Back to Journals » Clinical, Cosmetic and Investigational Dentistry » Volume 15
Association Between AIM2 and Pycard Genes Polymorphisms and Susceptibility to Periodontitis with Coronary Heart Disease
Authors Ali Daily Z, Al-Ghurabi BH, Al-Qarakhli AMA , Hussein HM
Received 16 September 2023
Accepted for publication 8 November 2023
Published 22 November 2023 Volume 2023:15 Pages 307—320
DOI https://doi.org/10.2147/CCIDE.S440577
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Christopher E. Okunseri
Zina Ali Daily,1 Batool Hassan Al-Ghurabi,2 Ahmed Makki A Al-Qarakhli,3 Hashim Mueen Hussein4
1Department of Periodontics, College of Dentistry, University of Baghdad, Baghdad, Iraq; 2Department of Basic Science, College of Dentistry, University of Baghdad, Baghdad, Iraq; 3Department of Oral Diagnosis, College of Dentistry, University of Anbar, Baghdad, Iraq; 4Department of Conservative Dentistry, College of Dentistry, University of Mustansiriyah, Baghdad, Iraq
Correspondence: Zina Ali Daily, Department of Periodontics, College of Dentistry, University of Baghdad, Baghdad, Iraq, Email [email protected]
Background: Numerous genetic variations in inflammasome components are linked to prevalent disorders in the general population, including periodontitis and cardiovascular illness. Polymorphisms in the genes play a critical in the initiation and development of inflammatory diseases. The limited study on AIM2 gene variation associated with inflammatory disease and no study of PYCARD gene variation associated with inflammatory disease.
Objective: This case-control study was to examine the association between the single nucleotide polymorphism of AIM2 and Pycard genes with susceptibility to periodontitis with and without coronary heart disease, to determine interleuken-18 and gasdermin D levels in the saliva of periodontitis with and without coronary heart disease patients, as well as their correlation with salivary interleuken-18 and gasdermin D levels and clinical periodontal parameters.
Methods: The present study recruited 120 participants: 30 were healthy subjects (control, C), 30 had generalized periodontitis (P), 30 had atherosclerosis coronary heart disease with clinically healthy periodontium (AS-C), and 30 had atherosclerosis coronary heart disease with generalized periodontitis (AS-P). All individuals’ demographic data recorded, saliva and blood samples collected, then periodontal characteristics were detailed. These parameters include plaque index, bleeding on probing, probing pocket depth, and clinical attachment loss. AIM2 and Pycard gene polymorphisms were analyzed by polymerase chain reaction assay, electrophoresis and sequencing. An enzyme-linked immunosorbent assay (ELISA) was conducted to determine the level of interleuken-18 and gasdermin D in their saliva.
Results: The study result of high frequency (T) in single-nucleotide polymorphisms. The high genotypes distribution of GT and TT genotypes in the AIM2 gene and the CT and TT genotypes in the Pycard gene were detected in the periodontitis, atherosclerosis coronary heart disease with healthy periodontium and atherosclerosis coronary heart disease with generalized periodontitis groups as compared to control group. Elevation of salivary interleuken-18 and gasdermin D levels in three patients’ groups compared to healthy controls. Both these single-nucleotide polymorphisms also significantly correlated with higher salivary interleuken-18 and gasdermin D levels and worse clinical indices of periodontitis.
Conclusion: Single-nucleotide polymorphisms in the AIM2 and Pycard genes are associated with an increased risk of developing periodontitis with and/or without coronary heart disease. Elevation of salivary interleuken-18 and gasdermin D levels associated and impacted on periodontitis with and/or without coronary heart disease. These single-nucleotide polymorphisms may provide evidence for a genetic role in the pathogenesis of periodontitis with and without atherosclerosis coronary heart disease.
