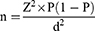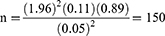Back to Journals » Clinical Audit » Volume 16
Assessment of the Usage, Storage, and Expiration Date Checking of Drugs at Dilla University Teaching Hospital
Authors Wonte MM , Aweke Z , Getachew H , Ali SA, Tadesse M
Received 8 September 2023
Accepted for publication 22 December 2023
Published 4 January 2024 Volume 2024:16 Pages 1—7
DOI https://doi.org/10.2147/CA.S435155
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zoka Milan
Mesay Milkias Wonte, Zemedu Aweke, Hailemariam Getachew, Siraj Ahmed Ali, Muhiddin Tadesse
Department of Anesthesiology, Dilla University College of Health Science and Medicine, Dilla, Ethiopia
Correspondence: Mesay Milkias Wonte, Department of Anesthesia, Dilla University College of Health Sciences and Medicine, PO. BOX: 419/13, Dilla, Ethiopia, Tel +251949180127, Email [email protected]
Introduction: Medications require suitable storage conditions to ensure the potency and efficacy of medicines. Appropriate putting-away conditions are required to ensure the potency of medications. Medication that is not maintained at the required temperature may further increase the unnecessary burden on the general population’s economy due to their lack of potency and efficacy. It is crucial to reduce any wastage, including that of drugs, to increase the efficient use of these scarce resources because there are significant health implications of drug waste.
Methodology: A descriptive study was done at Dilla University Teaching Hospital beginning November 02, 2022, to December 02, 2022. Twenty indicators were used to assess the practice of drug storage, utilization, and checking expiration dates were designed and variables requiring a definition for the fullness of the checklist were predefined. For each measure, the expected achievement rate was 100%. A less than 50% completion rate was seen as a critical area in need of development, while indicators with a greater than 90% attainment rate were rated as acceptable. To analyze the data, SPSS version 26 was used.
Results: The study found that none of the indications for the use, storage, and checking of drug expiration dates had a 100% completion rate. Among the indicators found to be below average (50%) were medical gas storage, medication storage, critical medication arrangement, storage of medications in refrigerators without using refrigerators for other purposes, storage facilities below 25°C, presence of lockable cupboards, and storage of internal medications such as oral liquids, injectable medications, rectal medications, and segregation.
Conclusion: In conclusion, the majority of the indications for the usage, storage, and checking of the expiration date of drugs were discovered to be nonexistent or done insufficiently. To improve the practice of medicine storage, use, and expiration date checking, we recommend several strategies such as regular evaluation and monitoring.
Keywords: drug storage, efficacy, medication wastage, potency
Introduction
Background
To ensure the quality and efficacy of medications, the proper storage conditions are crucial. Medication lacks the required potency and efficacy to effectively treat disease, if they are not stored at the required temperature (25°C), it might further raise the unnecessary burden on the economy of the general population.1 The development of acceptable storage settings, retest intervals, and drug shelf life benefits from knowledge of the consequences of ecological elements including temperature, humidity, and light.2
Loss of efficacy, safety, potency, and the production of dangerous compounds are all side effects of expired medications. They can also be a source of accidental poisoning and abuse (such as opioids), so there needs to be a proper mechanism in place for getting rid of them.3,4 A medicine is no longer potent enough to be used as manufactured after its expiration date. By examining the date of expiration on the pharmaceutical container, customers can determine the shelf life of a drug.5
Storage of expired medications could be brought about by inadequate prediction of future demand, poor management of a supply chain, and a lack of collaboration between the national supply system and the development partners proposing to supply medications. Similarly, patients may not be ready to take all of the prescribed prescriptions due to adverse effects, dosage changes, feeling well, or medication expiration dates.5
Medication waste is any pharmaceutical product that is not completely utilized or remains unused throughout the pharmaceutical supply and usage chain.6 There are various important public health implications of drug loss, rendering minimizing any wastage, including that of medications, a critical goal in improving the appropriate usage of these limited resources. Like the financial burden that unutilized pharmaceuticals place on people and communities, or the environmental problems linked with waste disposal and the way they affect society.7–10
Because it has a detrimental effect on the environment and the healthcare budget, drug waste generally burdens society. Reducing this unnecessary drug waste is an interesting solution to this issue. Implementing prescription waste-reduction strategies is a shared responsibility of manufacturers, distributors, doctors, pharmacists, and patients. To ensure a sustainable supply, usage, and effects of medication, health authorities need to force hospitals to take responsibility and work together.6
Any drug considered to be potentially dangerous should also be stored separately and, if possible, eliminated. Wherever possible, “sound-alike” medications (such as magnesium sulfate and potassium chloride, pancuronium, and oxytocin) should be physically separated from one another. To help prevent accidental mis-selection, the concentration of pharmaceuticals should be standardized (eg, heparin, morphine, and midazolam), and only one concentration should be kept on hand whenever possible.11 This descriptive study aims to enhance the practice of drug storage, utilization, and checking expiration dates at Dilla University Teaching Hospital.
