Back to Journals » International Journal of General Medicine » Volume 17
Association of Serum Uric Acid with Non-Valvular Atrial Fibrillation: A Retrospective Study in China
Authors Yuan HJ, Jiao HC, Liu XJ, Hao H, Liu Y, Xue YT, Li Y
Received 6 January 2024
Accepted for publication 15 April 2024
Published 22 April 2024 Volume 2024:17 Pages 1533—1543
DOI https://doi.org/10.2147/IJGM.S458089
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Hua-Jing Yuan,1 Hua-Chen Jiao,2 Xiu-Juan Liu,2 Hao Hao,2 Yang Liu,2 Yi-Tao Xue,2 Yan Li2
1Shandong University of Traditional Chinese Medicine, Jinan, Shandong Province, 250014, People’s Republic of China; 2Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, Shandong Province, 250014, People’s Republic of China
Correspondence: Yi-Tao Xue; Yan Li, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Shanshi East Road, Lixia District, Jinan City, Shandong Province, People’s Republic of China, Tel +86-531-82602943 ; +86-531-68611662, Email [email protected]; [email protected]
Purpose: The association between serum uric acid (SUA) and atrial fibrillation (AF) has been widely focused on and studied in recent years. However, the exact association between SUA and AF is unclear, and the effect of gender on the association between SUA levels and AF has been controversial. This study aimed to investigate the association between SUA levels and non-valvular AF (NVAF) and the potential effect of gender on it.
Patients and Methods: A total of 866 NVAF patients (463 males, age 69.44 ± 8.07 years) and 646 sex-matched control patients in sinus rhythm, with no history of arrhythmia were included in this study. t-test, ANOVA, and chi-square test were used for baseline data analysis. The receiver operating characteristic curve, logistic regression and Pearson correlation analysis were used for correlation analysis.
Results: Compared to controls, NVAF patients exhibited higher SUA (P< 0.001). After adjusting for confounders of NVAF, SUA remained significantly associated with NVAF, regardless of gender (OR= 1.31, 95% CI 1.18– 1.43, P< 0.001). SUA demonstrated higher predictability and sensitivity in predicting the occurrence of female NVAF compared to male (area under the curve was 0.68 (95% CI 0.64– 0.72, P< 0.001), sensitivity 87.3%), with the optimal cut-off point identified as 5.72 mg/dL. Furthermore, SUA levels correlated with APOA1, Scr and NT-proBNP in NVAF patients. SUA levels varied significantly among NVAF subtypes.
Conclusion: High SUA levels were independently associated with NVAF, regardless of gender. SUA exhibited higher predictability and sensitivity in predicting the occurrence of NVAF in females compared to males. High SUA levels may affect other NVAF-related factors and participate in the pathophysiological process of NVAF.
Keywords: serum uric acid, non-valvular atrial fibrillation, gender difference, retrospective study, predictive indicators
Introduction
Atrial fibrillation (AF) is a common cardiac arrhythmia. At present, there are more than 30 million AF patients worldwide, with its incidence and related mortality increased significantly.1,2 It is predicted that the number of adults with AF in Europe will reach 17.9 million by 2060.3 Atrial thrombus and subsequent embolic events, including ischemic stroke and other thromboembolisms, are serious complications of AF. Given that AF is widely recognized as a significant risk factor for ischemic stroke,4,5 its impact on the quality of life and health of patients and socioeconomic burden is significant. Despite the effective prediction of AF using ECG6 and its treatment with antiarrhythmic drugs and radiofrequency catheter ablation, high recurrence and mortality rates persist. Studies have shown that following a single radiofrequency catheter ablation, only 60.5% of patients maintain sinus rhythm after 37 months of follow-up, and after 42 months of follow-up after multiple radiofrequency catheter ablation, 74.9% of patients maintain sinus rhythm.7 Therefore, the prevention and treatment of AF cannot be ignored.
The pathological mechanism of AF is not yet clear, but inflammation and oxidative stress have been confirmed to play an important role in the occurrence and development of AF.8,9 Serum uric acid (SUA) is produced by purine catabolism, which induces inflammation in vascular endothelial cells and smooth muscle cells, as well as intracellular oxidative stress, leading to endothelial dysfunction and thus affecting thrombosis and inflammatory responses.10 The current study found that high SUA levels are strongly associated with cardiovascular disease (CVD) and its adverse events,11,12 and there is an association with traditional cardiovascular risk factors.13 SUA levels are significantly associated with inflammatory and oxidative stress pathways.14,15 It appears that SUA shares the same pathological progression as AF, and multiple studies support the significant association between SUA levels and AF. SUA discrete trajectories were significantly associated with the risk of atrioventricular block.16 Patients with high SUA levels have an increased risk of developing AF.17,18 However, SUA levels are affected by multiple complex and interrelated factors, notably gender. Assessing the correlation between SUA and cardiovascular risk factors based on gender differences seems necessary.19 Determining whether SUA acts as an independent risk factor or marker for AF is widely debated, which brings certain limitations to clinical application and reference.
