Back to Journals » Clinical Interventions in Aging » Volume 15
Assessment of Frailty and Occurrence of Anxiety and Depression in Elderly Patients with Atrial Fibrillation
Authors Uchmanowicz I , Lomper K , Gros M, Kałużna-Oleksy M, Jankowska EA , Rosińczuk J , Cyrkot T , Szczepanowski R
Received 17 April 2020
Accepted for publication 13 June 2020
Published 15 July 2020 Volume 2020:15 Pages 1151—1161
DOI https://doi.org/10.2147/CIA.S258634
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Izabella Uchmanowicz,1 Katarzyna Lomper,1 Małgorzata Gros,2 Marta Kałużna-Oleksy,3 Ewa A Jankowska,4,5 Joanna Rosińczuk,6 Tomasz Cyrkot,7 Remigiusz Szczepanowski7
1Department of Clinical Nursing, Wroclaw Medical University, Wroclaw, Poland; 2Student Research Circle in Nursing, Wroclaw Medical University, Wroclaw, Poland; 3 1st Department of Cardiology, University of Medical Sciences in Poznan, Poznan, Poland; 4Department of Heart Diseases, Wroclaw Medical University, Wroclaw, Poland; 5Centre for Heart Diseases, University Hospital, Wroclaw, Poland; 6Department of Nervous System Diseases, Wroclaw Medical University, Wroclaw, Poland; 7Department of Public Health, Wroclaw Medical University, Wroclaw, Poland
Correspondence: Katarzyna Lomper
Department of Clinical Nursing, Wroclaw Medical University, Faculty of Health Sciences, Wroclaw, Poland
Tel +48 71 784 18 05
Fax +48 71 345 93 24
Email [email protected]
Purpose: Atrial fibrillation (AF) is the most common cardiac arrhythmia, and its incidence increases with age. The elderly population is commonly affected by frailty syndrome (FS). FS syndrome along with anxiety and depressive symptoms are prevalent among elderly patients with AF. It is unclear whether depression contributes to AF or vice versa. The purpose of this study was to assess correlations between FS and the occurrence of anxiety and depression symptoms in a group of elderly patients with AF.
Patients and Methods: This cross-sectional study included 100 elderly patients (69 females, 31 males, mean age: 70.27 years) with AF. Standardized research instruments were used including the Tilburg Frailty Indicator (TFI) to assess FS, and two questionnaires to assess depression including the Geriatric Depression Scale (GDS), and the Hospital Anxiety Depression Scale (HADS).
Results: Mild FS was found in 38% and moderate FS in 29% of patients. Based on GDS scores, depression symptoms were found in 51% of patients’ sample. Based on HADS scores, 20% of patients were found to have anxiety symptoms, and 28% revealed depression symptoms. Single-factor analysis demonstrated a significant positive correlation between HADS anxiety symptoms (r=0.492), HADS depression symptoms (r=0.696), and GDS score (r=0.673) on the one hand, and overall TFI frailty score on the other. Multiple-factor analysis identified overall GDS score, education, and lack of bleeding as significant independent predictors of TFI scores (p< 0.05).
Conclusion: FS is common in the population of elderly patients with AF. We found evidence for the association between symptoms of anxiety and depression and the incidence of FS in this group of patients. Due to the risk of consequences which may in part be irreversible, screening for FS is recommended.
