Back to Journals » International Journal of Women's Health » Volume 16
Autologous Tumor-Infiltrating Lymphocyte Mono-Therapy Can Rapidly Shrank Tumor in Asian Patient with Stage III/IV Cervical Cancer: Two Cases Report
Authors Li F , Wang Y, Yan J, Wu H , Du X, Feng W, Zhang X, Xue Y, Wang H, Liu W
Received 26 October 2023
Accepted for publication 28 December 2023
Published 9 January 2024 Volume 2024:16 Pages 31—39
DOI https://doi.org/10.2147/IJWH.S446768
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Elie Al-Chaer
Fenge Li,1,2,* Yupeng Wang,1,* Jin Yan,1,* Huancheng Wu,3 Xueming Du,1 Weihong Feng,1 Xiaoqing Zhang,4 Yongming Xue,4 Huaqing Wang,5,6 Wenxin Liu7
1Department of Oncology, Tianjin Beichen Hospital Tianjin People’s Republic of China; 2Core Laboratory, Tianjin Beichen Hospital, Tianjin, People’s Republic of China; 3Department of Neurosurgery, Tianjin Beichen Hospital, Tianjin, People’s Republic of China; 4Department of Basic Research, Suzhou Lanma Biotechnology Co, Suzhou, People’s Republic of China; 5Department of Oncology, Tianjin Union Medical Center, Tianjin, People’s Republic of China; 6Department of Translational Medicine, Tianjin Union Medical Center of Nankai University, Tianjin, People’s Republic of China; 7Department of Gynecological Oncology, Tianjin Medical Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wenxin Liu, Department of Gynecological Oncology, Tianjin Medical Cancer Institute and Hospital, Huanhuxi Road, Tiyuanbei, Hexi District, Tianjin, People’s Republic of China, Tel +8618622221101, Email [email protected] Huaqing Wang, Department of Translational Medicine, Tianjin Union Medical Center of Nankai University, Jieyuan Road No. 190, Nankai District, Tianjin, People’s Republic of China, Tel +8618622221223, Email [email protected]
Introduction: Tumor-infiltrating lymphocytes (TILs) therapy is one of the most promising adoptive T cell therapies, which has shown great clinical efficacy against several solid malignancies. Nevertheless, clinical response to TILs mono-therapy in Asian patients with recurrent cervical cancer has not been well reported.
Case Presentation: Here, we report two patients who were diagnosed with metastatic cervical cancer and tumor progression following multiple conventional treatments. In particular, one of the patients has a history of severe myelosuppression after chemotherapy. The patients received lymphodepletion therapy, which consisted of cyclophosphamide (30mg/kg) for 2 days, followed by Fludarabine (25mg/m2) for 5 days, approximately 24 hr before receiving intravenous autologous TILs infusion. These two patients then received high doses of IL-2 for 10 days with the purpose of maintaining T cell survival and proliferation. Patient 1 experienced clinical partial response (PR) at 6 weeks post TILs infusion and a 33% tumor shrinkage at 12 weeks follow-up, and patient 2 was evaluated as stable disease (SD) at 6 weeks post treatment. Mild and manageable adverse events were observed and soon subsided after the TILs treatment. A time-course study examining the peripheral blood cell count and cytokine secretion demonstrated the persistence of infused TILs and long-term immune response.
Conclusion: These results suggest that TILs mono-therapy can be a promising treatment strategy for Asian patients with late-stage metastatic cervical cancer even with severe myelosuppression. TILs infusion can induce persistence and a long-term systematic immune response that reversed peripheral CD4+T and CD8+T percentages implying that TILs infusion increased cytotic T cell responses, which is consistent with clinical responses in these patients. Trial registration number: NCT05366478.