Methods and Materials
From November 02 to December 02, 2022, a descriptive study was carried out to evaluate the practice of medicine storage, use, and verifying expiration dates at DUTH. The Dilla University Teaching Hospital is situated 360 kilometers from Addis Ababa’s capital city in Dilla Town, Gedeo Zone, South Nation, Nationalities and Peoples Region. Patients hospitalized in the hospital for a variety of diseases can choose from a wide choice of services. Every year, on average, 3800 people have both elective and emergency surgeries.
For the effective use of these scarce resources, proper drug storage, use, and expiration date checking are essential; unfortunately, most hospitals, including our institution, pay little attention to this key issue. To evaluate the practice of medicine storage, use, and monitoring expiration dates among health professionals, we conducted a pilot study which yielded a proportion of 11%. To determine the sample size, a single population proportion formula was used. Since there was no previous study done similar to this topic, we have conducted a pilot study and took a proportion of 11% by assuming a 95% CI with a 5% margin of error,
Where z is the z score
d is the margin of error
N is the population size
P is the population proportion
Six anesthetists were recruited to collect the data from six different wards, to select the ward we first conducted stratified sampling, and after that simple random sampling technique was used to take the samples from each ward.
To evaluate these variables, checklists were designed based on a combination of criteria defined by the Australian and New Zealand College of Anesthetists (ANZCA),12 National Institute for Health and Care Excellence (NICE),13 and National Health System (NHS).14 (Table 1) A pilot study was conducted and required modifications were completed before the real data collection. Twenty indicators have been developed and checked as “Yes” if the predetermined criteria are met, “No” if the predetermined criteria are not met, and “NA” if the predetermined conditions are not obligatory if not met. (Table 2) The variables’ constituent parts served to specify the completeness of the indicators. For all factors, a 100% completion rate was projected. A completion rate of more than 90% is considered satisfactory, and less than 50% was deemed to be an area that needs significant improvement. The checklist was filled out by direct observation of medical professionals as they treated patients, verifying pharmaceutical expiration dates before use, and storing medications after use. The institutional review board (IRB) of Dilla University approved.
 |
Table 1 Standards Used to Assess to Practice of Drug Storage, Utilization and Checking Expiry Date at Dilla University Teaching Hospital, December, 2022 |
 |
Table 2 The Practice of Drug Storage, Utilization, and Checking the Expiry Date at Dilla University Teaching Hospital, December 2022 (N=150) |
After receiving permission from the Dilla University Teaching Hospital, the data were gathered. The study was conducted per the Helsinki Declaration. All data were retrieved, gathered, and safeguarded by avoiding personal identifications, and only the authors had access to the full set of data. At every stage of this study, confidentiality and privacy were maintained. Using SPSS version 26, the data were entered, coded, and cleaned. The analysis was descriptive. The data were displayed using descriptions and tables, and any pertinent conclusions were expressed as frequencies and percentages (%).
To evaluate the practice of drug storage, use, and expiration date checking, standards from the National Health System (NHS), Australian and New Zealand College of Anesthetists (ANZCA), and National Institute for Health and Care Excellence (NICE) were compiled. As noted in the discussion section of this paper, additional international guidelines and national guideline recommendations from industrialized countries were also examined.
Result
In this descriptive study, a total of 150 checking events from different units were taken and 20 indicators were involved during the study period.
Indicators to assess the practice of drug storage, utilization, and checking expiration date in Dilla University teaching Hospital Obstetrics and Gynecology Ward 28 (18.67), Intensive Care Unit 26 (17.3), Emergency Ward 20 (13.3), Pediatrics Ward 24 (16), Operation room 30 (20), Medical ward 22 (14.67). (Frequency and Percentage (n (%))), N= 150 (Table 2).
Discussion
This study found that none of the variables on the checklist of the practice of drug storage, utilization, and checking expiration date had a completion rate of 100%. Medical gas storage, storage of medications including intravenous fluid, arrangement of medications of critical medicines, storage of medications in refrigerators and not using refrigerators for other items, recording of the date the drug opened, recording the calculated expiry date on the medicine package/label, availability master key to the staffs, storage facilities below 25°C, medicines kept in ways designed to help their identification, presence of lockable cupboards and are locking when not being accessed, storage of internal medications like oral liquids, injectable medications, rectal medications, and segregation was among indicators found below average (<50%).