Therefore, in this study, we aimed to investigate the potential relationship between SUA levels and non-valvular AF (NVAF), as well as to examine SUA levels among different NVAF subtypes, to further understand the pathological process.
Materials and Methods
Patients and Methods
This was a cross-sectional retrospective design study. Patient case data were collected from the electronic medical record database of the Affiliated Hospital of Shandong University of Traditional Chinese Medicine from January 2005 to January 2022. A total of 1512 clinical data of patients were included, including 866 NVAF patients and 646 sex-matched patients with sinus rhythm and no history of arrhythmia. Inclusion criteria were as follows: (a) new-onset AF or patients with a history of AF who met the AF diagnostic criteria on admission;20 (b) age of 18–80 years. Exclusion criteria were as follows: (a) valve disease, hyperthyroidism, malignancy, recent surgery or other trauma, pregnancy, liver and kidney insufficiency; (b) the use of uric acid lowering drugs; (c) incomplete clinical data.
Data Collection
All data were obtained from the electronic medical records database of the Affiliated Hospital of Shandong University of Traditional Chinese Medicine. Baseline data were collected from medical records, including demographic data (age and gender), medical history (diabetes, hypertension, coronary heart disease (CHD), and ischemic stroke), medication use, CHA2DS2-VASc scores, and laboratory blood parameters. AF patients were divided into three groups according to gender, respectively, they included the male low-level group, <5.5 mg/dL; the intermediate-level group, 5.5–6.5 mg/dL; the high-level group, >6.5mg/dL; the female low-level group, 4.2 mg/dL; the intermediate-level group, 4.2–5.0 mg/dL; the high-level group, >5.0 mg/dL.
Laboratory blood parameters were obtained from fasting blood samples collected upon admission, which were subsequently analyzed at the Laboratory Department of the Affiliated Hospital of Shandong University of Traditional Chinese Medicine for determination. Specifically, SUA, serum creatinine (Scr), serum albumin, globulin, glucose (GLU), triglyceride (TG) and apolipoprotein A-1 (APOA1) were determined by the colorimetric method (OLYMPUS-AU2700, automatic biochemical analyzer). SUA is reported in μmol/L and converted to 1 mg/dL = 59.45 μmol/L, the upper limit of normal is 7 mg/dL for men and 6 mg/dL for women. D-dimer was determined by latex-enhanced photometric immunoassay (Stago automated coagulation instrument). White blood cell (WBC), neutrophil, and lymphocyte were determined by SYSMEX BC-5000, a hematology analyzer. International normalized ratio (INR) and fibrinogen (FIB) were measured by the instrumental method (Stago automated coagulation instrument). N-terminal pro-B-type natriuretic peptide level (NT-proBNP) was determined using electrochemiluminescence (Elecsys 2010 system, Roche Diagnostic).
Statistical Analysis
SPSS version 21.0 (IBM Corp, Armonk, NY, USA) was used for statistical analysis. Normally distributed continuous variables were presented as mean ± standard deviation (SD), and non-normally distributed continuous variables were presented as median (interquartile range [IQR]). Categorical variables were expressed as numerical values (percentages). Student’s t-test or Mann–Whitney U-test was used to compare continuous variables between two groups. ANOVA or the Kruskal–Wallis test was used to compare continuous variables between multiple groups. The Bonferroni method was used for multiple comparisons between multiple groups, which adjusts the P value of each test. Comparison of categorical variables was done by chi-square test. Pearson correlation analysis was used to explore interrelationships. Univariate and multivariate logistic regression were used to adjust for confounders to screen for independent risk factors. The receiver operating characteristic (ROC) curve was used to analyze the predictive ability of SUA for NVAF. GraphPad Prism version 9.0.0 (Inc, La Jolla, CA, USA) was used to visualize the results of the statistical analysis. Two-tailed P values <0.05 were considered statistically significant.