Keywords: tumor-infiltrating lymphocytes, clinical response, immune response, metastatic cervical cancer, treatment
Introduction
Adoptive T cell therapy has emerged as an area of extensive research and has revolutionized our approach to cancer treatments.1–3 Three major adoptive T cell therapies include tumor-infiltrating lymphocytes (TILs) therapy, Chimeric Antigen Receptor T-Cell (CAR-T) therapy, and T Cell Receptor-engineered T-Cell (TCR-T). In particular, TILs therapy differs from the other cell therapy modalities such that it leverages tumor-infiltrating lymphocytes isolated from solid tumor tissue, whereas most of the other cellular immunotherapies employ CAR-T or NK immune cells derived from peripheral blood. TILs therapy thus confers an absolute advantage as most of the TILs can recognize tumor cells, while only a few immune cells isolated from the peripheral blood can recognize tumors.4 Promising clinical responses to TILs therapy in treating metastatic melanoma, advanced non-small cell lung cancer (NSCLC), and cervical cancer in western countries have been reported with an overall response rate (ORR) of 34.3%, 23.1%, and 28%, respectively.5–7 Thus, TILs therapy shows great potential in improving the survival rate of a variety of metastatic cancers, which can reduce the probability of recurrence of patients.
Nevertheless, few preclinical and clinical studies of TILs therapy in Asian population exist. As a result, the safety, efficacy, and applicable population of TILs therapy in treating Asian patients with advanced malignant tumors have not been well examined. Here, we report two cases in which autologous tumor-infiltrating lymphocyte mono-therapy induced a rapid tumor shrinkage in an Asian patient with metastatic cervical cancer with a history of severe myelosuppression after chemotherapy. Peripheral blood cell count and cytokine secretion analysis over the course of the treatment revealed a persistent immune response in both patients. Together, this study suggests TILs mono-therapy as a promising treatment strategy for Asian patients with late-stage metastatic cervical cancer, even when presented with a history of severe myelosuppression following chemotherapy. Large scale clinical trials of TILs therapy in Asian patients are needed for further confirmation.
Cases Presentation
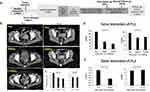
Patient 1 was a 68-year-old woman who was diagnosed with Stage IV cervical cancer when experiencing vaginal bleeding in November 2021. Pathological subtype of cervical squamous cell carcinoma was confirmed by biopsy at the General Hospital of Tianjin Medical University. Positron Emission Tomography-Computed Tomography (PET-CT) results were also consistent with a cervical cancer diagnosis. The patient received 10 cycles of AK104 (10mg/kg, Q3W), bevacizumab (15mg/kg, Q3W), carboplatin (AUC=5, Q3W), and paclitaxel (175mg/m2, Q3W) between October 2021 and July 2022 (Supplemental Figure 1). She experienced severe myelosuppression with leukopenia and thrombocytopenia (grade 3–4, CTCAE 5.0) after each cycle of chemotherapy and took about a month to recover. Pelvic MRI examination showed a cervical tumor of 3.6 cm 1 month before she received anti-tumor treatment, and an enhanced pelvic CT scan showed a cervical tumor of 5.2 cm when she received the first cycle of chemotherapy. Regular enhanced pelvic CT scan follow-ups revealed a cervical tumor of 5.0 cm on 01/17/2022, 4.5 cm on 02/27/2022, 4.0 cm on 04/12/2022, 3.6 cm on 06/06/2022 in the largest diameter. However, her disease progressed on 07/29/2022 with an enlarged cervical tumor of 4.5 cm and more larger metastatic lymphocyte notes near the iliac vessels, which were considered non-target lesions, along with aggravated symptom of bloody vaginal discharge. Patient 2 was a 24-year-old woman who was diagnosed with Stage IIIB cervical cancer when experiencing vaginal bleeding in November 2022. A biopsy diagnosis confirmed that her disease was pathologically adenocarcinoma with lymphocyte metastasis near the iliac vessels. She then received four cycles of radiotherapy, four cycles of chemotherapy