Keywords: periodontitis, coronary heart disease, single nucleotide polymorphisms, interleuken-18, gasdermin D
Introduction
The alveolar bone and connective tissues that keep teeth in place are destroyed by periodontitis, which is an infected inflammatory disease. The pathobionts also cause tissue damage by switching from a symbiotic relationship to a dysbiotic state.1,2 Genotypes significantly govern immunological responses while the dysbiotic flora plays a role in regulating the response. Genetic variables significantly affect immunological responses to bacterial infection by extension, disease distribution, severity, and spread variation.3
Atherosclerosis (AS) is an inflammatory disease that develops when the immune system initiates, spreads, and activates lesions across the cardiovascular system in response to an increase in circulating low-density lipoprotein (LDL) and cholesterol. Atherosclerosis is a manifestation of coronary heart disease (CHD). As the correlation between ASCHD and periodontitis has yet to be fully understood, this present study attempts to examine it from the perspective of gene polymorphisms.4,5
The AIM2 sensor protein, an adaptor molecule much like the apoptosis-associated speck-like containing a CARD domain (Pycard) with a Caspase recruitment domain (CARD) and caspase are components of the inflammasome. The AIM2 inflammasome is a protein complex that forms in response to either exogenous or sterile signals. The auto-proteolytic maturation of caspase-1 triggers the production of interleukin-1 (IL-1), IL-18, and gasdermin N-terminal; all of which are secreted into the extracellular space during the pyroptosis process. It also associates with inflammatory periodontal soft tissue damage, osteoclastogenesis, and atherogenesis.6–8
The pathogenesis of periodontal disease includes inflammatory and bacteria responses which may determine an increased host response subsequent to the presence of pathogenic oral biofilm in gingival tissues.9 More specifically, periodontal disease has been correlated an increase of levels of some systemic inflammatory mediators in serum and saliva, such as interleukin 1 (IL-1), IL-6, IL-18 and gasdermin D (GSDMD). It was demonstrated that the inflammatory mediators increased levels after exposure to pathogenic bacteria caused endothelial cell dysfunction and represent potent mediators of vascular inflammation. The expression of several proinflammatory cytokines were strongly associated in the gingival tissue and endothelial cells during periodontitis9,10.
During the last few decades, several evidences have analysed the association between periodontal disease, endothelial dysfunction, and increased risk of CHD and cardiovascular disease (CVD).11 For these causes, there is growing interest aimed to investigate in some other oral mediators that can impact the subclinical endothelial dysfunctions as an early sign of augmented risk of CHD and CVD.
Multiple studies have reported an association between single nucleotide polymorphisms (SNPs) in the component genes of inflammasomes and increased susceptibility to periodontitis and CHD across various ethnicities.12,13
Principally, the AIM2 inflammasomes and ASC adopter play important role in host defense against activating signals in periodontal disease and coronary heart disease.14,15
Few number of reports have been published about associations have also been found between genetic disorders; such as Beçhet illness, psoriasis, and periodontal disease; and variations in the AIM2 gene, which serves as double-stranded deoxyribonucleic acid (dsDNA) receptors.16 Some studies have also established an association between variations in the Pycard gene to cancer.17
Interestingly, there is no study has suggested an association between SNPs in the AIM2 and Pycard genes with susceptibility to periodontitis with and without CHD. Therefore, this gives light to the present study, and it is aimed to examine the associations between SNPs in the AIM2 and Pycard genes with susceptibility to periodontitis with and without CHD, to determine IL-18, GSDMD levels in the saliva of periodontitis with and without CHD patients, as well as assess the correlation between these SNPs with the level of IL-18, GSDMD and their clinical periodontal parameters.
Materials and Methods
Study Design
The current investigation is a case-control study. The study was conducted at the Department of Periodontics at Al-Ameed University Dentistry College and Hospital, Karbala Centre of Specialized Dental, the Imam Al-Hussain Medical City, or/and the Karbala Centre of Cardiovascular Diseases and Surgery between April to October 2022. The present study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) in terms of design of the study and results reporting.
In this study, we retrospectively analyzed 120 participants according to inclusion and exclusion criteria. The primary objective was to assess if there are correlations between SNPs of AIM2, Pycard genes and susceptibility to periodontitis with and without CHD. The secondary objective was to assess the level of IL-18 and GSDMD, the clinical periodontal parameters.
Study Subjects
The participants recruited in the present study were males and females, aged range (35–75) years, all with BMI <25kg/m2. At first, the diagnosis periodontitis case according to 2017 periodontitis classification, the participants who had detectable generalized extent, interdental CAL at ≥2 non-adjacent teeth, or ≥3 mm CAL on the buccal (facial) or lingual/palatal aspects, associated with PPD > 3 mm at ≥2 teeth periodontitis, stage (III, IV), grade (B, C) with unstable cases (PPD ≥ 4 mm with BOP or PPD >5 mm with or without BOP) for periodontitis groups.18 The CHD patients diagnosis via clinical dyspnea, chest ache and change electrocardiogram ECG record, laboratory raised the blood total cholesterol (TC), low-density lipoprotein LDL, triglyceride (TG) levels, decreased high-density lipoprotein (HDL) level, investigational via noninvasive screening tests coronary computerized tomography (CT) angiography with constructive plaque/vague outcomes in coronary arteries and diagnostic cardiac catheterization procedure with existing atherosclerotic plaque lesions by over 50%.19–22 Subsequently, recorded demographic data and saliva and blood samples were accumulated from all participants, then clinical examinations have been detailed.
Inclusion Criteria
The following participants were considered for recruitment in the study:
- A willingness to participate in the study.