Results
Baseline Characteristics of the Study Population
The study flowchart was shown in Figure 1. The mean age of 866 patients in the NVAF group was 69.44 ± 8.07 years, while the mean age of 646 control patients with sinus rhythm and no history of arrhythmia was 69.44 ± 8.81 years, both of which were not statistically significant (P=0.992). Compared to the control group, NVAF patients were more likely to have a history of hypertension, diabetes, CHD, and ischemic stroke. Additionally, NVAF patients exhibited higher levels of SUA, WBC, neutrophil to lymphocyte ratio (NLR), INR, FIB, D-dimer and Scr, along with lower levels of the albumin to globulin ratio (A/G), PCV, PDW, and APOA1 (Table 1). These differences were all statistically significant between the NVAF and control groups. However, gender and TG levels were not statistically significant between the two groups.
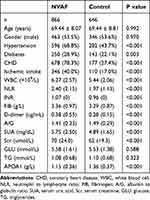 |
Table 1 Baseline Characteristics of the Study Population |
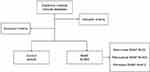 |
Figure 1 Study flowchart. Abbreviation: NVAF, non-valvular atrial fibrillation. |
We found that NVAF patients exhibited higher levels of SUA (Figure 2A) and there was still a significant difference between male and female NVAF patients (Figure 2B). Furthermore, in NVAF patients, we observed that SUA levels were positively correlated with Scr (P<0.001) and NT-proBNP (P<0.001), while negatively correlated with APOA1 (P<0.001) (Figure 3).
Correlation of SUA with NVAF
We used univariate logistic regression to assess the association between SUA and NVAF, and used multivariate logistic regression to adjust for confounding factors, aiming to improve the accuracy of the association between SUA and NVAF (Table 2). The results showed that SUA levels were significantly correlated with the occurrence of NVAF (OR= 1.41, 95% CI 1.31–1.51, P<0.001). After adjusting for CHD, diabetes, hypertension, and ischemic stroke, SUA levels were significantly associated with NVAF (OR=1.40, 95% CI 1.29–1.52, P<0.001). The adjusted WBC, NLR, INR, FIB, D-dimer, A/G, Scr and APOA1, SUA levels also showed a significant correlation with NVAF (OR=1.30, 95% CI 1.17–1.46, P<0.001). And after adjusting for the above confounders, SUA levels were still significantly associated with NVAF (OR=1.31, 95% CI 1.18–1.43, P<0.001). We also performed logistic regression analysis separately for male and female patients, adjusting for the above-mentioned confounders. The results showed that SUA was independently associated with NVAF in males (OR= 1.36, 95% CI 1.18–1.57, P<0.001) and females (OR=1.28, 95% CI 1.07–1.53, P=0.007), respectively (Table 2).
 |
Table 2 Association of SUA with NVAF by Logistic Regression |
In addition, we compared SUA levels according to different subtypes of NVAF (Table 3). Significant differences were observed in SUA levels among different NVAF subtypes (P<0.001). Specifically, compared to new-onset NVAF and paroxysmal NVAF, persistent NVAF were more likely to exhibit high SUA levels (adjusted P<0.001, Supplementary Table S1).
 |
Table 3 Correlation of SUA Levels with Subtypes of NVAF |
Differences in Different SUA Levels in NVAF Patients
We divided NVAF patients into six groups according to their SUA level and gender. Among them, we compared the differences in various parameters among different SUA levels in male and female patients (Supplementary Table S2). Regardless of gender, significant differences were observed in Scr and the use of diuretics among different SUA levels. Among male patients, age (P<0.001), FIB (P=0.002), and the use of β-blockers (P=0.010) were independently associated with SUA levels. Among female patients, higher SUA levels were more likely to have higher CHA2DS2-VASc (P=0.006). And there was a significant difference in the use of CCBs (P=0.008). In addition, NLR (P<0.001), INR (P<0.001), D-dimer (P=0.018), A/G (P=0.013), APOA1 (P<0.001), and NT-proBNP (P<0.001) were all significantly correlated with SUA levels.