Cisplatin (50mg/m2)+Paclitaxel (175mg/Kg), and two cycles of immunotherapy of Pembrolizumab (200mg, Q3W) for anti-cancer treatment. However, her disease was still rapidly progressed which is likely due to her poorly differentiated pathological cancer type. The two patients were then enrolled in a clinical trial of TILs therapy for further treatment after meeting the inclusion criteria and provided signed informed consent for the trial. Both patients received a lymphodepletion regimen consisting of cyclophosphamide (30mg/kg) for 2 days, followed by Fludarabine (25mg/m2) for 5 days, approximately 24 hr before receiving intravenous autologous TILs infusion. She then received high doses of IL-2 for 10 days according to the clinical trial protocol (Figure 1A). Patient 1 exhibited an objective clinical response at 6 weeks post treatment, which persisted until 12 weeks post TILs infusion (Figure 1B and C) as well as a disappearance of vaginal discharge. Her metastatic lymphocyte notes near the iliac vessels were stable Patient 2 was evaluated as a stable disease (SD) at 6 weeks post treatment (Figure 1B and C). Levels of tumor monitoring biomarkers CA125 and CA153 decreased after treatment suggesting an improvement in the patients’ disease condition (Figure 1D and E). Both patients were alive without severe clinical symptoms until the time of last follow-up and will be followed up longer according to the protocol when applicable.
Material and Methods
Autologous TILs Product Processing and Expansion
The TILs products used in treating the patients were manufactured according to the standard Good Manufacturing Practice of Medical Products (GMP) procedure provided by the Suzhou Lanma Biotechnology Co. Briefly, freshly resected patient tumor was placed in sterile RPMI media and delivered to the GMP manufacturing facility. The tumor was cut into 2–3mm3 fragments and were placed into culture containers (5–10 fragments per container) in media supplemented with 6000 IU/mL of IL-2 and 10% human AB serum. TILs were then split into a new culture container when they proliferated to ≥2X106/mL. Media containing 6000 IU/mL IL-2 was supplemented every 2–5 days to maintain TILs growth for 10–14 days according to a pre-Rapid Expansion Protocol (pre-REP). TILs obtained from the pre-REP culture were cryopreserved and recovered for a further rapid expansion (REP) when the patient is ready to receive the treatment. TILs were cultured in a medium containing anti-CD3 antibody (CDE-M120a, ACROBiosystems), irradiated feeder cells and 6000IU/mL IL-2 for a rapid 14-day expansion. During the rapid expansion (REP) process, TILs were also split into a new culture container when they proliferated to ≥2X106/mL every 2–3 days. Pathogen detection tests were performed on Day 0, −7 and −14 staring on the first day of REP. The final TILs products containing 3×109 and 5.1×109 cells of patients 1 and 2 aliquoted in 100 mL and 170mL infusion medium were shipped to the hospital in less than 12 hr and infused into the patients. T cell subtypes of the TILs products were characterized by staining with cell surface markers followed by flow cytometry analysis.
Enzyme-Linked Immunosorbent Assay (ELISA)
Peripheral blood was collected from patients at different time points according to the clinical trial protocol. Serum was isolated from the peripheral blood by centrifugation and was further analyzed for cytokine expressions. The expression levels of multiple cytokines were measured using pre-coated ELISA Kits (IFN-γ, Biolegend 430,107; TNF-α, Dakewe 1,117,202; IL-2, Biolegend 431,807; IL-4, Biolegend 430,307; IL-5, Multi Sciences 70-EK105-96; IL-6, Biolegend 430,507; IL-7, Multi Sciences 70-EK107-96; IL-10, Biolegend 430,607; IL-13, Multi Sciences 70-EK113-96; IL-15, Biolegend 435,107; IL-21, Biolegend 433,807; TGF-β1, Biolegend 437,707) with standard samples according to the manufacturer’s instructions. Plate reading was performed using a SpectraMax 190 Microplate Reader (Molecular Devices, LLC) with absorbance at 450 nm and 570 nm, and the concentrations were calculated using SoftMax Pro 7.1.2 (Molecular Devices, LLC).
