehabilitation Medicine Department, Northern Jiangsu People’s Hospital Affiliated to Yangzhou University, Yangzhou City, Jiangsu Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xing Jin; Zhaoxiang Meng
Rehabilitation Medicine Department, Northern Jiangsu People’s Hospital Affiliated to Yangzhou University, Yangzhou City, Jiangsu Province, People’s Republic of China
Email [email protected]
Zhaoxiang Meng Email [email protected]
Background: Meg3 has been shown to attenuate T2DM bone autophagy by activating p62 to inhibit bone formation. However, whether exercise can reverse this process to promote T2DM bone formation and its mechanism remains unknown.
Methods: A T2DM mouse model was established by a high-fat diet and STZ injection, and the mice were trained with 8-week HIIT and downhill running exercise. Micro-CT was used to scan the bone microstructure. Bone morphology was observed by HE staining, and the osteoblast (OB) activity in bones was observed by AKP staining. Calcium ion and phosphorus concentration in serum was detected by ELISA; RT-PCR was used to detect the mRNA level, and Western blot was used to detect the protein level of related indexes in Meg3/p62/Runx2 pathway.
Results: The inhibition of bone autophagy, in the bones of T2DM mice, resulted in the degradation of the bone tissue morphology and structure, with the increase of the expressions of Meg3, PI3K, Akt, mTOR, p62 and NF-κB. However, 8-week HIIT and downhill running could reverse this process, especially downhill running, manifested with the up-regulation of miR-16 mRNA level, along with Beclin-1, LC3 II and Runx2 mRNA and protein level.
Conclusion: T2DM leads to pathology in model mice. Eight-week HIIT and downhill running exercise can inhibit Meg3, activate autophagy of osteoblasts and promote bone formation in T2DM mice.
Keywords: Meg3, type 2 diabetes, autophagy of bone, exercise, p62, bone formation
Introduction
As a secondary systemic disease caused by metabolic disorders of energy, type 2 diabetes mellitus (T2DM) is generally accompanied by various symptoms, such as bone loss and enhanced bone fragility.1 In the course of this, pancreatic β cells have to over-secrete insulin to cope with excessive sugar in the body. However, this long-term situation causes damage to the pancreatic β cells, leading to a decrease in the function and dosage of insulin secretion. At the same time, the body gradually adjusts to the high-insulin environment, which forms insulin resistance (IR).2 Osteoblasts are newly confirmed insulin action sites, and their IR leads to IR affecting the whole body. Insulin resistance and secretion defects inhibit osteoblast-led bone formation and accelerate the process of bone resorption led by osteoclasts, which breaks the dynamic balance of bone metabolism, leading to T2DM osteoporosis (OP).3–5
 |
Figure 1 Experimental procedure. N, normal control group;, T, T2DM control group;, H, T2DM+HIIT group;, D, T2DM+downhill running group. |
In the field of sports medicine, exercise has slightly been the preferred treatment method for improving diabetes and osteoporosis due to its economic characteristics, high efficiency and low side effects.6,7 Exercise not only consumes excessive energy in the body to improve energy metabolism disorders, but also directly affects hormone secretion and bone cell-related signal pathways to regulate bone formation through muscle contraction generated by mechanical external force and ground counter-impact force.8,9 A large number of studies in T2DM models have previously confirmed that exercise, such as swimming, running and high-intensity intermittent training (HIIT), during the entire range of T2DM, can significantly promote bone formation.10–12 Additionally, weight-bearing exercise can promote bone formation through the Wnt signaling pathway.13 Half-marathon running upregulates the expression of miRNA-21-5p (miR-21-5p) and miR-378-5p which promote osteogenic differentiation, along with the increase of protein kinase B(Akt)/pAkt, recombinant mothers against decapentaplegic homolog 4 (Smad4) and runt-related transcription factor 2 (Runx2).14 However, most previous studies have focused on how exercise regulates bone metabolism, whereas bone autophagy, an important part of the bone formation process, has not been paid sufficient attention.14 The effects of exercise on regulating bone autophagy remain unclear.
The process of bone autophagy includes three steps: autophagy induction, autophagosome formation and maturation degradation.15,16 Autophagy will maintain a basic level under normal physiological conditions, and is capable of being initiated in the case of exercise stimulation, inflammation and oxidative stress.17,18 Interestingly, in this process, the total amount of p62 is negatively correlated with autophagy activity because p62, a key autophagy factor, is selectively degraded by autophagy lysosomes.19,20 Recent studies have confirmed that bone autophagy is decreased in the process of T2DM osteoporosis.21 Transmission electron microscope observation of bone marrow mesenchymal stem cells (BMSCs) and osteoblasts (OBs) in a high-glucose environment showed that autophagosomes in bone tissues were reduced, the expression of Beclin1 protein decreased, the expression of p62 autophagy marker protein increased and autophagy levels decreased in the high-glucose group, compared with those in the normal control group.22 This indicates that the high-glucose environment will reduce the level of bone autophagy, that is, T2DM can decrease the level of bone autophagy in the body, weaken bone formation and cause OP.