Keywords: atrial fibrillation, frailty syndrome, aging, anxiety, depression
Introduction
Atrial fibrillation (AF) is the most common arrhythmia in the general population. However, available epidemiological data indicate that its incidence is highest among elderly patients. The prevalence of AF increases with age: it affects 0.1% of the population under 55 years of age, 3.3% of the population over 60, and 10% of the population over 80.1 It is estimated that due to aging, AF will affect approx. 6–12 million people in the US by the year 2050, and approx. 17.9 million in Europe by 2060.2 In the light of the most recent guidelines, the increasing prevalence of AF is attributed to improved diagnosis of new cases, increasingly common occurrence of arrhythmia risk factors, and population aging.3
Moreover, frailty syndrome (FS) has been suggested as a significant predictor of cardiovascular disease in elderly patients with AF. FS is defined as a geriatric syndrome presenting with clinically identifiable physical alterations, such as loss of muscle mass and strength, decreased energy and exercise tolerance, and reduced physiological reserves.4 FS is among the main risk factors for complications among elderly patients. FS typically involves reduced physiological reserves, dysfunctions in multiple organs, and the resulting changes. In the literature, FS is described as a clinical syndrome resulting in weakness, slowness, reduced physical activity, fatigue, and weight loss. FS also refers to an accumulation of deficits that may be identified in a comprehensive geriatric assessment.5
The etiopathogenesis of FS includes biological (hormonal, inflammatory), social (poor financial status, social isolation/exclusion), and clinical factors (osteoporosis, sarcopenia, multiple comorbidities).6 FS is part of a continuum, at one end of which lies perfect health and independence, while at the opposite end there is an increased need for medical care and dependency on others. In FS, homeostasis may be disturbed due to increased intensity of stressors, such as a severe depressive episode or infection, that may result in patients’ disability. Research on FS supports the shared concept of a pathophysiological pathway leading to disability through the progressive loss of physiological reserve and physical fitness with age.7 FS analyzed in the context of disability is an important prognostic measure in terms of the need for care and therapy. Furthermore, an accurate distinction between FS and disability allows for implementing interventions to reverse the associated changes or prevent their progression.7–10
The prevalence of FS increases with age and may significantly affect both treatment success and the ultimate outcomes of AF.11 It is estimated at 7–12% of the elderly population. Between the ages of 56 and 74, FS is diagnosed in 3.9% of patients, but in patients aged over 85, this proportion increases to 25%.12
Older age is also associated with a greater intensity of stressful life situations, from the change of one’s professional status to the death of one’s life partner and in many situations potentially result in loneliness, and risk of financial difficulties. All of these situations may result in the development of anxiety or depressive disorders. Nowadays as indicated by the World Health Organization (WHO) depressive disorders are the second most common cause of disability, after cardiovascular disease.13
Depression and FS are widespread in the elderly population, but their mutual correlation has not yet been widely investigated. Cross-sectional studies have demonstrated a four-fold increase in depression risk in elderly patients diagnosed with FS. The opposite association has also been observed – depressed patients have a four times greater risk of becoming frail.14
Medical recommendations for diagnosis and treatment of AF emphasize the mood disorders occurring in the elderly population.3 Research on depression demonstrates that it is a major cause of emotional distress in older age and a factor potentially contributing to the pathogenesis of a variety of diseases.15,16 Depressed elderly patients often experience a deterioration of their daily functioning and quality of life (QoL), as well as low moods.17 Additionally, the recent reports show that both depressive and anxiety symptoms are important for effective therapeutic process in patients with AF.17 Anxiety and depression symptoms are aggravated with each episode of AF.18 Moreover, they are associated with a greater severity of AF symptoms and lower QoL19 compared to patients with other cardiac disorders.20 AF is also associated with an increased risk of death and increased demand for health care.20
The purpose of the study was to assess correlations between FS and the occurrence of anxiety and depression in a group of elderly patients with AF.
Patients and Methods
Study Design and Setting
This cross-sectional study was performed between February 2019 and June 2019 in the Wroclaw University Hospital in Poland. The STROBE guidelines (Strengthening the Reporting of Observational Studies in Epidemiology) were followed.
Study Participants and Qualification
The sample of 100 patients diagnosed with AF (mean age 70.27 years) was recruited from 235 eligible patients and enrolled in the study as indicated in the flow diagram (Figure 1). All patients were asked to complete the questionnaires during hospitalization, with a cardiac nurse present. Inclusion criteria were as follows: age above 65 years, no comorbidities associated with severe hemodynamic instability, no cognitive impairment preventing unassisted completion of the questionnaires, and informed consent to participate in the study.
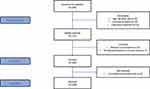 |
Figure 1 STROBE flow diagram of study participants. |
Research Tools
In the study, an original questionnaire was used to collect basic socio-demographic (ie, age, sex, education, residence, professional activity, marital status), and clinical data (ie, duration of illness, previous cardioversions, hospitalizations, AF symptoms, comorbidities). The following standardized instruments were also applied: the Tilburg Frailty Indicator (TFI), the Geriatric Depression Scale (GDS), and the Hospital Anxiety Depression Scale (HADS).