- Adult individuals age range (35–75) years.
- The adult subjects with BMI <25kg/m2.
- Patients who apply the diagnostic criteria which are as follows:
- The subjects had at least 20 healthy teeth.
- The subjects have good overall health with no history of any systemic diseases other than ASCHD in atherosclerotic coronary heart disease groups.
- The participants were diagnosed with periodontitis stages III and IV, grades B and C with unstable status patient should be included in periodontitis patient groups.
- The systemically healthy patients, with clinically healthy periodontium, who were included in this study had BOP < 10%, PPD ≤ 3mm, and intact periodontium,23 for the control group participants.
Exclusion Criteria
The following patients were excluded from the study:
- Patients with systemic diseases that are known to affect the development of periodontal disease.
- Patients had periodontal treatment in the last six months.
- Patients were smokers or had other behavioural variables.
- Patients had been treated with corticosteroids or antibiotics in the last three months.
- Patients were pregnant or nursing at the time of the study.
- Patients had implants or dentures.
- Patients had periodontitis with the molar-incisor pattern.
Sample Size
Based on the data obtained from the primary outcome of the pilot study to the genetic marker (gene polymorphism of AIM2 and Pycard) for the study groups. The sample size was calculated utilizing Odd/Ratio and https://epitools.ausvet.com.au/casecontrolss. A total number of 120 participants (30 subjects per Group) were calculated with a test power of 80% and an α probability of 0.05.
Periodontal Parameters Examination
The periodontal parameters evaluated clinically for every tooth were presented in their mouths, involving full mouth plaque index (PLI),24 full mouth BOP,25 PPD, and CAL. A Hu‐Friedy University of North Carolina (UNC) 15 probe was used to assess periodontal parameters. The percentage of bleeding that occurred at six sites per tooth; namely, the mesiofacial, facial, distofacial, mesiolingual, lingual, and distolingual regions; was assessed to determine the PPD and CAL and recorded to the nearest millimeter. The percentage of O’Leary PLI scores at four surfaces visually was also recorded. Exclusion from the examination was the wisdom teeth.
Calibration
The calibrated periodontal examinations were completed before the study started and conducted at the Department of Periodontology at the University of Al-Ameed, College of Dentistry and Hospital. The reliability of this present study depended on an analysis of intra‐examiner calibration. Calibration sessions for the clinical periodontal parameters data were recorded twice within an hour of examining five non‐study patients who had generalized periodontitis. The intraclass correlation coefficient (ICC) was 0.94 for pocket probing depth (PPD) and 0.88 for clinical attachment level (CAL), whereas the average kappa coefficients value was 0.89 for bleeding on probing (BOP) and 0.91 for plaque index (PLI). Thus, the study’s degree of reliability was satisfactory.
Saliva Sample Collection and Analysis
The participants were encouraged to practice good oral hygiene by brushing their teeth and flossing. Prior to the oral examination, saliva samples were collected in a clean plastic cup via passive drooling at 09:00 to 12:00 hours. A micropipette was then used to transfer 300 μL of the saliva sample into an Eppendorf tube for centrifugation at 3000 G rpm for 20 min (Thermo Scientific, USA) before being stored at −20°C.
An ELISA (MyBioSource©, San Diego, California, USA) was carried out according to the manufacturer’s instructions to determine the level of IL-18 and GSDMD present in the saliva.
Blood Sample Collection and Genotype Estimation
2 mL of peripheral blood was collected within 30 seconds and placed in a tube containing ethylenediaminetetraacetic acid (EDTA). A ReliaPrep™ Blood gDNA Miniprep System (Promega, USA) DNA extraction kit was used according to the manufacturer’s instructions to extract the genomic DNA from the blood samples. The DNA concentration of the blood was assessed using a Macrogen® QuantiFluor® dsDNA System Primer (Korea). A functional primer solution was obtained by dissolving the lyophilized form in nuclease-free water. The optimal annealing temperature for the primer was investigated. Using the identical Forward and Reverse primer combination at the annealing temperature, the DNA template was amplified.
A Veriti™ Thermal Cycler (USA) polymerase chain reaction (PCR) assay was used to detect polymorphisms. This was amplified before an agarose gel electrophoresis was conducted to confirm the presence of amplifications. The PCR products were Sanger sequenced in a Macrogen® ABI3730XL Automated DNA Sequencer (Korea). Geneious software for sequence data analysis was then used to analyze the results.
Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the University of Baghdad, College of Dentistry, Iraq (number 652,622) that gave their stamp of approval to the study’s moral foundations. All the human research protocols complied with the ethical standards laid out in the Helsinki Statement and its later modifications. Each participant signed an informed consent form after being provided detailed information about the study and its purposes.