Flow Cytometry Analysis
Approximately 1×106 of REP TILs were taken out from the final TILs products and washed twice with 1× phosphate buffered solution (PBS) prior to staining. Cells were resuspended in 100 µL of FACS staining buffer (PBS+0.5% FBS) containing 1:50 diluted antibodies and stained for 30 min at 4°C in the dark. Cells were then washed twice with 1×PBS and analyzed using the BD FACSCanto II flow cytometer (BD Biosciences, USA). Data analysis was performed using FlowJo V10 (BD Biosciences, USA). The antibodies used in this test include FITC anti-human CD3 (Biolegend 300,406), PE anti-human CD4 (Biolegend 317,410), PerCP anti-human CD8 (Biolegend 344,708), APC anti-human CD326 (EpCAM) (Biolegend 369,810), and PE/Cyanine7 anti-human TCRα/β (Biolegend 306,720).
Meanwhile, peripheral blood was collected from the patients at different time points and red blood cells were removed by RBC Lysis Buffer (Solarbio R1010). Samples were then washed, stained, and analyzed in the same way as TILs mentioned above. Antibodies including FITC anti-human CD3 (300,406, Biolegend), PE anti-human CD4 (317,410, Biolegend), PerCP anti-human CD8 (344,708, Biolegend), APC anti-human CD25 (356,110, Biolegend), APC anti-human CD69 (310,910, Biolegend), PE anti-human CD39 (328,208, Biolegend), PE anti-human CD103 (Integrin αE) (350,206, Biolegend), APC anti-human CD197 (CCR7) (353,214, Biolegend), and PE anti-human CD352 (NTB-A) (332,304, Biolegend) were used in this assay.
Laboratory Testing for CA125, CA153
Expression levels of cancer antigen 125 (CA125) and cancer antigen 153 (CA153) in peripheral blood serum were determined using the special CA125/CA153 antigen determination kit (Product number 386357, Beckman, United States).
Results
Autologous Tumor-Infiltrating Lymphocyte Mono-Therapy Induced an Objective Clinical Response with Mild Side Affects
Patient 1 exhibited an objective clinical response at 6 weeks post treatment (Partial Response, PR), which persisted until 12 weeks post TILs infusion (Figure 1B and C). Besides, her metastatic lymphocyte notes near the iliac vessels were measured as stable disease. Patient 2 was evaluated as a stable disease (SD) at 6 weeks post treatment for both target and nontarget lesions (Figure 1B and C). Expression levels of tumor biomarkers CA125 and CA153 decreased in patient 1 after treatment suggesting an improvement in the patient’s condition (Figure 1D). There was a slight decrease of CA125 expression while not showing reduction in terms of CA153 in patient 2 (Figure 1E). Only a few adverse events were observed in the patients including nausea, vomiting, inappetence, leukopenia, thrombocytopenia, fever, rash, herpes labialis, edema of both lower limbs, and diarrhea (Supplemental Table 1). Further, timeline of the detailed side effects after TILs infusion of both patients is shown in Supplemental Figure 2. Most of these side effects subsided in 7 days after the IL-2 infusion treatment, suggesting that the TILs therapy was safe and well tolerated. A recovery from leukopenia and thrombocytopenia took approximately 1-month post TIL treatment, which agrees with patient 1’s previous history of severe myelosuppression after chemotherapy (Supplemental Figure 3A). Importantly, symptoms of abnormal vaginal discharge gradually disappeared 4 weeks post TILs treatment of both patients.