Surprisingly the majority of the indicators are far from the standards. This points out that the health care providers gave minimal or no weight to the proper storage of medications, their utilization, and recording of the date the drug opened as well as recording calculated expiration date which directly influences the quality of patient outcomes and indirectly affects the economy of the individuals and the hospital. This result is coherent with the study done in Malta.15
The other indicator here which was given less emphasis was the safe and secure storage of medical gases in cylinders, 36% and 48.7% respectively which shows the practice of medical gas in cylinder storage is very poor, despite the risk the fire and other damage. This study is in line with a study done in Italy.16 Medical oxygen by itself is not harmful, but when it comes into contact with oils, greases, or fats (including gels and antiseptics), it has a tendency to self-ignite and strongly encourage the burning of substances. Other fire-resistant materials, such as compounds, may burn severely in oxygen that has been increased or purified.17
Even though they seem to happen more frequently in operating rooms where oxygen is regularly administered, fires involving medical oxygen are not a recent occurrence. The prevention of fires in operating rooms has drawn considerable attention from the American fire prevention and anesthesiology communities ever since the Emergency Care Research Institute (ECRI), a global non-profit patient safety organization with headquarters in the United States, placed surgical fires third on its list of the top ten health technology hazards. At the time, ECRI predicted that there would be 500–600 surgical fires annually in the United States alone.18
The short-circuit-related fire mostly destroyed the air conditioning system in locations such as the operating room, the storehouse for biomedical equipment, the X-ray room, the neonatology incubator, the pediatric ICU, the children’s ward, the dialysis ward, and the intensive care unit (ICU) receiving ventilator support. These sites may occasionally contain electrical devices, such as ventilators, with high and fluctuating power loads that put them at risk of short-circuiting. The main cause of a lot of flames is air enriched with oxygen. The amount of ignition energy needed to start a fire lowers in the presence of higher O2 concentrations, and any heat or spark might set one off.19
Storage of drugs under circumstances that ensure their quality up until usage or administration (48.7%), Storage areas below 25°C (42%) There is adequate airflow in refrigerators and freezers (64.7%), The areas that have received less attention yet have a significant impact on patient outcomes include refrigerators and freezers used exclusively for the storage of medications. This result is consistent with the findings of the Pakistani study.20
The other indicator that was also given less concern is the recording of the date the drug was opened and the recording of the calculated expiration date of the drug, the study done in Saudi Arabia is inconsistent with this result.21 This poor practice might significantly affect patients and increase the workload on healthcare providers.22 Additionally, it might affect patient outcomes by inadequately treating diseases and increasing the time of hospital stay. Generally, the practice of drug storage, utilization, and checking expiration date in our study was found poor and others have zero performance at all in most of the tools; so great emphasis should be given to handling these finite resources. Due to its small sample size and single-center design, this study cannot be readily extrapolated to other hospitals. To address this serious issue, we recommend a large sample sized, multi-center study.
Conclusion
In conclusion, the majority of the indicators of the practice of drug storage, utilization, and checking expiration date were found not performed and below the standards. The improper disposal of medication increases the chance of inadvertent overdose or drug abuse, pollutes water supplies, and releases chemicals into the environment. The administration should launch a convenient medicine take-back program issue directives, conduct educational initiatives, and educate both the general public and experts working in healthcare settings about proper disposal techniques. We recommend various approaches like consistent evaluation and monitoring to enhance the practice of drug storage, utilization, and checking expiration dates. Follow the CDC guidelines to decrease the wastage of multiple vial drugs (www.cdc.gov/injectionsafety/1anonly.hmtl). A safe and secure place should be prepared for medical gas storage. The refrigerators have to be used only for the storage of medications. All medications, including intravenous fluid, have to be stored in a safe place and unless they were not used their packaging should not be removed. Critical medicines should be placed safely and should be accessed easily when they are needed, all other medications should be arranged according to their class to avoid misidentification.
Abbreviations
ANZCA, Australian and New Zealand College of Anesthetists; DUTH, Dilla University Teaching Hospital; GC, Gregorian calendar; ICU, Intensive Care Unit; NHS, National Health System; NICE, National Institute for Health and Care Excellence; OR, Operation Room.
Data Sharing Statement
The corresponding author can provide all the necessary materials upon request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
For this work, no funding source was found.
Disclosure
The authors confirm that there are no competing interests in this work.
References
1. Ali SA, Ali SA, Suhail N. Importance of storing medicines on required temperature in pharmacies and role of community pharmacies in rural areas: literature review. I-Manag J Nurs. 2016;6(2):32.