The ROC Curve
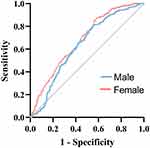
The ROC curve analysis was used to analyze the predictive ability of SUA for NVAF. As shown in Figure 4, in male patients, the area under the ROC curve was 0.63 (95% CI 0.60–0.67, P < 0.001), with the optimal cut-off point for SUA of 6.37 mg/dL, and the sensitivity of 81.5% (95% CI 77.1–85.2%). In female patients, the area under the ROC curve was 0.68 (95% CI 0.64–0.72, P < 0.001), with the optimal cut-off point for SUA of 5.72 mg/dL, and the sensitivity of 87.3% (95% CI 83.1–90.6%). These findings indicated that SUA has a diagnostic value for the occurrence of NVAF. Compared to male NVAF, SUA demonstrates higher predictability and sensitivity in the occurrence of female NVAF.
Discussion
In this retrospective study, we observed a significant association between SUA levels and the occurrence of NVAF which were significantly associated in both male and female patients. After multiple adjustments for confounders, the association of SUA levels with NVAF remained significant regardless of gender. Additionally, in NVAF patients, SUA levels were found to be positively correlated with Scr and NT-proBNP, and negatively correlated with APOA1. Patients with persistent NVAF were more likely to exhibit higher SUA levels compared to new-onset NVAF and paroxysmal NVAF. Additionally, SUA has a diagnostic value for the occurrence of NVAF, with higher predictability and sensitivity in female NVAF compared to male NVAF. The optimal cut-off point for SUA in predicting NVAF occurrence was determined to be 5.72 mg/dL.
SUA is the final product of purine metabolism, which is regulated by xanthine oxidase (XO). Male generally have higher SUA levels than female of the same age, which is related to the urination effect of estrogen.21,22 Hyperuricemia occurs when the balance between the production and excretion of SUA in the body is disrupted. In general, SUA levels above 7 mg/dL in males and 6 mg/dL in females are considered hyperuricemia.23 However, studies have found that SUA not only plays an important role in gout and joint damage but is also an independent risk factor for CVD. A growing number of clinical studies support the association between SUA levels and CVD.10,24,25 SUA is involved in oxidative stress, inflammation, endothelial dysfunction, and other pathophysiological processes of CVD.13 A cohort study involving 36,313 subjects showed that SUA was significantly associated with cardiovascular mortality,26 and a Mendelian randomization study based on 3315 patients suggested that high SUA levels were causally associated with adverse cardiovascular outcomes.27
At present, the pathophysiological mechanism of AF still needs to be further explored, but the roles of inflammation and oxidative stress in AF have been widely demonstrated.8,28,29 XO, a key enzyme in SUA metabolism, also serves as an important source of reactive oxygen species (ROS) that cause oxidative damage in the heart.30 The accumulation of ROS occurs concurrently with the production of SUA.31 Studies have indicated a correlation between oxidative stress and atrial remodeling in AF, with SUA and XO playing important roles in this process.28 Normal SUA levels act as antioxidants, however, in hyperuricemic states, SUA acts as pro-oxidants.32 High SUA levels can inhibit the Nrf2 signaling pathway, increase the production of ROS, and promote oxidative stress.24,33 In addition, the accumulation of ROS can also lead to the activation of inflammation and endothelial dysfunction.34 And SUA levels were significantly correlated with pro-inflammatory factors including TNF-α, IL-6, and CRP.35 Experimental evidence suggests that high SUA concentrations can activate MAPK to upregulate the expression of inflammatory markers, and effectively activate the NLRP3 inflammasome through NF-kB activation to damage the cardiovascular system.24,36 Moreover, the inflammatory response is involved in atrial fibrosis, atrial remodeling, and atrial conduction in AF.29 In addition, studies have found that the accumulation of SUA can enhance the expression of KCNA5, the main channel of AF induction and recurrence, thereby affecting the electrophysiological mechanism of the myocardium.37 It is evident that SUA plays an important role in the pathological progression of AF.
Clinical studies have found that high SUA levels are associated with the risk of the occurrence of AF.38 However, SUA levels are affected by gender, and the impact of gender on the association of SUA levels with AF remains controversial. In a cohort study involving 6308 subjects with a follow-up period of 10.8 years, after adjustment for cardiovascular risk factors and other parameters, SUA was associated with an increased risk of AF, regardless of gender.39 And in a cross-sectional study based on 1056 elderly individuals, high SUA levels were associated with the prevalence of AF in the general elderly, with no significant different between male and female (P=0.401). In the present study, we conducted adjustments for AF-related confounders and found that the association of SUA levels with AF was significant in both males and females, which is consistent with previously reported findings. However, in a cohort study involving 7155 subjects, SUA was independently associated with AF only in female patients after adjustment for AF-related factors.40 Conversely, another clinical study suggested that SUA is independently associated with AF only in males.41 In addition, our ROC curve analysis found higher predictability and sensitivity in predicting the occurrence of female NVAF compared to male (area under the curve was 0.68 (95% CI 0.64–0.72, P<0.001), sensitivity 87.3%), with an optimal cut-off point of 5.72 mg/dL. A meta-analysis involving 44 prospective cohort studies with 1,134,073 participants concluded that SUA levels were significantly associated with CVD mortality, which was stronger in females compared to males.42 Although the effect of gender on the association between SUA and AF is currently controversial, our results indicated that female patients with high SUA should be more concerned about the occurrence of NVAF. Currently, it is recommended to target an SUA level of 5.0 mg/dL for long-term control in patients with CVD.23,43 However, because SUA levels may affect CVD differently in males and females, larger prospective studies are needed to determine optimal target levels of SUA in patients of different genders for long-term management of CVD.