Autologous Tumor-Infiltrating Lymphocyte Mono-Therapy Induced a Systematic Immune Response
The infused TILs product for the two patients contained 97% and 99.6% CD3+ T cells, 56.6% and 62.8% CD3+CD8+ T cells, 28.5% and 11.7% CD3+CD4+ T cells, 88.9% and 90.2% CD3+TCR-αβ+ T cells, respectively (Figure 2A). We monitored the numbers of lymphocytes in peripheral blood during treatment and found that there was a continuously high percentage of lymphocytes ranging from 40% to 70% started on Day 17–22 after TILs infusion until 12 weeks post treatment (Figure 2B). We further characterized CD4+ and CD8+ T cell subtypes in the peripheral blood after treatment and found that TILs infusion led to a high proportion of CD8+ T cells on and after day 15 post infusion (Figure 3Aa), suggesting both the persistence and expansion of infused TILs in the peripheral blood. Terminally exhausted CD8+CD39+ and NTB-A−CD69+ T cells were maintained at low levels on and after day 4 post TILs infusion (Figure 3Ab). In addition, percentages of CD103+ memory T cells and CCR7+ naive T cells were low right after infusion (Figure 3Ac). Percentage of regulatory T cells (CD3+CD4+CD25+) decreased on day 15 after infusion (Figure 3Ad). Measurements of peripheral T cell sub-types of Patient 2 showed similar trends as Patient 1 (Figure 3B). Next, we characterized the Th1 and Th2 cytokine milieu in the peripheral blood of the two patients over the course of treatment. We observed that the level of transforming Growth Factor-β1 (TGF-β1) decreased right after TILs infusion, suggesting a decreased tumor activity in the patient (Figure 4). The sharp increase in levels of IFN-γ and TNF-α around 4–6 weeks after TILs infusion reflected the antitumor activity and may explain the continuous regression of tumor at weeks 6 and 12. These results suggest that TILs infusion altered the composition of systematic T cell subtypes in the patient by enhancing its anti-tumor immune reactivity. Moreover, a high neutrophil count (Supplemental Figure 3B) and a high level of C-reactive protein (CRP) expression (Supplemental Figure 4) starting at 4 days post treatment suggested an activated immune response in the patients. Levels of CRP expression largely returned to normal 1 month after treatment.
Discussion
Cervical cancer is one of the top lethal cancers for women. While early screening and vaccination programs have effectively reduced the number of new cases in many countries,8–10numbers of new cases and related deaths remain high in developing countries. Autogenic tumor-infiltrating lymphocytes (TILs) therapy has shown more specificity and potency to inhibit tumor growth in cervical cancers.6,11,12 Moreover, TILs are directly harvested and expanded from individual patients. This allows a personalized and patient-specific approach to recognize and target tumor cells after infusion. So far, the clinical efficacy of TILs therapy has only been reported in Stage III and IV cancer patients with advanced melanoma, lung cancer, and cervical cancer mostly in western countries.5–7 These patients usually have worse performance status or have poor bone marrow condition after cycles of standard chemotherapy or radiotherapy. Clinical studies on TILs therapy are currently focusing on patients without standard treatment options including radiochemotherapy (RCT). It has been shown that there was a restructuring of tumor infiltrating T cells occurred after RCT, which may lead to insufficient number of T cells to perform anti-tumor response. Expanding TILs in vitro to a greater number and infusing them back might increase the cytotic score of CD8+T cells.13 The main objectives of this current case study are to 1) report clinical research models of TILs therapy for advanced cervical cancer along with worse bone marrow conditions, 2) carry out preliminary toxicological and systematic research over time on TILs infusion, and 3) explore the safety, effectiveness, and applicable population of TILs therapy and then informing future studies. Here, we revealed that TILs infusion induced changes in systematic immune response in the treated patients. We observed alterations in T cell subtypes and cytokine profiles, which are consistent with the tumor shrinkage of Patient 1. Although few studies have revealed the clinical efficacy of TILs therapy, systematic responses induced by TILs therapy were not well reported.5–7 We found that peripheral CD8+T and CD4+T percentages switched after 8 days post TILs infusion leading to dominate CD8+T cell population in both patients (Figure 3A and B), suggesting that ascendant CD8+T cells might be the key factor to induce effective anti-tumor response for TILs therapy, and basic studies are needed to further understand the anti-tumor response induced by TILs therapy in molecular or genetic level. However, there are a few shortcomings in the current study that need to be further replenished by TCR clone sequencing on infused TILs and peripheral blood post treatment, which are important to track TILs precisely over time.