Long non-coding RNA Meg3 (Meg3) has been a research hot spot in the field of biology and medicine in recent years, and much has been reported about its effects on regulating bone metabolism through interaction with downstream microRNA16 (miR-16).23 Studies have shown that Meg3 can act as a competing endogenous RNA (ceRNA) to negatively regulate its downstream target gene miR-16, and then initiate the process of bone autophagy dominated by p62 to affect bone metabolism through phosphatase inositol 3ʹ-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR).21 So, does the expression of Meg3, miR-16, PI3K, Akt, mTOR, p62 and Runx2 change during the process of T2DM? What role does exercise play in this process? These questions are still waiting to be resolved by research.
In the present study, we used the high-fat-diet (HFD) and STZ-induced T2DM model with downhill running and HIIT to evaluate the effects of exercise, both downhill running and HIIT, on Meg3/p62/Runx2 signaling path in the bones of T2DM mice. We were expecting to find a better exercise method to promote bone recovery in T2DM mice, and hoping this study can provide theoretical guidance for the treatment and prevention of T2DM OP.
Materials and Methods
Animals and Experimental Design
Fifty-two 4-week-old male C57BL/6 male mice (Code: SCXK: Su 2017–0007) were purchased from Yangzhou University (Yangzhou, China). All mice were provided with a normal chow diet (casein 19.0%, L-cystine 0.2%, corn starch 47.9%, maltodextrin 11.8%, sucrose 6.5%, cellulose 4.7%, soybean oil 2.4%, lard 1.9%, mineral mix 0.95%, dicalcium phosphate 1.2%, calcium carbonate 0.5%, potassium citrate 1.6%, vitamin mix 0.95%, choline bitartrate 0.2%; purchased from Jiangsuxietong, Nanjing, Jiangsu, China) or a high-fat diet (HFD; casein 25.8%, L-cystine 0.4%, sucrose 9%, maltodextrin 16.3%, cellulose 6.5%, soybean oil 3.4%, lard 31.7%, mineral mixture 1.6%, dicalcium phosphate 1.4%, calcium carbonate 0.8%, potassium citrate 2.3%, vitamin mixture 1.3%, choline bitartrate 0.3%; purchased from Jiangsuxietong, Nanjing) for 18 weeks. At the 9th week, three doses of 40 mg/kg streptozotocin (STZ) (48 h/dose, purchased from Sigma, St. Louis, Missouri, USA) was dissolved in 10 mmol/L citrate buffer (pH 4.5) and injected intraperitoneally into HFD-fed mice. Random glucose levels in tail veins of mice were continuously measured. A blood glucose level of ≥16.7 mmol/L was considered as a successful T2DM model. A blood glucose level of ≤6.8 mmol/L was considered as normal. All mice were housed individually in standard plastic cages under conventional laboratory conditions (23±2°C, 55±5% humidity), with ad libitum access to food and water, and maintained on a standard 1:1 day/night cycle. Mice were randomly assigned to four groups: normal control group (N, n=13); T2DM control group (T, n=13); T2DM+HIIT group (H, n=13); T2DM+downhill running group (D, n=13). All experiments were approved by the Animal Ethics Committee of Yangzhou University (approval number: YZU-TYXY-0031). The experiments were in adherence to the ARRIVE guidelines for experiments on animals. The experimental procedures were in agreement with the “Regulations for the Administration of Affairs Concerning Experimental Animals“ China, for welfare of experimental animals issued by the government of the People's Republic of China under “Laws of the People’s Republic of China”.
Exercise Protocol
HIIT and downhill running were adapted from previous research.24,25 The specific protocol is slightly modified according to the actual situation. Group H participated in 8 weeks' HIIT, of which the speed is 85%Smax for 2 min and 45%Smax for 3 min, in a 6-round cycle, with warm-up and relax at 40%Smax before and after exercise for 5 min. The slope of Group H is 0°. Group D participated in 8 weeks' downhill running, of which the speed is 60%Smax for 30 min, with warm-up and relax at 40%Smax before and after exercise for 5 min. The slope of Group D is −10°. In addition, adaptive training (13.3 m/min, 15 min/d) was performed on both Group H and Group D before the formal training program for 3 days. No electrical stimulation was used in the exercise program. Smax is the average of the maximum speeds of all tested mice. The Smax was retested every two weeks and the speed of exercise adjusted based on the new Smax. The Smax test method is as follows: the initial speed was 8 m/min, increased by 1 m/min every 2 min until exhaustion (the mice were whipped to run; the running speed would be considered as the maximum speed if the mice cannot run at a certain speed with the time exceeding 10 s).