Statistical Analysis
GraphPad® Prism version 9.0 (GraphPad Software Inc., La Jolla, CA) was used to conduct the statistical analysis. Continuous and categorical variables were analyzed using univariate descriptive statistics revealing the means and standard deviations. A one-way analysis of variance (ANOVA) was also conducted on each of the independent variables of the four groups. While a chi-square test for Hardy-Weinberg equilibrium (HWE) was conducted on each of the SNPs of the four groups separately. The univariable correlations between genetic variations and susceptibility to periodontitis or CHD were assessed using the chi-squared test while the OR was used to estimate the genotype distribution and allele frequency. The AIM2 G/T(rs2793845), the Pycard C/T (rs8056505) genes and the variables were correlated using the Binary logistic regression. For the present study, P < 0.05 was taken to indicate statistical significance.
Results
Study Population
A total of 1370 patients screened,387 individuals refrained from participating in the present study while 863 were excluded as present in exclusion criteria. The study included 120 participants who met the eligibility criteria and agreed to be recruited in the present study. They were equally divided into four groups: 30 individuals serving as healthy controls (group C), 30 in the generalized periodontitis group (P), 30 in the AS coronary heart disease with clinically healthy periodontium group (AS-C), and 30 in the AS coronary heart disease with generalized periodontitis group (AS-P), as illustrated in (Figure 1).
 |
Figure 1 Flowchart of the study design for CHD and periodontitis patients. |
Patient Demographics and Periodontal Parameters
The demographic characteristics of all the participants; namely, age, BMI, and gender were non-significantly different among the study groups. The participants recruited in the present study were 95 males and 25 females. Male patients are predominant, representing 80% of all participants included in the study. The mean age was 56.17 years. We observed a predominance of BMI of patients representing 21kg/m2 included in the study. The clinical periodontal parameters were demonstrated to be statistically greater for the P and AS-P groups with and without diagnosed CHD, compared to the periodontal healthy participants, C and AS-C (all p < 0.001) as revealed in (Table 1).
Salivary IL-18 and GSDMD Protein Levels
The P (65.862 pg/mL), AS-C (102.418 pg/mL), and AS-P (145.773 pg/mL) groups had higher significant mean levels of IL-18 in their saliva than the C group (6.594 pg/mL) (Table 1) (Figure 2).
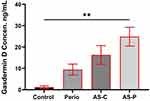
This study revealed a significant elevation in the mean salivary levels of GSDMD in (P, 8.647 ng/mL; AS-C, 14.772 ng/mL; AS-P, 20.894 pg/mL) groups compared to the control group (C, 1.019 ng/mL) (p < 0.001) as revealed in (Table 1) (Figure 3).
AIM2 and Pycard Single Nucleotide Polymorphisms Analysis
The genotype distributions of SNPs in the AIM2 G/T (rs2793845) and Pycard C/T (rs8056505) gene did not differ significantly from those obtained from the chi-square test for Hardy-Weinberg equilibrium (HWE) of all the groups (Table 2 and Table 3).
An examination of the genotype distributions in the SNP of the AIM2 gene of the 120 participants revealed that the OR of the TT and GT genotypes were higher significantly (p ≤ 0.01) in the P, AS-C, and AS-P groups than in the Control group (Table 2, Figure 4).
The T allele of the SNP of the AIM2 G/T (rs2793845) gene appeared more frequently in the P (0.85), AS-C (0.72), and AS-P (0.80) groups than Control group (Table 2).
An examination of the genotype distributions in the SNP of the Pycard gene of the 120 participants revealed that the OR of the TT and CT genotypes were higher significantly (p ≤ 0.01) in the P, AS-C, and AS-P groups than the Control group (Table 3, Figure 5).
The T allele of the SNP of the Pycard C/T (rs8056505) gene appeared more frequently in the P (0.74), AS-C (0.8), and AS-P (0.7) groups than the Control group (Table 3).
The Correlations Between SNPs of AIM2 G/T (rs2793845), Pycard C/T (r rs8056505), and IL-18, Demographic Data and Clinical Variables
Table 4 provides the correlations between the AIM2 G/T (rs2793845) and Pycard C/T (rs8056505) genes with the age, BMI, gender, clinical periodontal parameters, and IL-18 levels of all the groups. Both the PPD, and CAL of the P group, and AS-P group revealed positive and significant correlations with the genes. Further, a positive and significant correlation was observed between the genes and the IL-18 and GSDMD of the P group, AS-C group, and AS-P group with the two genes, which had a significant impact on the numbers (P<0.05) based on the Binary logistic regression.
 |
Table 4 The Association Between Mean Values of Age, Gender, BMI, the Clinical Parameters, IL-18, GSDMD and SNPs of AIM2 G/T (rs2793845), Pycard C/T (r rs8056505) of Patients Groups |
Discussion
This present study found the associations between inflammation-related human genetic variants and increase susceptibility to development periodontitis with and without CHD via their pathological genotypes effecting, which contribute to the pathogenesis of both diseases. Further, there was a significant increase in the mean levels of IL-18 and GSDMD in the saliva of periodontitis with and without CHD patients than the control group that determined the association and impact on periodontitis and CHD. A significant correlation was also observed between these genes and salivary IL-18, GSDMD levels and clinical periodontal parameters.