We also gained several insights of TILs therapy from this case study: 1) advanced cervical cancer patients with severe myelosuppression may tolerate lymphocyte depletion and benefit from TILs therapy with improved survival; 2) adverse events in TILs therapy were well-tolerated, mild, and manageable; 3) TILs therapy can induce systematic immune responses, which might be persistent for a long period. Meanwhile, treating solid tumor with adoptive cell therapy has been challenging due to the high intratumoral heterogeneity and complexity of the immune-suppressive tumor microenvironment. TILs therapy shows significant advantages in treating solid tumors owing to its composition of multiple TCR clone subsets that simultaneously target multiple tumor antigens with high specificity. However, there remain important questions for future research on TILs therapy. First, while TILs therapy induces a long-term anti-tumor response, studies on its efficacy for treating early-stage cancers are in dire need. Second, combining TILs therapy with other immunotherapy or targeted therapy may improve overall treatment efficacy and need to be further examined. Third, genetically editing TILs to improve their anti-tumor function may be crucial for further improving their efficacy. Fourth, whether and how high-risk-HPV infection correlated with clinical response of TILs therapy. Finally, the exact molecular mechanisms underlying the anti-tumor activity of TILs therapy and the potency assay of TILs remain open questions. A most recent study uncovered nine sub-clusters of tumor infiltrating T cells via single-cell RNA sequencing of 76,911 individual cells from 13 human cervical squamous cell carcinoma samples showing that TH17 and TNFRSF9high Treg cells were relatively more abundant in advanced cervical cancer patients. These two populations of cells both exhibited high and specific expression of CCL20, which is correlated with short survival of these patients.14 Future studies are still needed to decipher the molecular regulation of tumor infiltration of TH17 and TNFRSF9high Treg cells and other T cell subtypes, and in turn to provide targets for T cell manipulation.
It is worth noting that the results of the present study will add evidence for setting up the standards of suitable population who may likely benefit from TILs therapy in Asian cervical cancer patients. Oncologists can make more precise judgements for choosing treatment strategies based on these information during clinical practice.
Conclusion
We revealed that TILs mono-therapy can be a promising treatment strategy for Asian patients with late-stage metastatic cervical cancer even with severe myelosuppression. TILs infusion can induce persistence and a long-term systematic immune response, which is consistent with clinical responses in advanced cervical cancer patients. Further, TILs treatment reversed peripheral CD4+T and CD8+T percentages implying that TILs infusion increased cytotic T cell responses in recurrent cervical cancer patients.
Data Sharing Statement
Raw data will be provided by corresponding authors upon reasonable request.
Statement of Ethics
Study approval statement: This trial was a study that was approved by the ethical review board of the ethics committee of Tianjin Beichen Hospital (Approval number: 2022170213).
Consent to participate statement: written informed consents were obtained from both patients to participate in the study.
Written informed Consent for Publication statement: written informed consent was obtained from both patients for publication of the details of their medical case and any accompanying images.
Funding
This work was supported by the Tianjin Beichen Hospital (Funding No. Beichen District Health System Technology Projects, SHGY-2020024, SHGY-2021006, SHGY-2022003), National Natural Science Foundation of China (Funding No. 82070206), Tianjin Key Medical Discipline (Specially) Construction Project, Foundation of Tianjin Science and Technology Commission of China (Grant NO.20JCZXJC00100 to W. L.), Foundation of Tianjin Municipal Education Commission of China (Grant No. 2019ZD033 to W. L.).