Tissue Preparation
The specimens were collected 12 h after the last exercise training (fasting for 8 h before collection). Firstly, the mice were anesthetized with intraperitoneal injection of 4% chloral hydrate at a dose of 0.1 mL/10 g before eyeballs were removed and blood collected. Then, the blood was stored in a 1.5 mL enzyme-free EP tube at 4°C overnight before centrifuging at 4000 rpm×10 min, at 4°C. The upper serum was extracted and placed in the refrigerator at −80°C for ELISA-related indexes determination; left and right hind limbs of the mice were selected and the attached soft tissue removed for detecting the bone tissue morphometric indexes by micro-CT. The right femur was removed and decalcified with 10% EDTA, then paraffin-embedded and HE stained; BMSCs were extracted from the left tibia for primary culture and differentiation to OB induced for later alkaline phosphatase (AKP) staining; the left femur was prepared for later RT-PCR detection and Western blotting detection.
Micro-CT
The bone of the right right femur of mice was fixed with 4% paraformaldehyde for 24 hours, and its connective tissue was removed and stored in 75% alcohol for later detection. The micro-CT supporting Skyscan system was used to calculate the morphometric indexes of femoral cancellous bone and cortical bone of mice in each group at the size of 18 μm/frame. Finally, three-dimensional structure diagrams, relevant data of tissue morphometric index of cancellous bone and cortical bone were obtained.
Hematoxylin-Eosin (HE) Staining
The bone of right hind limb of the mouse was cleaned by 0.01 M PBS, fixed with 4% paraformaldehyde for 24–36 hours, then cleaned again with 0.01 M PBS, and then the bone tissue was decalcified with 10% EDTA solution for 25 days, and the fluid was changed once every 5 days (until the bone tissue softened). After decalcification, the bone tissue was stained, dehydrated, replaced and sealed with neutral resin according to the standard procedure of HE staining. Sequential pictures were taken with a microscope.
Phosphatase (AKP) Staining
The ends of the femur of each group of mice were removed by ophthalmic scissors, and the bone marrow was blown out of the bone marrow cavity with a 1 mL syringe, and the blood cell count plate was used to count. According to the standard of 50 µL/well level, the bone marrow was evenly placed in a 24-well plate, and the BMSCs primary culture was carried out in the petri dish (37°C, 5% CO2). The liquid was changed once every two days. After 6 days, vitamin C (1000×) and β-glycerophosphoric acid (100×) were added to induce BMSCs to differentiate into OB, and the liquid was changed once every 2 days. After 13 days, the medium was aspirated, fixed with 4% PFA for 10 minutes and rinsed with PBS twice.
To prepare AKP dye solution, 12 mg fast red violet LB salt and 4 mg AS-MAX were added to 10 mL Tris-HCl (pH=8.3) and 10 mL ddH2O . Then the differentiated OB was stained with AKP staining solution.26
ELISA
According to the requirements of the enzyme-linked immunosorbent assay kit, Ca2+ ELISA Kit (Nanjing Jiancheng C004-2-1), P5+ELISA Kit (Nanjing Jiancheng C006-1), insulin (Beijing Solarbio SEKM0141) and AKP ELISA Kit (Nanjing Jiancheng A059-2) were used to assess the relative level in serum of mice.
Real-Time PCR
According to the reagent manual, mRNA was extracted from the right femur by Trizol (TianGen, Beijing, China), and the purity and concentration of mRNA were detected by ultraviolet spectrophotometer. And then, the reverse kit PrimeScriptTMRT reagent Kit was used with gDNA Eraser (Takara, Shiga, Japan) to convert mRNA to cDNA. For Meg3 and miR-16 we used LnRcute LncRNA First-Stand cDNA Synthesis Kit (TianGen, Beijing, China) and miRcute Plus miRNA First-Stand cDNA Kit (TianGen, Beijing, China), respectively. PCR amplification was performed with TB Green® Premix Ex TaqTMII (Takara, Shiga, Japan). For Meg3 and miR-16 we used LnRcute LncRNA qPCR Detection Kit (TianGen, Beijing, China) and miRcute Plus miRNA qPCR Kit (TianGen, Beijing, China), respectively. After amplification, β-actin was used as the internal reference. The related primers were designed by Primer Premier primer design software and synthesized by Sangon Biotech Co., Ltd (Shanghai, China) We used the method of 2−ΔΔCt to calculate the expression of each gene’s mRNA. The primer sequences are shown in Table 1.