In our study, we found SUA levels were negatively correlated with APOA1 and positively correlated with Scr and NT-proBNP in NVAF patients. Scr and the use of diuretics showed significant differences among different SUA levels in NVAF patients. Age, FIB, and the use of β-blockers were significantly associated with SUA levels only in male patients. NLR, INR, D-dimer, A/G, APOA1, NT-proBNP, CHA2DS2-VASc and the use of CCBs were significantly associated with SUA levels only in female patients. The important role of inflammation and oxidative stress in AF is self-evident. Serum albumin is widely involved in human physiological functions and regulates inflammation and oxidative stress.44,45 A/G has been used as an indicator to assess inflammatory response, with studies showed that serum albumin is independently associated with AF,46 and A/G may affect the sympatho-vagal imbalance and lead to the occurrence of AF.47 In our study, NVAF patients exhibited significant levels of A/G, but among NVAF patients with different SUA levels, A/G showed significant differences only in female patients. APOA1 is the major apolipoprotein of HDL, which has anti-inflammatory and antioxidant properties.48 Consistent with previous reports,49 our study findings indicated significantly lower APOA1 levels in AF patients compared to healthy subjects. These observations highlight the complex interaction between inflammatory and oxidative pathways in the pathogenesis of AF.
An increasing number of studies explores the interactions between inflammation, oxidative stress, endothelial dysfunction, thrombosis and AF. Studies have found high SUA levels are an independent risk factor for left atrial thrombus in patients with NVAF,50 and elevated SUA levels are associated with an increased risk of recurrent venous thromboembolism.51 In some clinical scenarios, SUA is considered a pre-thrombotic marker.31 However, our study found that FIB showed significant differences among different SUA levels in male NVAF, and CHA2DS2-VASc. D-dimer showed significant differences among different SUA levels in female NVAF. Ischemic stroke showed no significant differences among different SUA levels. Therefore, the effect of SUA on the prothrombotic status of NVAF patients needs to be validated in larger clinical studies, and gender may also affect the association of SUA with NVAF prothrombotic status.
In addition, our study also found a significant association between SUA levels and subtypes of NVAF. Specifically, compared with new-onset NVAF and paroxysmal NVAF, persistent NVAF were more likely to have high levels of SUA. This observation may be attributed to higher levels of inflammation and oxidative stress damage in patients with persistent NVAF. However, few studies with large sample sizes have investigated the association between SUA and AF subtypes. Studies with larger sample sizes may uncover more interesting situations among NVAF subtypes.
This study is a single-center retrospective study investigating the potential association of SUA with NVAF. Moreover, we analyzed the potential association of SUA with other parameters in NVAF patients and found that SUA is more likely to be an effective predictor in female NVAF patients. However, several limitations should be acknowledged. Firstly, the small sample size and single-center nature of our study may introduce bias. Secondly, parameters such as markers of inflammation, oxidative stress and echocardiography were lacking. This was due to it not being measured on admission or lacking too much. Finally, due to the limitations of retrospective analysis, there may still be some potential confounding factors. Future research efforts should aim to overcome these limitations to provide a more robust understanding of the association between SUA and NVAF.
Conclusion
In the present study, high SUA levels were significantly associated with NVAF, regardless of gender. Compared to male NVAF, SUA has higher predictability and sensitivity in predicting the occurrence of female NVAF. Furthermore, we found that in NVAF patients, SUA levels were negatively correlated with APOA1 and positively correlated with Scr and NT-proBNP. SUA levels were significantly different among NVAF subtypes. Compared to new-onset NVAF and paroxysmal NVAF, persistent NVAF was more likely to have high levels of SUA. These findings contribute to a further understanding of the pathological progression of NVAF and provide references for clinical treatment.