TFI
The TFI allows for a comprehensive and reliable evaluation of FS, including its physical, psychological, and social components. The questionnaire includes three subscales. The physical subscale (0–8 points) measures physical health, unintentional weight loss, difficulty in walking, balance, hearing and vision problems, grip strength, and physical fatigue. The psychological subscale includes such factors as memory problems, feeling down, feeling nervous or anxious, and inability to cope with problems. The social subscale includes three factors: living alone, lack of social relations, and lack of social support. Eleven items have two response categories, “yes” and “no”, and four items also have the response category “sometimes”. After recoding, score ranges are as follows: 0–15 (overall frailty), 0–8 (physical frailty), 0–4 (psychological frailty), and 0–3 (social frailty). The total TFI score ranges between 0 and 15 points, and scores over 5 points are considered diagnostic for FS.21 The instrument was adapted and translated for the Polish cultural setting by Uchmanowicz et al Cronbach’s Alpha internal consistency for this measurement was 0.74.22
GDS
The GDS was developed in 1983 by Yesavage et al23 and it is a screening instrument evaluating the intensity of depressive symptoms in elderly patients. For the present study, the 15-item version was used. Each response is scored 1 or 0, and scores of 0–5 points indicate no depression, 6–10 points – mild depression, and 11–15 points – severe depression (α = 0.94).24 We used a Polish version of GDS adapted by Albinski et al.25 This GDS measure has proven to be a valid and reliable screening instrument, providing an indication for further detailed diagnostics.25
HADS
The HADS is a self-reported screening scale developed for non-psychiatric patients at risk of anxiety or affective disorders.26 This instrument measures anxiety as a state and not a trait.27 Due to its high sensitivity and specificity, it is one of the most commonly used screening instruments in medical settings.28 It has been adapted for the Polish cultural setting by Majkowicz et al29 It comprises two separate subscales, assessing anxiety (α = 0.79) and depression (α = 0.74). Answers are given using a 4-item Likert scale, where the minimum score is 0, and the maximum is 21. Scores of 0–7 points indicate no anxiety or depression; 8–10 points are considered a borderline result, while 11–21 points indicate a marked disorder.27
Ethical Considerations
The study was approved by the independent Bioethics Committee of the Wrocław Medical University, Poland. All patients were informed on the study protocol and provided informed consent to participate. The study protocol was developed in accordance with the Declaration of Helsinki.30
Data Analysis
Quantitative variables were analyzed using means, standard deviations, and median, quartile, minimum, and maximum values. Qualitative variables (ie, sex, education, residence, professional activity, marital status, AF symptoms, comorbidities) were analyzed using the counts and percentages of each value. Correlations between quantitative variables were analyzed using Pearson’s or Spearman correlation coefficients. Correlation strength was interpreted as follows: |r| ≥ 0.9 – very strong correlation, 0.7 ≤ |r| < 0.9 – strong correlation, 0.5 ≤ |r| < 0.7 – moderately strong correlation, 0.3 ≤ |r| < 0.5 – weak correlation, |r| ≥ 0.3 – very weak (negligible) correlation.
Multivariate analysis of independent impact of the variables being studied on the quantitative variable was performed using linear regression. The results are shown as regression model parameter values with a 95% confidence interval (CI). Due to the large number of potential predictors, a selection process was applied. First, the number of parameters that may be satisfactorily estimated was determined. The most popular guidelines recommend 1 parameter per 10 patients, which gives 10 parameters for the group of 100 patients analyzed here.31 Scores from the GDS and the two HADS subscales were included in the multiple-factor analyses. This requires an estimation of 3 parameters, leaving 7 “unused” parameters. In the next step, single-factor regression analyses were performed for each variable that could be included in multiple-factor analysis. They were put in an order of decreasing significance of impact on the TFI score (ie, from highest to lowest p-value). Then, variables from the top of this “ranking table” were included in multiple-factor analysis in such a way that estimation was required for 7 parameters. This process was then repeated for each TFI subscale, hence the inclusion of different variables in each multiple-factor analysis. Variable distribution normality was verified using the Shapiro–Wilk test. All analyses used a significance threshold of 0.05. The analyses were performed using R software, version 3.5.2.