Significantly higher T allele frequencies and GT and TT genotype distributions in the AIM2 (rs2793845) gene were found in the blood samples of the P, AS-C, and AS-P groups than GG genotype distributions as compared to the control group. Therefore, this pathological genotype was associated with an increased risk of developing periodontitis, CHD, or both. The examination of the SNP revealed the association between the downstream regulation area of the AIM2 gene and the onset of periodontitis and CHD. Therefore, this SNP affects the abnormal gene expression, activates the AIM2 protein and host mediators, leading to periodontal tissue breakdown, dysfunctional endothelial cells and atherosclerotic plaque forming. The SNP alleles affect the inflammatory process of periodontitis and CHD by varying the modified regulatory function.
Only a handful of human genetic variations of the AIM2 gene have been studied concerning inflammatory disorders. According to Marchesan et al, the allele frequency and genotype distributions of the SNP of the AIM2 gene may be a major factor in what causes periodontal disease.26 However, Figueira et al conversely reported that individuals with changes in the SNP of the AIM2 gene had a significantly lower risk of contracting pulmonary tuberculosis (PTB) than the healthy control group.27
Significantly higher T alleles frequencies and CT and TT genotype distributions in the Pycard gene (rs8056505) gene were found in the blood samples of the P, AS-C, and AS-P groups than CC genotype distributions which were compared to the control group. Therefore, this pathological genotype was associated with an increased risk of developing periodontitis, CHD, or both. The examination of the SNP of the Pycard gene revealed that it is located in the 5’ untranslated promotor region; where the majority of the regulatory components are contained including the proximal promoter, which contains the cis-regulatory elements for immunological and tissue-resident cells. In both periodontitis and atherogenesis, an epigenetic modifier has been related to causing the abnormal expression of certain proteins (ASC protein) which, in turn, causes the degeneration of critical cellular processes. Furthermore, Pycard gene variation with other factors may collectively trigger the process of pyroptosis. The study of Šutić et al found that the promotor regions of the Pycard gene may help distinguish between lung cancer and non-cancerous tissue samples.28 Mamoor similarly found that sequence variations in the Pycard gene may play a role in the understanding of luminal breast cancers in humans.29
Salivary IL-18 levels were considerably greater in the P, AS-C, and AS-P groups as compared to the control group. Therefore, IL-18 may play a regulatory role in inflammation and tissue damage as both a pro-inflammatory and anti-inflammatory cytokine. Higher levels of IL-18 have been linked to a decline in periodontal health, malfunction in the blood vessel cells and development of atherosclerosis plaque. Similar findings were reported by Zhang et al and Jefferis et al, who found that the pleiotropic functions of IL-18, the excessive levels of IL-18 have been found to damage the bone and soft tissues of the periodontium, malfunction the cells of the blood vessels, causing inflammation, and facilitate the development of atherosclerotic plaques.30,31
This study found a significant elevation in salivary GSDMD levels in P, AS-C, AS-P patient groups compared to control group. The explanation of that GSDMD is a proinflammatory pore-forming mediator that plays a vital regulatory role in inflammation and tissue damage by carrying out the execution phase of pyroptosis. Periodontal disease, endothelial cell malfunction, and cardiomyocyte pyroptosis all resulted from its rise. This was in accordance with the studies by Zhuang et al and the study of Weng et al. They found that activating GSDMD altered the pyroptotic rate of human periodontal ligament cells, which in turn resulted in the formation and release of interleukin (IL)-1 and interleukin (IL)-18.32,33 This study result is similar to the study of Shi et al that revealed GSDMD levels were high in the blood serum, it produced as a result of hypoxia/reoxygenation in cardiomyocytes that undergo pyroptosis.34
Multiple studies have reported a correlation between periodontitis and atherosclerosis CHD. However, subgingival periodontal pathogens are the primary cause of the inflammatory processes via increased levels of systemic inflammatory mediators that subsequently lead to the destruction of periodontal tissue. When periodontal pathogens have entered the blood stream, they can cause bacteraemia and/or endo-toxaemia as well as inflammatory changes in vessel walls, induce the expression of inflammatory cytokines; such as IL-1β; upregulate endothelial adhesion molecules, and create a prothrombotic environment; all of which could contribute to inflammation-associated atherosclerosis.35,36 Patients with atherosclerosis and CHD have been seen a significant increase IL-1β levels due to oxLDL and live bacteria have been recovered from atherosclerotic plaques, which directly stimulate atheroma formation and maturation and can exacerbate periodontal destruction.19,37 Thus, the impact of periodontal pathogens and elevation of inflammatory mediators including IL-18 and GSDMD are increased the risk of CHD.