Abbreviations
SUA, serum uric acid; AF, atrial fibrillation; NVAF, non-valvular AF; CVD, cardiovascular disease; CHD, coronary heart disease; Scr, serum albumin; Glu glucose; TG, triglyceride; APOA1, apolipoprotein A-1; INR, international normalized ratio; WBC, white blood cell; FIB, fibrinogen; NT-proBNP, N-terminal pro-B-type; SD, standard deviation; IQR, interquartile range; NLR, neutrophil to lymphocyte ratio; A/G, albumin to globulin ratio; ROC, receiver operating characteristic; XO, xanthine oxidase; ROS, reactive oxygen species.
Data Sharing Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Ethics Statement
This study was conducted by the principles of the Declaration of Helsinki and was approved by the Medical Research Ethics Committee of the Affiliated Hospital of Shandong University of Traditional Chinese Medicine, approval number was 2021-022-KY. The informed consent of this study was waived by the Medical Research Ethics Committee of the Affiliated Hospital of Shandong University of Traditional Chinese Medicine. All patient data was anonymized and maintained with confidentiality.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No.81603609) and the Natural Science Foundation of Shandong Province (Grant No. ZR2023MH053).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129(8):837–847. doi:10.1161/CIRCULATIONAHA.113.005119
2. Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int J Stroke. 2021;16(2):217–221. doi:10.1177/1747493019897870
3. Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013;34(35):2746–2751. doi:10.1093/eurheartj/eht280
4. Danese E, Montagnana M, Cervellin G, et al. Hypercoagulability, D-dimer and atrial fibrillation: an overview of biological and clinical evidence. Ann Med. 2014;46(6):364–371. doi:10.3109/07853890.2014.912835
5. Hyman DA, Siebert V, Jia X, et al. Risk assessment of stroke in patients with atrial fibrillation: current shortcomings and future directions. Cardiovasc Drug Ther. 2019;33(1):105–117. doi:10.1007/s10557-018-06849-7
6. Karakayali M, Artac I, Omar T, et al. Assessment of the efficacy of the electrocardiographic P-wave peak time in predicting atrial high rate episode in patients with cardiac implantable electronic devices. J Electrocardiol. 2023;80:40–44. doi:10.1016/j.jelectrocard.2023.05.001
7. Wang Y, Xu Y, Ling Z, et al. Radiofrequency catheter ablation for paroxysmal atrial fibrillation: outcomes during a 3-year follow-up period. J Int Med Res. 2019;47(4):1636–1648. doi:10.1177/0300060519828522
8. Pinho-Gomes AC, Reilly S, Brandes RP, et al. Targeting inflammation and oxidative stress in atrial fibrillation: role of 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibition with statins. Antioxid Redox Sign. 2014;20(8):1268–1285. doi:10.1089/ars.2013.5542
9. Samman TA, Sandesara PB, Hayek SS, et al. Association between oxidative stress and atrial fibrillation. Heart Rhythm. 2017;14(12):1849–1855. doi:10.1016/j.hrthm.2017.07.028
10. Saito Y, Tanaka A, Node K, et al. Uric acid and cardiovascular disease: a clinical review. J Cardiol. 2021;78(1):51–57. doi:10.1016/j.jjcc.2020.12.013
11. Diallo A, Diallo MF, Carlos-Bolumbu M, et al. Uric acid-lowering effects of sodium-glucose cotransporter 2 inhibitors for preventing cardiovascular events and mortality: a systematic review and meta-analysis. Diabetes Obes Metab. 2024;26(5):1980–1985. doi:10.1111/dom.15483
12. Cui K, Song Y, Yin D, et al. Uric acid levels, number of standard modifiable cardiovascular risk factors, and prognosis in patients with coronary artery disease: a large cohort study in Asia. J Am Heart Assoc. 2023;12(20):e030625. doi:10.1161/JAHA.123.030625
13. Ndrepepa G. Uric acid and cardiovascular disease. Clin Chim Acta. 2018;484:150–163. doi:10.1016/j.cca.2018.05.046