Results
Participants’ Characteristics
Based on an analysis of basic socio-demographic characteristics, most of the patients studied were female (61%), had completed vocational (34%) or primary education (29%), lived in urban areas (55%), were professionally inactive (85%) and married (59%). The data are shown in Table 1.
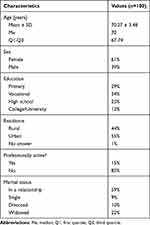 |
Table 1 Sociodemographic Characteristics of the Patients Studied (n=100) |
Table 2 presents the clinical data for the group. The mean time from AF diagnosis was 3.96 years. The patients had undergone electrical cardioversion 3.32 times on average. The most common arrhythmia symptoms included heart palpitations (83%), fatigability (33%), and chest pain (25%). The most common comorbidities were hypertension (82%) and diabetes (31%). Most patients had been hospitalized due to their AF between 3 and 5 times (48%).
 |
Table 2 Clinical Data of the Patients Studied (n=100) |
Table 3 presents analysis results for the TFI, HADS, and GDS scores. The mean score on the physical subscale of the TFI was 3.22 points, on the psychological subscale – 2.23 points, and on the social subscale – 1.34 points. Mild frailty was identified in 38% of patients, and moderate frailty in 29%. Based on HADS scores, 20% of patients were found to have anxiety symptoms, and 28% of the sample showed depression symptoms. In turn, based on GDS scores, depression was identified in a larger number of patients (51%).
 |
Table 3 TFI, HADS, and GDS Scores in the Patients Studied (n=100) |
Single-Factor Analysis
Single-factor analysis was performed for selected variables and TFI scores. The anxiety score from HADS was found to be significantly positively correlated (r=0.492; p<0.001) with the overall frailty score, as well as its physical and social component (p<0.05); indicating that greater severity of anxiety symptoms is associated with more severe frailty, both overall and in the physical and social dimensions. The depression score from HADS was found to be significantly positively correlated with the overall frailty score, as well as its physical and social component (p<0.05), indicating that greater severity of depressive symptoms is associated with more severe frailty, both overall and in the physical and social dimensions. The GDS score and the anxiety and depression scores from HADS were found to be significantly positively correlated with the overall frailty score, as well as its physical and social component (p<0.05), indicating that greater severity of anxiety and depression symptoms is associated with more severe frailty, both overall and in the physical and social dimensions. Notably, though, the correlation with the social dimension was considerably weaker than the remaining correlations (Table 4).
 |
Table 4 Single-Factor Analysis for Selected Variables and FS (TFI) |
Multiple-Factor Analysis
Overall Tilburg Frailty Indicator Score
Multiple-factor linear regression results demonstrated that independent predictors of the overall TFI score (p<0.05) include: total GDS score – each additional point in the GDS questionnaire increases the TFI score by an average of 0.206 points; college/university education – compared with primary education, it decreases the score by an average of 1.546 points; no bleeding – decreases the score by an average of 0.933 points (Table 5).
 |
Table 5 Multiple-Factor Analysis for the Overall TFI Score |
Physical Components of the Tilburg Frailty Indicator
The linear regression model demonstrated that independent predictors of the physical TFI score (p<0.05) include: The GDS score – each additional point in the GDS questionnaire increases the TFI score by an average of 0.212 points; age – each additional year increases the score by an average of 0.183 points; manner of taking medication – unassisted taking of medication from labeled boxes decreases the score by an average of 0.991 points, compared to medication being prepared and given by another person (Table 6).
 |
Table 6 Multiple-Factor Analysis for the Physical TFI Score |
Psychological Components of the Tilburg Frailty Indicator
The linear regression model demonstrated that independent predictors of the psychological TFI score (p<0.05) include: heart failure – decreases the score by an average of 0.421 points; and dizziness – decreases the score by an average of 0.408 points (Table 7).