 |
Table 1 List of Primer Sequences |
Western Blotting
After the soft tissue on the tibia of mice had been removed, tibias were cut by ophthalmic scissors and 80g weighed by electronic scale. The tibia of each sample was placed in the grinding tube, to which had been added grinding beads and lysis buffer, and placed in the tissue homogenizer for grinding. The supernatant and was gotten for examining concentration of total protein by bicinchoninic acid method(BCA). Polyacrylamide gel electrophoresis was performed to separate the target proteins from the samples with the equal amount of total protein. Proteins were transferred to PVDF membrane (Invitrogen) and closed non-specific binding with 5% skimmed milk. The protein was added with primal antibodies of anti-PI3K (1:1000, Abcam), anti-Akt (1:1000, Abcam), anti-pAkt (1:1000, Abcam), anti-mTOR (1:1000, Sigma), anti-Beclin-1 (1:1000, Proteintech), anti-LC3 II (1:1000, Proteintech), anti-p62 (1:1000, Proteintech), anti-NF-κB (1:1000, Proteintech), anti-Runx2 (1:1000, Sigma), anti-beta actin (1:1000, Abcam) and secondary antibodies (Abcam) in turn, and then washed with tris-buffered saline Tween (TBST). The PVDF film was developed and photographed by Alpha gel imaging system. Beta-actin was used as an internal reference, and the data were analyzed by self-contained software.
Statistical Analysis
Excel was utilized to organize data. SPSS 20.0 and GraphpadPrism 5.0, respectively, were used for statistics and image generation. Data were expressed as Mean ± SD. Comparisons between groups were analyzed by one-way ANOVA analysis of variance. Significance was set at P<0.05.
Results
HIIT and Downhill Running Alleviate the T2DM Pathology
As shown in Table 2, the body weight, fasting blood glucose level and fasting blood insulin level of mice in each group at the 19th week were recorded. The T2DM model was established by a high-fat diet combined with intraperitoneal STZ injection. Compared with the N group, T group manifested significantly lower body weight (P<0.01), lower fasting blood insulin level (P<0.01) and higher fasting blood glucose (P<0.01), indicating that the T2DM mouse model induced by HFD and STZ was successful.27
 |
Table 2 Animal Characteristics |
However, compared to T group, both H group and D group all exhibited higher body weight (P<0.05), lower fasting blood glucose (P<0.05) and higher fasting blood insulin level (P<0.01), significantly, suggesting that 8-week HIIT and downhill running alleviated the T2DM pathology.
HIIT and Downhill Running Ameliorate T2DM Bone Morphology
As shown in (Figure 1, Figure 2) in cancellous bone, we found that the density of cancellous bone trabecular in T group was significantly lower than that in N group after paraffin-embedded sections of the femurs of mice in each group were stained with HE staining. Compared with T group, the density of cancellous bone trabecular increased significantly in H group and D group, especially in the downhill running group.
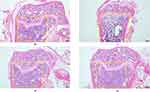 |
Figure 2 HE staining of femur. |
HIIT and Downhill Running Improve Cancellous Bone Microstructure in T2DM Model Mice
Three-dimensional structure is strong evidence reflecting the bone microstructure.28 To further explore the impacts of T2DM on bone tissue, we scanned femurs of each group and found that, as shown in Figure 3 in cancellous bone, the bone mineral density (BMD, P<0.01), bone volume fraction (BV/TV, P<0.01), trabecular number (Tb.N, P<0.01) and trabecular thickness (Tb.Th, P<0.05) in T group were significantly lower than those in N group, while the trabecular separation (Tb.Sp, P<0.01) was considerably higher than that in N group, indicating that T2DM damaged the cancellous bone microstructure in mice.
Compared with T group, H group and D group exhibited significantly higher BMD (P<0.05), higher BV/TV (P<0.05), higher Tb.N (P<0.05) and lower Tb.Sp (P<0.05), while, additionally, D group manifested higher Tb.Th (P<0.05), suggesting 8-week HIIT and downhill running reversed the negative effects of T2DM on cancellous bone microstructure, especially downhill running. What is worthy of note is that, compared to H group, D group exhibited higher BMD (P<0.05), higher BV/TV (P<0.05) and higher Tb.Th (P<0.05), which also supports the first sentence.
Effects of HIIT and Downhill Running on AKP Activity, Ca2+ and P5+ in Serum
Literature has confirmed that, as osteogenesis marker, alkaline phosphatase (AKP) in serum is closely related to bone formation while a high concentration of Ca2+ and P5+ (essential ions for bone formation) in serum predicts a decline in osteogenesis. As shown in Figure 4, compared with N group, T group exhibited significantly lower AKP activity (P<0.01), with the concentration of Ca2+ (P<0.05) and P5+ (P<0.05) increased.
Compared to T group, after 8-week HIIT and downhill running, in serum, H group and D group both manifested significantly higher AKP activity (P<0.05), lower concentration of Ca2+(P<0.05) and lower concentration of P5+(P<0.05). In addition, AKP activity in D group was significantly higher than that in H group.