The demographic variables assessed in this present study; specifically, age and BMI; were relevant in the four groups. The mean value of BMI was within the ordinary range for all experimental groups to prevent confounding effects.
Most studies on the Iraqi population report a higher prevalence of periodontitis in males than females. This may be due to the differences in the hygiene practices or socioeconomic status of the two groups.38–42
In this present study, periodontal parameters; such as PLI, BOP, PPD, and CAL; were higher in the AS-P group than in the other three groups. Al-Taweel et al,19 Yagnik et al,20 Al-Ghurabi et al,43 and Shaker and Hashem44 reported similar findings and concluded that the inflammatory response due to plaque biofilms, which produce an extensive variety of bacterial by-products; such as toxins, enzymes, and hydrogen sulphide (H2S); illicit accelerate periodontal tissue deterioration; such as periodontal ligaments loss, pocket formation, bone resorption, and tooth loss. This facilitates the onset and progression of CHD and may change the periodontal environment via primary inflammatory pathways.
Binary logistic regression results of this present study indicate that the SNPs of the AIM2 and Pycard genes significantly correlated with clinical periodontal parameters and salivary IL-18 and GSDMD. This was due to the presence of underlying inflammatory and molecular pathways that explain the pathogenesis of periodontitis and atheroma in CHD.
As the SNPs of the AIM2 and Pycard genes have been found to be associated with disease development and severity, not only do they provide light on how diseases manifest, but they also have the potential to be used as a new therapeutic method in the fight against and treatment of conditions including periodontal disease and coronary heart disease.
a-Data Sharing Statement: All the genetic sequences are available at (http:/www.ncbi.nlm,nih.gov/genbank). The Gene Bank accession numbers are: LC741257, LC741258, LC741259, LC741260, LC741261, LC741262, LC741263, LC741264, LC741265, LC741266, LC741267, LC741268, LC741269, LC741270, LC741271, LC741272, LC741354, LC741355, LC741356, LC741357, LC741358, LC741359, LC741360 and LC741361.
Conclusion
Highly pleiotropic genetic variations in the T allele of the GT and TT genotypes of the AIM2 inflammasome and the CT and TT genotypes of the Pycard gene in periodontitis with and without CHD indicate an increased susceptibility to both conditions. These SNPs in the AIM2 and Pycard genes may alter epigenetic gene regulation leading to aberrant cytokine production in periodontitis and CHD. The IL-18 and GSDMD pro-inflammatory cytokines of the host response are produced against activating signals in coronary heart disease and periodontal disease that caused inflammation and tissue damage. Periodontitis with and without CHD patients have significantly higher levels of salivary IL-18 and GSDMD. These biomarkers are associated and impacted on periodontitis with and without CHD. Moreover, these two SNPs are significantly correlated with salivary IL-18, GSDMD levels and periodontal parameters. This correlation may be explained by the existence of underlying inflammatory and molecular pathways of the pathogenesis of both illnesses.
Funding
Self-funded.
Disclosure
The authors declare no conflicts of interest related to this work.
References
1. Orlandi M, Graziani F, D’Aiuto F. Periodontal therapy and cardiovascular risk. Periodontal. 2000;83:107–124.
2. Abdulkareem AA, Abdulbaq HR, Milward MR. In vitro homeostasis of rat oral epithelial cell cultures following withdrawal of periodontal pathogens. Braz Dent J. 2020;31(2):135–142. doi:10.1590/0103-6440202002561
3. Laine ML, Crielaard W, Loos BG. Genetic susceptibility to periodontitis. Periodontal. 2000;58(1):37–68. doi:10.1111/j.1600-0757.2011.00415.x
4. Herrera D, Molina A, Buhlin K, Klinge B. Periodontal diseases and association with atherosclerotic disease. Periodontal. 2000;83:66–89.
5. Zardawi F, Gul S, Abdulkareem A, Sha A, Yates J. Association between periodontal disease and atherosclerotic cardiovascular diseases: revisited. Front Cardiovasc Med. 2021;7:625579. doi:10.3389/fcvm.2020.625579
6. Al-Obaidi MJ, Al-Ghurabi BH. Potential role of NLRP3 inflammasome activation in the pathogenesis of periodontitis patients with type 2 diabetes mellitus. Med Chem Sci. 2023;6(3):522–531.