Disclosure
Authors Xiaoqing Zhang and Yongming Xue were employed by Suzhou Lanma Biotechnology Co. All the other authors have no conflict of interest to declare.
References
1. Tsimberidou A-M, Van Morris K, Henry Hiep V, et al. T-cell receptor-based therapy: an innovative therapeutic approach for solid tumors. J Hematol Oncol. 2021;14(1):102. doi:10.1186/s13045-021-01115-0
2. Mirzaei HR, Rodriguez A, Shepphird J, Brown CE, Badie B. Chimeric Antigen receptors t cell therapy in solid tumor: challenges and clinical applications. Front Immunol. 2017;8:1850. doi:10.3389/fimmu.2017.01850
3. Leon E, Ranganathan R, Savoldo B. Adoptive T cell therapy: boosting the immune system to fight cancer. Semin Immunol. 2020;49:101437. doi:10.1016/j.smim.2020.101437
4. Paijens ST, Vledder A, de Bruyn M, Hans W. Nijman.Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol Immunol. 2021;18(4):842–859. doi:10.1038/s41423-020-00565-9
5. Creelan BC, Wang C, Teer JK, et al. Landin, et al.Tumor-infiltrating-lymphocyte treatment for anti-PD-1 resistant metastatic lung cancer: a Phase I trial. Nat Med. 2021;27(8):1410–1418. doi:10.1038/s41591-021-01462-y
6. Tang Y, Zhang AXJ, Chen G, Wu Y, Gu W. Prognostic and therapeutic TILs of cervical cancer Current advances and future perspectives. Mol Ther Oncolytics. 2021;22:410–430. doi:10.1016/j.omto.2021.07.006
7. Rohaan MW, van den Berg JH, Kvistborg P, Haanen JBAG. Adoptive transfer of tumor-infiltrating lymphocytes in melanoma: a viable treatment option. J Immunother Cancer. 2018;6(1):102. doi:10.1186/s40425-018-0391-1
8. Brisson M, Kim JJ, Canfell K, et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395(10224):575–590. doi:10.1016/S0140-6736(20)30068-4
9. Finocchario-Kessler S, Wexler C, Maloba M, Mabachi N, Ndikum-Moffor F, Bukusi E. Cervical cancer prevention and treatment research in Africa: a systematic review from a public health perspective. BMC Women's Health. 2016;16:29. doi:10.1186/s12905-016-0306-6
10. T GD, Giannini A, Bogani G, et al. Prevention, screening, treatment and follow-up of gynecological cancers: state of art and future perspectives. Clin Exp Obstet Gynecol. 2023;50(8):160. doi:10.31083/j.ceog5008160
11. Zhu Y, Zhou J, Zhu L, Wenjing H, Liu B, Xie L. Adoptive tumor infiltrating lymphocytes cell therapy for cervical cancer. Hum Vaccin Immunother. 2022;18(5):2060019. doi:10.1080/21645515.2022.2060019
12. Ruan H, Oike T, Sato H, Ando K, Ohno T. Association between Tumor Mutational Burden, Stromal CD8+ Tumor-Infiltrating Lymphocytes, And Clinical Factors In Cervical Cancers Treated with Radiotherapy. Cancers. 2023;15(4):1210. doi:10.3390/cancers15041210
13. Liu C, Li X, Huang Q, et al. Single-cell RNA-sequencing reveals radiochemotherapy-induced innate immune activation and MHC-II upregulation in cervical cancer. Signal Transduct Target Ther. 2023;8(1):44. doi:10.1038/s41392-022-01264-9
14. Liu C, Zhang M, Yan X, et al. Single-cell dissection of cellular and molecular features underlying human cervical squamous cell carcinoma initiation and progression. Sci Adv. 2023;9(4):eadd8977. doi:10.1126/sciadv.add8977
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.