 |
Table 7 Multiple-Factor Analysis for the Psychological TFI Score |
Social Components of the Tilburg Frailty Indicator
The linear regression model demonstrated that independent predictors of the social TFI score (p<0.05) include: The HADS depression score – each additional point in this scale increases the TFI score by an average of 0.093 points; marital status – compared to being married: being single increases the score by an average of 1.1 points; being widowed increases the score by an average of 0.605 points; male sex – decreases the score by an average of 0.685, compared to female sex; manner of taking medication – unassisted taking of medication from labeled boxes increases the score by an average of 0.613 points, compared to medication being prepared and given by another person (Table 8).
 |
Table 8 Multiple-Factor Analysis for the Social TFI Score |
Discussion
Increasing life expectancy in the general population results in an increased prevalence of cardiovascular diseases, including AF. The incidence of AF rises with age.1 The high incidence of this arrhythmia in elderly patients is associated with degenerative processes occurring in the heart, including wall stiffening, increased left atrial pressure, and changes in the cardiac conduction system. Another risk factor for AF in the elderly population is multimorbidity ie, concurrent hypertension, diabetes mellitus, ischemic heart disease, heart failure, and valvular disease.32 Age is associated not only with a higher risk of AF but also that of FS,33 as well as with more frequent situations potentially contributing to anxiety-depressive disorders.
The purpose of the present study was to assess correlations between FS and the occurrence of anxiety and depression in a group of elderly patients with AF. In the study, frailty was found in a decisive majority of patients’ sample, ie, mildly frail patients (38%) and moderately frail patients (29%). Additionally, GDS measures indicated the presence of depression symptoms in 51% of the AF participants, whereas the HADS questionnaire showed either depression (28%) and anxiety symptoms (20%) in the investigated group of patients. There was the positive significant correlation between anxiety and depression symptoms and the overall frailty score in AF patients as indicated by the single-factor analysis. Regression analysis showed also that the GDS score of AF patients contributed significantly to the prediction of frailty syndrome in this patients’ population.
Frailty is quite common in the elderly population, but the available literature reflects a controversy regarding patients with AF. The prevalence of FS in this population is estimated at between 4.4% and 75.4%.34 Therefore, the actual prevalence of frailty among AF patients remains poorly understood.
Common symptoms of AF include heart palpitations, chest pain, and shortness of breath, dizziness, or even fainting. During an arrhythmic episode, patients typically complain of a rapid, irregular, and pounding heartbeat.35 Patients in the present study reported heart palpitations, fatigability, and chest pains during an episode as the most common symptoms. The frequent and unexpected occurrence of symptoms leads to numerous hospitalizations. AF is also associated with considerable mortality, as well as high health-care costs. Moreover, patients are affected by a psycho-social burden, including anxiety and depression.36 The analysis of HADS questionnaire scores demonstrated a definite anxiety disorder in 20% of patients and a depressive disorder in 28%.
Anxiety and depression in patients with cardiovascular disease are associated with poorer clinical prognosis, but its impact on AF treatment success is not clear. So far, the correlation of anxiety and depression with stroke and bleeding has been studied in this patient group. Diagnosed depression and anxiety disorder were associated with a greater risk of both ischemic stroke and intracranial hemorrhage.37
It is unclear whether depression contributes to FS or vice versa, or if both disorders co-occur in the same patients independently of one another, but research confirms the pathophysiological processes and biomarkers present in both. The present study corroborates the available literature data. Elderly patients were found to be at increased risk of depressive symptoms and disability in terms of basic activities.38 Frailty in hospitalized elderly patients may be associated with depressive symptoms and functional disability. Systematic reviews indicate that disease with concurrent depressive symptoms is a risk factor for FS.39 Research to date shows a correlation between frailty and depression in the elderly population. Approximately 4–16% of frail patients aged 65 and above have concurrent symptoms of serious depression, and this percentage increases to 35% when patients aged 75 and above are considered.4 Depression concurrent with frailty leads to a number of adverse outcomes, including deteriorated QoL, more hospitalizations, and increased morbidity and mortality.14 The present findings demonstrate a significant correlation between frailty (as measured by the TFI), including its social and physical dimensions, and depression and anxiety (as measured by the HADS). Further regression analysis revealed that depression symptoms (as measured by the GDS) are a strong predictor of frailty, as opposed to anxiety symptoms.