7. Turer CC, Durmus D, Balli U, Guven B. Effect of non-surgical periodontal treatment on gingival crevicular fluid and serum endocan, vascular endothelial growth factor-A, and tumor necrosis factor-alpha levels. Periodontal. 2017;88:493–501.
8. Al-Ghurabi BH. The role of soluble TLR-2 in the immunopathogenesis of Gingivitis. Internat Med. 2021;28(1):37–39.
9. Isola G, Polizzi A, Alibrandi A, Indelicato F, Ferlito S. Analysis of Endothelin-1 Concentrations in Individuals with Periodontitis. Sci Rep. 2020;10(1):1652. doi:10.1038/s41598-020-58585-4
10. Isola G, Polizzi A, Alibrandi A, Williams RC, Giudice AL. Analysis of galectin-3 levels as a source of coronary heart disease risk during periodontitis. J Periodontal Res. 2021;56(3):597–605. doi:10.1111/jre.12860
11. Holtfreter B, Empen K, Gläser S, et al. Periodontitis is associated with endothelial dysfunction in a general population: a cross-sectional study. PLoS One. 2013;8(12):e84603. doi:10.1371/journal.pone.0084603
12. Wang Y, Liu X, Shi H, et al. NLRP3 inflammasome, an immune-inflammatory target in pathogenesis and treatment of cardiovascular diseases. Clin Transl Med. 2020;10(1):91–106. doi:10.1002/ctm2.13
13. Schunk SJ, Kleber ME, März W, et al. Genetically determined NLRP3 inflammasome activation associates with systemic inflammation and cardiovascular mortality. Eur Heart J. 2021;42(18):1742–1756. doi:10.1093/eurheartj/ehab107
14. Sharma BR, Karki R, Kanneganti T-D, et al. Role of AIM2 inflammasome in inflammatory diseases, cancer and infection. Eur J Immunol. 2019;49(11):1998–2011. doi:10.1002/eji.201848070
15. Marchesan JT, Girnary MS, Moss K, et al. Role of inflammasomes in the pathogenesis of periodontal disease and therapeutics. J Periodontol. 2000;82(1):93–114. doi:10.1111/prd.12269
16. Wang J, Gao J, Huang C, et al. Roles of AIM2 Gene and AIM2 Inflammasome in the Pathogenesis and Treatment of Psoriasis. Front Genet. 2022;13:929162. doi:10.3389/fgene.2022.929162
17. Koizumi M, Watanabe T, Masumoto J, et al. Apoptosis-associated speck-like protein containing a CARD regulates the growth of pancreatic ductal adenocarcinoma. J Scient Repor. 2021;11(1):22351.
18. Tonetti MS, Greenwell H, Kornman KS. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. Periodontal. 2018;89(1):159–s172.
19. Al-Taweel FBH, Saliem SS, Abd OH, Whawell SA. Assessment of Serum Interleukin-1β and Interleukin-6 Levels in Patients with chronic periodontitis and coronary heart disease. Eur J Gen Dent. 2021;10:78–83. doi:10.1055/s-0041-1732954
20. Yagnik K, Mahendra J. The Periodontal‐Cardiovascular alliance: evaluation of miRNA‐146a in subgingival plaque samples of chronic periodontitis patients with and without coronary heart disease. J Invest Clin Dent. 2019;00:e12442. doi:10.1111/jicd.12442
21. Bagavad Gita J, George AV, Pavithra N, Chandrasekaran SC, Latchumanadhas K, Gnanamani A. Dysregulation of miR-146a by periodontal pathogens: a risk for acute coronary syndrome. J Periodontol. 2019;90(7):756–765. doi:10.1002/JPER.18-0466
22. Temelli B, Yetkin Z, Savas H, et al. Circulation levels of acute phase proteins pentraxin 3 and serum amyloid A in atherosclerosis have correlations with periodontal inflamed surface area. J Appl Oral Sci. 2018;26:e20170322.
23. Chapple ILC, Mealey BL, Van Dyke TE, et al. Periodontal health and gingival diseases and conditions on an intact and a reduced periodontium: consensus report of workgroup 1 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. Clin Periodontal. 2018;45(20):S68–S77.
24. O’Leary TJ, Drake RB, Naylor JE. The plaque control record. J Periodontol. 1972;43(1):38. doi:10.1902/jop.1972.43.1.38
25. Mühlemann HR, Son S. Gingival sulcus bleeding--a leading symptom in initial gingivitis. Helv Odontol Acta. 1971;15:107–113.