14. Ruggiero C, Cherubini A, Ble A, et al. Uric acid and inflammatory markers. Eur Heart j. 2006;27(10):1174–1181. doi:10.1093/eurheartj/ehi879
15. Kurajoh M, Fukumoto S, Yoshida S, et al. Uric acid shown to contribute to increased oxidative stress level independent of xanthine oxidoreductase activity in MedCity21 health examination registry. Sci Rep. 2021;11(1):7378. doi:10.1038/s41598-021-86962-0
16. Li N, Cui L, Shu R, et al. Distinct uric acid trajectories are associated with incident cardiac conduction block. Arthritis Res Ther. 2024;26(1):59. doi:10.1186/s13075-024-03288-8
17. Kwon CH, Lee SH, Lee JY, et al. Uric acid and risk of atrial fibrillation in the Korean general population. Circ J. 2018;82(11):2728–2735. doi:10.1253/circj.CJ-18-0748
18. Wang X, Hou Y, Wang X, et al. Relationship between serum uric acid levels and different types of atrial fibrillation: an updated meta-analysis. Nutr Metab Cardiovas. 2021;31(10):2756–2765. doi:10.1016/j.numecd.2021.05.034
19. Redon P, Maloberti A, Facchetti R, et al. Gender-related differences in serum uric acid in treated hypertensive patients from central and east European countries: findings from the Blood Pressure control rate and CArdiovascular Risk profilE study. J Hypertens. 2019;37(2):380–388. doi:10.1097/HJH.0000000000001908
20. Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation). J Am Coll Cardiol. 2006;48(4):854–906. doi:10.1016/j.jacc.2006.07.009
21. Borges RL, Ribeiro AB, Zanella MT, et al. Uric acid as a factor in the metabolic syndrome. Curr Hypertens Rep. 2010;12(2):113–119. doi:10.1007/s11906-010-0098-2
22. Watanabe E. Uric acid and atrial fibrillation - cause or other association? Circ J. 2012;76(3):584–585. doi:10.1253/circj.CJ-12-0057
23. Borghi C, Domienik-Karlowicz J, Tykarski A, et al. Expert consensus for the diagnosis and treatment of patient with hyperuricemia and high cardiovascular risk: 2021 update. Cardiol J. 2021;28(1):1–14. doi:10.5603/CJ.a2021.0001
24. Yu W, Cheng JD. Uric acid and cardiovascular disease: an update from molecular mechanism to clinical perspective. Front Pharmacol. 2020;11:582680. doi:10.3389/fphar.2020.582680
25. Karabag Y, Rencuzogullari I, Cagdas M, et al. Association of serum uric acid levels with SYNTAX score II and long term mortality in the patients with stable angina pectoris who undergo percutaneous coronary interventions due to multivessel and/or unprotected left main disease. Int J Cardiovas Imag. 2019;35(1):1–7. doi:10.1007/s10554-018-1446-6
26. Zhang W, Iso H, Murakami Y, et al. Serum uric acid and mortality form cardiovascular disease: EPOCH-Japan Study. J Atheroscler Thromb. 2016;23(6):692–703. doi:10.5551/jat.31591
27. Kleber ME, Delgado G, Grammer TB, et al. Uric acid and cardiovascular events: a Mendelian randomization study. J Am Soc Nephrol. 2015;26(11):2831–2838. doi:10.1681/ASN.2014070660
28. Korantzopoulos P, Kolettis TM, Galaris D, et al. The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. Int J Cardiol. 2007;115(2):135–143. doi:10.1016/j.ijcard.2006.04.026
29. Hu YF, Chen YJ, Lin YJ, et al. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12(4):230–243. doi:10.1038/nrcardio.2015.2
30. Deng Y, Liu F, Yang X, et al. The key role of uric acid in oxidative stress, inflammation, fibrosis, apoptosis, and immunity in the pathogenesis of atrial fibrillation. Front Cardiovasc Med. 2021;8:641136. doi:10.3389/fcvm.2021.641136
31. Tapoi L, Salaru DL, Sascau R, et al. Uric acid-an emergent risk marker for thrombosis? J Clin Med. 2021;10(10):2062. doi:10.3390/jcm10102062
32. Maharani N, Kuwabara M, Hisatome I. Hyperuricemia and atrial fibrillation. Int Heart J. 2016;57(4):395–399. doi:10.1536/ihj.16-192
33. Sun X, Jiao H, Zhao J, et al. Unexpected effect of urate on hydrogen peroxide-induced oxidative damage in embryonic chicken cardiac cells. Free Radical Res. 2017;51(7–8):693–707. doi:10.1080/10715762.2017.1362106