Therefore, the severity of depressive symptoms predicts the symptoms associated with a greater severity of FS. Moreover, a lower frequency of bleeding (physical dimension) is associated with a lesser severity of these symptoms. It seems fairly obvious and intuitive that better health contributes to the development of resources allowing for better coping with stress, thus relieving the symptoms of frailty. Interestingly, a significant correlation was also found between education and less severe frailty symptoms. A college or university education predicts a lower severity of FS. Better education may be associated with better cognitive and intellectual functioning. These resources in turn favor better coping with stress.40 However, the patients’ intellectual functioning was not evaluated here, which is a limitation of the present study. Other predictors of FS identified here include age and living alone. The older the patient, the greater the likelihood of more severe symptoms. A similar tendency is observed in patients without support provided by a partner.
Findings on sex are interesting from the psychological point of view. Male patients were found to have less severe frailty symptoms. However, from a psychological perspective, declarations in a questionnaire are burdened with a certain bias. For instance, symptoms of psychological disorders may be reported differently by male and female patients. Respondents tend to show themselves in the best possible light by providing socially acceptable responses. Factors involved in this process may include self-deception and other-deception. Hence, the possible underestimation of frailty symptoms in self-reports by male patients.
Notably, symptoms such as fatigue, weight loss, slowness, and reduced physical activity are present both in depressive disorders and in FS. The so-called “overlap syndrome” hypothesis suggests that attempts to clinically distinguish between depression and FS may be less successful in the elderly population. Still, recent studies employing confirmatory latent class analyses have demonstrated that depression and FS are clearly distinct syndromes, rather than a single construct. Therefore, one of the most accurate methods of studying correlations between the two clinical syndromes involves narrowing the scope to a specific population of patients with a cardiovascular disorder (subgroup of frail individuals with AF symptoms) and including additional pathological factors, such as psychological distress.
The present study focused on an analysis of associations between frailty and psychological distress in a group of patients with AF, taking a cross-sectional approach. Notably, psychological distress in AF patients is a trigger for AF episodes. The analysis of psychological distress, including anxiety and depression, demonstrates that in a large portion of the AF population these factors contribute to a greater severity of symptoms, lower QoL, and recurrence of AF episodes. In analyses of correlations between depression and frailty, a critical part involves determining the causality, and specifically whether one syndrome precedes the other or whether both syndromes occur independently of one another. Longitudinal studies on the topic have not yet conclusively addressed this issue. Experimental data from longitudinal studies demonstrate that depressive symptoms are a significant predictor of concurrent FS, but also that an opposite correlation may exist as well.
In the present study, depressive symptoms were considered a significant predictor of FS. Symptoms occurring in elderly individuals interfere with their normal functioning in the long term and may contribute to the development of FS. In a cross-sectional analysis of frail patients aged 65 and above using the GDS questionnaire, depressive symptoms were found to be positively correlated with FS. It is worth noting that depressive symptoms are not typically diagnosed in elderly patients at all, especially when they have multiple health issues, which means these symptoms are not adequately treated either. In particular, lack of diagnostics for depression in cardiovascular patients, including AF patients, may result in poorer adherence to treatment, more risky behaviors, and greater risk of mortality and future cardiac events.
To sum up, the present study discusses and investigates frailty in patients with AF aged 65 or above by estimating and analyzing critical factors associated with FS in this population, such as depression. There are a few key study limitations that should be discussed. Namely, the present study used only self-reported tests to evaluate patient’s conditions. One has also to bear in mind that nurses’ assistance with filling in the study questionnaires might have affected patients’ responses. Also, potential limitation is the fact that the study sample consisted of patients from a single center. Thus, our findings should be carefully extrapolated to the multicentre or institutional studies. Another limitation is that our study does not resolve a diagnostic issue of the presence or absence of the full-blown anxiety and depression, because a full diagnosis in the AF patients’ sample would need to employ DSM (The Diagnostic and Statistical Manual of Mental Disorders) criteria for mental disorders and relevant measures of such psychopathology.