26. Marchesan JT, Jiao Y, Moss K, et al. Common polymorphisms in the IFI16 and AIM2 genes are associated with periodontal disease. Periodontal. 2017;88(7):663–672.
27. Figueira MBD, de Lima DS, Boechat AL, et al. Single-Nucleotide Variants in the AIM2 – absent in Melanoma 2 Gene (rs1103577) AssociatedWith Protection for Tuberculosis. Front Immunol. 2021;12:604975. doi:10.3389/fimmu.2021.604975
28. Šutić M, Motzek A, Bubanović G, Linke M, Sabol I, Vugrek O. Promoter methylation status of ASC/TMS1/PYCARD is associated with decreased overall survival and TNM status in patients with early stage non-small cell lung cancer (NSCLC). Transl Lung Cancer Res. 2019;8(6):1000–1015. doi:10.21037/tlcr.2019.12.08
29. Mamoor S. A single nucleotide variant on chromosome 16 residing within PYCARD distinguishes patients with luminal A breast cancer. Cancer. 2022.
30. Zhang Y, Kuang W, Li D, et al. Natural killer-like B cells secreting interleukin-18 induces a proinflammatory response in periodontitis. Front Immunol. 2021;12:
31. Jefferis BJMH, Papacosta O, Owen CG, et al. Interleukin 18 and coronary heart disease: prospective study and systematic review. Atheroscl J. 2011;217(1):227–233. doi:10.1016/j.atherosclerosis.2011.03.015
32. Zhuang J, Wang Y, Qu F, Wu Y, Zhao D, Xu C. Gasdermind played a critical role in the cyclic stretch–induced inflammatory reaction in human periodontal ligament cells. BMC Immunity Ageing J. 2019;42:548–558.
33. Weng Y, Ye B, Lin J, et al. Elevated circulating levels of gasdermin D are related to acute myocardial infarction and pyrogptosis. BMC Cardiovascular Disorders J. 2022;22(1):554. doi:10.1186/s12872-022-02998-8
34. Shi H, Gao Y, Dong Z. GSDMD-mediated cardiomyocyte pyroptosis promotes myocardial I/R Injury. Circ Res. 2021;129(3):383–396. doi:10.1161/CIRCRESAHA.120.318629
35. Schulz S, Schlitt A, Hofmann B, Schaller H, Reichert S. Periodontal pathogens and their role in cardiovascular outcome. J Clin Periodontol. 2020;47(2):173–181. doi:10.1111/jcpe.13224
36. Meurman JH, Sanz M, Janket SJ. Oral health, atherosclerosis, and cardiovascular disease. Crit Rev Oral Biol Med J. 2004;15(6):403–413. doi:10.1177/154411130401500606
37. Zoheir N, Kurushima Y, Lin G, Nibali L. Periodontal infectogenomics: a systematic review update of associations between host genetic variants and subgingival microbial detection. J Clin Oral Investig. 2022;26(3):2209–2221. doi:10.1007/s00784-021-04233-8
38. Hussein HM, Mahmood AA, Alberaqdar FA. The prevalence and relationship of root caries depth and gingival recession among different Iraqi groups. Mustansiria Dental J. 2015;12(1):144–155.
39. Saeed NA, Hussein HM, Mahmood AA. Prevalence of dental anxiety in relation to sociodemographic factors using two psychometric scales in Baghdad. Mustansiria Dental J. 2017;14(1):38–50. doi:10.32828/mdj.v14i1.753
40. Fadhil R, Al Ghurabi BH, AL-Ghaban NMH. Nuclear Factor-kappa B Gene Polymorphism and Interleukin-8 in Iraqi Population with Severe Chronic Periodontitis. Glob Pharm Technol. 2019;11(9):181–186.
41. Abdulkareem AA, Abdulbaqi HR, Nayyef HK, Saleem SS. Investigation of the consistency between reported chief complaint and periodontal health status of Iraqi patients in relation to age and gender (A retrospective study). J Bagh College Dentistry. 2019;31(2):65–69. doi:10.26477/jbcd.v31i2.2626
42. Abbas MJ, Albaaj FS, Hussein HM, Mahmood AA. Importance of preventive dentistry in the elderly: a personal approach. Dent Res J. 2022;19:11. doi:10.4103/1735-3327.338774
43. Al-Ghurabi BH. Evaluation of serum anti-Cardiolipin antibody, hs-CRP and IL-6 levels in chronic periodontitis as possible risk factors for cardiovascular diseases. J Bagh College Dentistry. 2012;24(2):161–165.
44. Shaker ZF, Hashem BH. Study the role of proinflammatory and anti- inflammatory cytokines in Iraqi chronic periodontitis patients. J Bagh College Dentistry. 2012;24(1):164–169.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.