Effect of HIIT and Downhill Running on Relative mRNA Expression in Meg3/P62/Runx2 Pathway in T2DM Mice
As shown in Figure 5, compared to N group, the mRNA expression of Meg3 (P<0.01), PI3K (P<0.05), Akt (P<0.05), mTOR (P<0.05), p62 (P<0.01) and NF-κB (P<0.05) in bone of T group increased significantly, while miR-16 (P<0.05), Beclin-1 (P<0.01), LC3 II (P<0.01) and Runx2 (P<0.01) decreased significantly.
Compared with T group, in both H group and D group, the expressions of Meg3 (P<0.05), Akt (P<0.05), mTOR (P<0.05), p62 (P<0.05) and NF-κB (P<0.05) decreased significantly, with a significant increase of miR-16 (P<0.05), Beclin-1 (P<0.05), LC3 II (P<0.05) and Runx2 (P<0.05). Additionally, the mRNA expression of PI3K (P<0.05) in D group also decreased significantly. What is worth mentioning is that, compared to H group, the expressions of Meg3 (P<0.05) and PI3K (P<0.05) decreased significantly, along with a significant increase of LC3 II (P<0.05) and Runx2 (P<0.05).
Effect of HIIT and Downhill Running on Relative Protein Expression in Meg3/P62/Runx2 Pathway in T2DM Mice
As shown in Figure 6, compared to N group, the protein expression of PI3K (P<0.01), p-Akt (P<0.05), mTOR (P<0.01), p62 (P<0.01) and NF-κB (P<0.05) in T group was significantly up-regulated, while the protein expression of Beclin-1 (P<0.01), LC3 II (P<0.01) and Runx2 (P<0.01) was significantly decreased, suggesting that T2DM inhibited bone autophagy via Meg3/p62/Runx2 pathway to inhibit bone formation, which led to T2DM OP.
Compared to T group, the protein expression in both H group and D group decreased significantly, such as PI3K (P<0.05) and p62 (P<0.05), while Beclin-1 (P<0.05), LC3 II (P<0.05) and Runx2 (P<0.05) increased significantly. In addition, p-Akt (P<0.05), mTOR (P<0.05) and NF-κB (P<0.05) in D group were significantly decreased. Compared to H group, D group exhibited lower PI3K (P<0.05), lower p62 (P<0.05), lower NF-κB (P<0.05) and higher Beclin-1 (P<0.05), higher LC3 II (P<0.05) and higher Runx2 (P<0.05), indicating that both HIIT and downhill running can reverse the negative influence of T2DM OP to a certain extent, especially downhill running.
Effect of HIIT and Downhill Running on OB Differentiation in T2DM Model Mice
The BMSCs of mice in each group were primary cultured and induced to differentiate into OB by vitamin C and β-glycerophosphate, and the OB staining was performed by AKP. As shown in Figure 7, the result of AKP staining in the T group significantly showed lower activity than that in N group, indicating a significant decrease in the activity of OB produced by differentiation in T group. Compared with T group, the results of AKP staining in H group and D group showed significantly increased activity, and the increase in D group was more significant.
 |
Figure 7 Changes of OB activity produced by differentiation in each group. |
Discussion
In the present study, we examined the effects of long-term (8-week) HIIT and downhill running on promoting bone autophagy to improve the ability of bone formation in HFD and STZ-\induced T2DM model mice. And we found that 8-week exercise like HIIT and downhill running mitigated T2DM OP in T2DM model mice. Exercise-induced improvement in these effects was associated with inhibited Meg3/p62/Runx2 signaling pathway in bone tissue of T2DM model mice. In this process, the up-regulation of p62, a marker gene of bone autophagy, whose high expression predicts low level of bone autophagy, plays an important role. This is a point we will discuss later.
In this study, we found that peripheral blood glucose level was increased, peripheral insulin level and weight were reduced in the HFD and STZ-induced T2DM model mice, while 8-week HIIT and downhill running exercise significantly improved the insulin level, blood glucose level and weight of T2DM mice. And these outcomes are consistent with previous studies.29 Additionally, these results were also reflected in the changes of AKP activity, Ca2+ and P5+ in serum and further confirmed that long-term HIIT and downhill running exercise could ameliorate the T2DM pathology in T2DM mice. T2DM inhibits bone formation ability of mice, resulting in an increase in free Ca2+ and P5+ in blood.
In addition, large numbers of studies have shown that the bone is another organ with damage induced by T2DM, characterized by osteoporosis.30 Walsh et al found that the occurrence of T2DM is accompanied by bone loss and increased bone fragility.31 In our study, we found that the bone microstructure of model mice is impaired, along with the decrease of BMD, BV/TV, Tb.N and Tb.Th. Long-term (8-week) HIIT and downhill running exercise could significantly improve the situation in T2DM mice, especially downhill running exercise. And these findings are consistent with previous studies.32 These results suggest that long-term HIIT and downhill running exercise could enhance both bone microstructure and morphology in T2DM mice.