34. Doehner W, Landmesser U. Xanthine oxidase and uric acid in cardiovascular disease: clinical impact and therapeutic options. Semin Nephrol. 2011;31(5):433–440. doi:10.1016/j.semnephrol.2011.08.007
35. Lyngdoh T, Marques-Vidal P, Paccaud F, et al. Elevated serum uric acid is associated with high circulating inflammatory cytokines in the population-based Colaus study. PLoS One. 2011;6(5):e19901. doi:10.1371/journal.pone.0019901
36. Xiao J, Zhang XL, Fu C, et al. Soluble uric acid increases NALP3 inflammasome and interleukin-1beta expression in human primary renal proximal tubule epithelial cells through the Toll-like receptor 4-mediated pathway. Int J Mol Med. 2015;35(5):1347–1354. doi:10.3892/ijmm.2015.2148
37. Taufiq F, Maharani N, Li P, et al. Uric acid-induced enhancements of Kv1.5 protein expression and channel activity via the Akt-HSF1-Hsp70 pathway in HL-1 atrial myocytes. CIRC J. 2019;83(4):718–726. doi:10.1253/circj.CJ-18-1088
38. Kobayashi T, Kokubo Y, Higashiyama A, et al. Uric acid and incident atrial fibrillation of 14 years population-based cohort study: the Suita Study. J Arrythm. 2021;37(5):1215–1219. doi:10.1002/joa3.12612
39. Nyrnes A, Toft I, Njolstad I, et al. Uric acid is associated with future atrial fibrillation: an 11-year follow-up of 6308 men and women--The Tromso Study. Europace. 2014;16(3):320–326. doi:10.1093/europace/eut260
40. Suzuki S, Sagara K, Otsuka T, et al. Gender-specific relationship between serum uric acid level and atrial fibrillation prevalence. Circ J. 2012;76(3):607–611. doi:10.1253/circj.CJ-11-1111
41. Sun GZ, Guo L, Wang J, et al. Association between hyperuricemia and atrial fibrillation in rural China: a cross-sectional study. BMC Cardiovasc Disor. 2015;15(1):98. doi:10.1186/s12872-015-0089-y
42. Rahimi-Sakak F, Maroofi M, Rahmani J, et al. Serum uric acid and risk of cardiovascular mortality: a systematic review and dose-response meta-analysis of cohort studies of over a million participants. BMC Cardiovasc Disor. 2019;19(1):218. doi:10.1186/s12872-019-1215-z
43. Li Q, Li X, Wang J, et al. Diagnosis and treatment for hyperuricemia and gout: a systematic review of clinical practice guidelines and consensus statements. BMJ OPEN. 2019;9(8):e026677. doi:10.1136/bmjopen-2018-026677
44. Arques S. Human serum albumin in cardiovascular diseases. Eur J Intern Med. 2018;52:8–12. doi:10.1016/j.ejim.2018.04.014
45. Sheinenzon A, Shehadeh M, Michelis R, et al. Serum albumin levels and inflammation. Int J Biol Macromol. 2021;184:857–862. doi:10.1016/j.ijbiomac.2021.06.140
46. Liao LZ, Zhang SZ, Li WD, et al. Serum albumin and atrial fibrillation: insights from epidemiological and Mendelian randomization studies. Eur J Epidemiol. 2020;35(2):113–122. doi:10.1007/s10654-019-00583-6
47. Pal GK, Shyma P, Habeebullah S, et al. Association of albumin-globulin ratio with sympathovagal imbalance in pregnancy-induced hypertension. Indian J Physiol Pharmacol. 2011;55(2):128–138.
48. Du R, Winarsih I, Ho B, et al. Lipid-free apolipoprotein A-I exerts an antioxidative role against cell-free hemoglobin. Am J Clin Exp Immuno. 2012;1(1):33–48.
49. Cinar T, Tanik VO, Gurkan K. Comparison of apolipoprotein-A1 levels between paroxysmal atrial fibrillation patients and healthy subjects. J Cardiovasc Thorac. 2020;12(2):140–144. doi:10.34172/jcvtr.2020.23
50. Tang RB, Dong JZ, Yan XL, et al. Serum uric acid and risk of left atrial thrombus in patients with nonvalvular atrial fibrillation. Can J Cardiol. 2014;30(11):1415–1421. doi:10.1016/j.cjca.2014.06.009
51. De Lucchi L, Nardin C, Sponchiado A, et al. Serum uric acid levels and the risk of recurrent venous thromboembolism. J Thromb Haemost. 2021;19(1):194–201. doi:10.1111/jth.15139
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.