2. Sengupta P, Chatterjee B, Tekade RK. Current regulatory requirements and practical approaches for stability analysis of pharmaceutical products: a comprehensive review. Int J Pharm. 2018;543(1–2):328–344. doi:10.1016/j.ijpharm.2018.04.007
3. Gul A, Nazish S, Sabir S, Nazish H, Masood T. Expired drugs-awareness and practices of outdoor patients. J Rawalpindi Med Coll. 2016;20:45–48.
4. Kahsay H, Ahmedin M, Kebede B, Gebrezihar K, Araya H, Tesfay D. Assessment of knowledge, attitude, and disposal practice of unused and expired pharmaceuticals in community of Adigrat city, Northern Ethiopia. J Environ Public Health. 2020;2020:6725423. doi:10.1155/2020/6725423
5. Sarla GS. Efficacy and disposal of drugs after the expiry date. Egypt J Immunol. 2019;31(4):431–434. doi:10.4103/ejim.ejim_110_19
6. Smale EM, Egberts TC, Heerdink ER, van den Bemt BJ, Bekker CL. Waste-minimising measures to achieve sustainable supply and use of medication. Sustain Chem Pharm. 2021;20:100400. doi:10.1016/j.scp.2021.100400
7. West LM, Diack L, Cordina M, Stewart D. A systematic review of the literature on ‘medication wastage’: an exploration of causative factors and effect of interventions. Int J Clin Pharm. 2014;36(5):873–881. doi:10.1007/s11096-014-9981-2
8. Hussain A. Medicine storage trends & practices: a literature review. J Appl Pharm. 2017;9:1–14. doi:10.21065/19204159.9.1
9. Bekele KM, Abay AM, Mengistu KA, et al. Knowledge, attitude, and practice on over-the-counter drugs among pharmacy and medical students: a facility-based cross-sectional study. Integrat Pharm Res Pract. 2020;9:135. doi:10.2147/IPRP.S266786
10. Ebrahim AJ, Teni FS, Yimenu DK. Unused and expired medications: are they a threat? A facility-based cross-sectional study. J Prim Care Community Health. 2019;10:2150132719847857. doi:10.1177/2150132719847857
11. Mackay E, Jennings J, Webber S. Medicines safety in anaesthetic practice. BJA Educ. 2019;19(5):151. doi:10.1016/j.bjae.2019.01.001
12. Australian and New Zealand College of anesthetists. Guidelines for the safe management and use of medications in anaesthesia; 2018. Available from: www.anzca.edu.au.
13. Excellence NIfHa C. Professional guidance on the safe and secure handling of medicines; 2018. Available from: www.nice.org.uk.
14. WB. National health system Grampian storage of medicines within clinical areas policy; 2020. Available from: www.nhs.org.uk.
15. West LM. Medication wastage: the current situation. 2015.
16. Wood MH, Hailwood M, Koutelos K. Reducing the risk of oxygen-related fires and explosions in hospitals treating Covid-19 patients. Process Saf Environ Prot. 2021;153:278–288. doi:10.1016/j.psep.2021.06.023
17. Wróblewski W, Tuśnio N, Wolny P, et al. Fire safety of healthcare units in conditions of oxygen therapy in covid-19: empirical establishing of effects of elevated oxygen concentrations. Sustainability. 2022;14(7):4315. doi:10.3390/su14074315
18. USA - The Emergency Care Research Institute (ECRI) releases top health technology hazards for 2010. Int J Health Care Qual Assur. 2010;23(3).
19. Chowdhury K. Fires in Indian hospitals: root cause analysis and recommendations for their prevention. J Clin Anesth. 2014;26(5):414–424. doi:10.1016/j.jclinane.2013.12.014
20. Shah SSAM, Naqvi BS, Fatima M, Khaliq A, Sheikh AL, Baqar M. Quality of drug stores: storage practices & regulatory compliance in Karachi, Pakistan. Pak J Med Sci. 2016;32(5):1071–1076. doi:10.12669/pjms.325.9705
21. Bashatah A, Wajid S. Knowledge and disposal practice of leftover and expired medicine: a cross-sectional study from nursing and pharmacy students’ perspectives. Int J Environ Res Public Health. 2020;17(6):2068. doi:10.3390/ijerph17062068
22. Zemedkun A, Mulugeta H, Getachew H, Destaw B, Mola S, Milkias M. Assessment of manual intraoperative anesthesia record-keeping practice at Dilla university referral hospital, Dilla, Ethiopia. Open Access Surg. 2021;14:1. doi:10.2147/OAS.S298387
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.