Previous animal studies reported that autophagy is altered in bone tissue during the generation of T2DM, and this shift of the osteoblasts towards a non-mineralizing, osteocyte phenotype appears to be coordinated by Beclin1-mediated autophagy.33 It demonstrates a correlation between the impaired ability of bone formation and autophagy. Moreover, p62, a key gene of autophagy, was found up-regulated in the bone of T2DM model mice in our study. It is suggested that there is an inhibition of autophagy during the appearance of T2DM. In addition, in the current study, we found that, compared with normal control mice, levels of PI3K, Akt/p-Akt and mTOR in the bones of the T group were significantly increased. The current results further strengthen the observation that T2DM results in low autophagy level in bones. What is worth mentioning is that Akt does not change the level of protein expression, but is reflected in the level of phosphorylation.
Interestingly, we found that 8-week HIIT and downhill running could both inhibit p62 and activate autophagy to improve bone formation, especially downhill running exercise, which reduces Meg3, PI3K, Akt, mTOR, p62 and NF-κB mRNA levels, and increases secretion of miR-16, Beclin-1, LC3 II and Runx2. Our results are consistent with previous findings that exercise can improve OP due to T2DM.34 In addition, Pezhman et al found that swim training could partially compensate for T2DM-associated changes of bone.35 On the macro level, Aoi Ikedo et al found that a 6-week resistance training regimen effectively increased BMD and improved bone quality in T2DM model rats, which is also consistent with our study.
Another interesting question regarding the current study is why the effect of downhill running exercise seems better than HIIT exercise on improving bone formation in T2DM model mice. Viggers et al found that exercise differs in type, mechanical load, and intensity, as does the osteogenic response to exercise. A combination of weight-bearing aerobic and resistance exercise may be preventive against excessive bone loss with T2DM.36 As a sensitive mechanical organ, bone can transform the mechanical stress stimulation caused by downhill running and HIIT exercise into the regulation of cytokines related to bone metabolism. As mentioned earlier, exercise can down-regulate the expression of Meg3, up-regulate its downstream miR-16, to inhibit PI3K/Akt/mTOR pathway, increase the bone autophagy initiator mTORC1 receptor, down-regulate the expression of p62, inhibit NF-kappa B and then increase Runx2 and activate OB autophagy. Downhill running and HIIT exercise are effective in improving bone histomorphology and increasing bone mass in T2DM, and the effect of the former is better than that of the latter. The reason for this result may be related to the mode of exercise. HIIT improves the function of heart, lung and other organs in mice, while downhill running has more mechanical stress stimulation and thus the treadmill produces greater effect on the mice.
In conclusion, bone autophagy was seen to exist in T2DM OP. The work also confirms for the first time that HIIT and downhill running exercise are beneficial via down-regulation of the Meg3 gene and promoting miR-16 mRNA level to inhibit PI3K/Akt/mTOR pathway, activating p62-mediated autophagy to promote Runx2 and bone formation. There are also many limitations in our study, one of which is that we failed to observe the presence of autophagosomes in bone tissue with transchromatic electron microscope. This means that our work lacks a certain degree of persuasiveness in the macroscopic aspect when we study the situation of bone tissue autophagy. Our research team will work to resolve this point from cell experiments in the future.
Conclusion
We examined the protective role of 8-week HIIT and downhill running exercise in a high-fat diet and STZ-induced T2DM OP. We provided evidence that T2DM-induced OP was associated with increased Meg3 activity, and aerobic exercise attenuates these effects, which may be influenced or partially mediated by down-regulating the Meg3/miR-16/PI3K/Akt/mTOR/p62/NF-κB/Runx2 pathway.
Abbreviations
Akt, protein kinase B; AKP, alkaline phosphatase; BMD, bone mineral density; BMSCs, bone marrow mesenchymal stem cells; BV/TV, bone volume fraction; ceRNA, competing endogenous RNA; DMR, differentially methylated region; HE, hematoxylin–eosin; HIIT, high-intensity intermittent training; Meg3, long non-coding RNA Meg3; micro-CT, microcomputed tomography; miR-16, microRNA16; mTOR, mammalian target of rapamycin; mTORC1, mammalian target of rapamycin complex 1; NF-κB, nuclear factor kappa-B; OB, osteoblast; OP, osteoporosis; PI3K, phosphatidyl inositol 3ʹ-kinase; STZ, streptozotocin; T2DM, type 2 diabetes mellitus; Tb.N, trabecular number; Tb.Sp, trabecular separation; TBST, tris buffered saline Tween; Tb.Th, trabecular thickness.
Data Sharing Statement
The data will be available after request to the university.
Funding
This work was supported by grants from the project supported by The China Postdoctoral Science Foundation (project number: 2019M661957).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Alharbi KK, Abudawood M, Khan IA. Amino-acid amendment of Arginine-325-Tryptophan in rs13266634 genetic polymorphism studies of the SLC30A8 gene with type 2 diabetes-mellitus patients featuring a positive family history in the Saudi population[J]. J King Saud Univ Sci. 2021;33(1):101258. doi:10.1016/j.jksus.2020.101258
2. Macdonald IA. A review of recent evidence relating to sugars, insulin resistance and diabetes[J]. Eur J Nutr. 2016;55(2):17–23. doi:10.1007/s00394-016-1340-8
3. Roberts FL, Rashdan NA, Phadwal K, et al. Osteoblast-specific deficiency of ectonucleotide pyrophosphatase or phosphodiesterase-1 engenders insulin resistance in high-fat diet fed mice[J]. J Cell Physiol. 2020;236(6):4614–4624. doi:10.1002/jcp.30194
4. Razny U, Polus A, Goralska J, et al. Effect of insulin resistance on whole blood mRNA and microRNA expression affecting bone turnover[J]. Eur J Endocrinol. 2019;181(5):525–537. doi:10.1530/EJE-19-0542
5. Parker L, Lin X, Garnham A, et al. Glucocorticoid-Induced Insulin Resistance in Men Is Associated With Suppressed Undercarboxylated Osteocalcin[J]. J Bone Miner Res. 2019;34(1):49–58. doi:10.1002/jbmr.3574
6. Balducci S, Sacchetti M, Haxhi J, et al. Physical exercise as therapy for type 2 diabetes mellitus[J]. Diabetes Metab Res Rev. 2014;1:13–23. doi:10.1002/dmrr.2514
7. Benedetti MG, Furlini G, Zati A, et al. The Effectiveness of Physical Exercise on Bone Density in Osteoporotic Patients[J]. Biomed Res Int. 2018;2018:4840531. doi:10.1155/2018/4840531
8. Pagnotti GM, Styner M, Uzer G, et al. Combating osteoporosis and obesity with exercise: leveraging cell mechanosensitivity[J]. Nat Rev Endocrinol. 2019;15(6):339–355.
9. Yuan Y, Chen X, Zhang L, et al. The roles of exercise in bone remodeling and in prevention and treatment of osteoporosis[J]. Prog Biophys Mol Biol. 2016;122(2):122–130. doi:10.1016/j.pbiomolbio.2015.11.005
10. Li L, He D, Jiang K, Zhao Y. Effects of forced swimming stress on expression and phosphorylation of PI3K/Akt signal pathway in pancreas of type 2 diabetic rats[J]. Ann Transl Med. 2020;8(16):1006. doi:10.21037/atm-20-5304
11. Minematsu A, Hanaoka T, Takeshita D, et al. Long-term wheel-running can prevent deterioration of bone properties in diabetes mellitus model rats[J]. J Musculoskelet Neuronal Interact. 2017;17(1):433–443.
12. Alvarez C, Ramirez-Campillo R, Martinez-Salazar C, et al. Low-Volume High-Intensity Interval Training as a Therapy for Type 2 Diabetes[J]. Int J Sports Med. 2016;37(9):723–729. doi:10.1055/s-0042-104935
13. Cheng L, Khalaf AT, Lin T, et al. Promotes the Osteoinduction of HA/β-TCP Biomaterials via the Wnt Signaling Pathway[J]. Metabolites. 2020;10(3):90. doi:10.3390/metabo10030090
14. Valenti MT, Deiana M, Cheri S, et al. Physical Exercise Modulates miR-21-5p, miR-129-5p, miR-378-5p, and miR-188-5p expression in progenitor cells promoting osteogenesis[J]. Cells. 2019;8(7):742. doi:10.3390/cells8070742
15. Ma Y, Qi M, An Y, et al. Autophagy controls mesenchymal stem cell properties and senescence during bone aging[J]. Aging Cell. 2018;17(1):e12709. doi:10.1111/acel.12709
16. Jaber FA, Khan NM, Ansari MY, et al. Autophagy plays an essential role in bone homeostasis[J]. J Cell Physiol. 2019;234(8):12105–12115. doi:10.1002/jcp.27071
17. Cinque L, Forrester A, Bartolomeo R, et al. FGF signalling regulates bone growth through autophagy[J]. Nature. 2015;528(7581):272–275. doi:10.1038/nature16063
18. Moscat J, Diaz-Meco MT. P62 at the crossroads of autophagy, apoptosis, and cancer[J]. Cell. 2009;137(6):1001–1004. doi:10.1016/j.cell.2009.05.023
19. Long M, Li X, Li L, et al. Multifunctional p62 Effects Underlie Diverse Metabolic Diseases[J]. Trends Endocrinol Metab. 2017;28(11):818–830. doi:10.1016/j.tem.2017.09.001
20. Zhang P, Zhang H, Lin J, et al. Insulin impedes osteogenesis of BMSCs by inhibiting autophagy and promoting premature senescence via the TGF-β1 pathway[J]. Aging. 2020;12(3):2084–2100. doi:10.18632/aging.102723
21. Bartolomé A, López-Herradón A, Portal-Núñez S. Autophagy impairment aggravates the inhibitory effects of high glucose on osteoblast viability and function [J]. Biochem J. 2013;455(3):329–337. doi:10.1042/BJ20130562
22. Xu J, Xu YZ. The LncRNA Meg3 downregulation leads to osteoarthritis progression via miR-16/SMAD7 axis[J]. Cell Biosci. 2017;7(8):7. doi:10.1186/s13578-017-0195-x
23. Pu Z, Wu L, Guo Y, et al. LncRNA Meg3 contributes to adenosine-induced cytotoxicity in hepatoma HepG2 cells by downregulated ILF3 and autophagy inhibition via regulation PI3K-AKT-mTOR and beclin-1 signaling pathway[J]. J Cell Biochem. 2019;120(10):18172–18185. doi:10.1002/jcb.29123
24. Wang N, Liu Y, Ma Y, et al. High-intensity interval versus moderate-intensity continuous training: superior metabolic benefits in diet-induced obesity mice[J]. Life Sci. 2017;191:122–131. doi:10.1016/j.lfs.2017.08.023
25. Ai L, Luo W, Yuan P, et al. Liver macrophages mediate effects of downhill running and caloric restriction on nonalcoholic fatty liver disease of high fat diet-fed mice[J]. Life Sci. 2020;256:117978. doi:10.1016/j.lfs.2020.117978
26. Chen XH, Sun P, Chen AG, et al. Effects of different exercises on the cAMP/CREB/Atf4 pathway and bone formation in the bones of type 2 diabetic mice[J]. Chine J Sports Med. 2017;36(11):977–983.
27. Zhang C, Deng J, Liu D, et al. Nuciferine ameliorates hepatic steatosis in high-fat diet/streptozocin-induced diabetic mice through a PPARα/PPARγ coactivator-1α pathway[J]. Br J Pharmacol. 2018;175(22):4218–4228. doi:10.1111/bph.14482
28. Bouxsein ML, Boyd SK, Christiansen BA, et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography[J]. J Bone Miner Res. 2010;25(7):1468–1486. doi:10.1002/jbmr.141
29. Li S, Huang Q, Zhang L, et al. Effect of CAPE-pNO2 against type 2 diabetes mellitus via the AMPK/GLUT4/GSK3β/PPARα pathway in HFD/STZ-induced diabetic mice[J]. Eur J Pharmacol. 2019;853:1–10. doi:10.1016/j.ejphar.2019.03.027
30. Napoli N, Chandran M, Pierroz DD, et al.; IOF Bone and Diabetes Working Group. Mechanisms of diabetes mellitus-induced bone fragility[J]. Nat Rev Endocrinol. 2017;13(4):208–219. doi:10.1038/nrendo.2016.153
31. Walsh JS, Vilaca T. Obesity, Type 2 Diabetes and Bone in Adults[J]. Calcif Tissue Int. 2017;100(5):528–535. doi:10.1007/s00223-016-0229-0
32. Viggers R, Al-Mashhadi Z, Fuglsang-Nielsen R, et al. The impact of exercise on bone health in type 2 diabetes mellitus-a systematic review[J]. Curr Osteoporos Rep. 2020;18(4):357–370. doi:10.1007/s11914-020-00597-0
33. Rendina-Ruedy E, Graef JL, Lightfoot SA, et al. Impaired glucose tolerance attenuates bone accrual by promoting the maturation of osteoblasts: role of Beclin1-mediated autophagy[J]. Bone Rep. 2016;5:199–207. doi:10.1016/j.bonr.2016.08.001
34. Ikedo A, Kido K, Ato S, et al. The effects of resistance training on bone mineral density and bone quality in type 2 diabetic rats[J]. Physiol Rep. 2019;7(6):e14046. doi:10.14814/phy2.14046
35. Pezhman L, Sheikhzadeh Hesari F, Ghiasi R, et al. The impact of forced swimming on expression of RANKL and OPG in a type 2 diabetes mellitus rat model[J]. Arch Physiol Biochem. 2019;125(3):195–200. doi:10.1080/13813455.2018.1446178
36. Pagnotti GM, Styner M, Uzer G, et al. Combating osteoporosis and obesity with exercise: leveraging cell mechanosensitivity. Nat Rev Endocrino. 2019;15(6):339–355.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