Conclusion
FS is common in the population of elderly patients with AF. Frailty assessment in elderly patients is recommended due to the associated cascade of irreversible alterations ultimately resulting in disability or hospitalizations. In the clinical practice, health-care providers should recognize elderly patients with concurrent frailty, requiring intensified therapeutic interventions tailored to their individual needs and expectations. Our study shows that anxiety and depression may contribute to the incidence of FS in this group. The important finding from our study is that severity of depressive symptoms predicts the symptoms associated with a greater severity of FS. Our study clearly implicates that clinical assessments in elderly AF patients should include the measures of anxiety and depression symptoms in order to prevent the occurrence and severity of FS.
Abbreviations
AF, atrial fibrillation; FS, frailty syndrome; GDS, Geriatric Depression Scale; HADS, Hospital Anxiety Depression Scale; QoL, quality of life; STROBE, Strengthening the Reporting of Observational Studies in Epidemiology; TFI, Tilburg Frailty Indicator; WHO, World Health Organization.
Disclosure
No conflicts of interest have been declared by the authors.
References
1. Sardar P, Chatterjee S, Chaudhari S, Lip GYH. New oral anticoagulants in elderly adults: evidence from a meta-analysis of randomized trials. J Am Geriatr Soc. 2014;62(5):857–864. doi:10.1111/jgs.12799
2. Schnabel RB, Yin X, Gona P, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet Lond Engl. 2015;386(9989):154–162. doi:10.1016/S0140-6736(14)61774-8
3. Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg. 2016;50(5):e1–e88. doi:10.1093/ejcts/ezw313
4. Buigues C, Padilla-Sánchez C, Garrido JF, Navarro-Martínez R, Ruiz-Ros V, Cauli O. The relationship between depression and frailty syndrome: a systematic review. Aging Ment Health. 2015;19(9):762–772. doi:10.1080/13607863.2014.967174
5. Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interv Aging. 2014;9:433–441. doi:10.2147/CIA.S45300
6. Afilalo J. Frailty in patients with cardiovascular disease: why, when, and how to measure. Curr Cardiovasc Risk Rep. 2011;5(5):467–472. doi:10.1007/s12170-011-0186-0
7. Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm–issues and controversies. J Gerontol a Biol Sci Med Sci. 2007;62(7):731–737. doi:10.1093/gerona/62.7.731
8. Hogan DB, MacKnight C, Bergman H. Steering committee, Canadian initiative on frailty and aging. Models, definitions, and criteria of frailty. Aging Clin Exp Res. 2003;15(3 Suppl):1–29.
9. Aminzadeh F, Byszewski A, Dalziel WB, Wilson M, Deane N, Papahariss-Wright S. Effectiveness of outpatient geriatric assessment programs: exploring caregiver needs, goals, and outcomes. J Gerontol Nurs. 2005;31(12):19–25. doi:10.3928/0098-9134-20051201-06
10. Hallberg IR, Kristensson J. Preventive home care of frail older people: a review of recent case management studies. J Clin Nurs. 2004;13(6B):112–120. doi:10.1111/j.1365-2702.2004.01054.x
11. Zathar Z, Karunatilleke A, Fawzy AM, Lip GYH. Atrial fibrillation in older people: concepts and controversies. Front Med. 2019;6. doi:10.3389/fmed.2019.00175.
12. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol a Biol Sci Med Sci. 2001;56(3):M146–M156. doi:10.1093/gerona/56.3.M146
13. World Health Organization. Mental Health: A Call for Action by World Health Ministers. Geneva, Switzerland: World Health Organization; 2014. Available from: https://www.mhinnovation.net/resources/mental-health-call-action-world-health-ministers.
14. Soysal P, Veronese N, Thompson T, et al. Relationship between depression and frailty in older adults: a systematic review and meta-analysis. Ageing Res Rev. 2017;36:78–87. doi:10.1016/j.arr.2017.03.005
15. Casey DA. Depression in the elderly: a review and update. Asia Pac Psychiatry. 2012;4(3):160–167. doi:10.1111/j.1758-5872.2012.00191.x
16. Casey DA. Depression in older adults: a treatable medical condition. Prim Care. 2017;44(3):499–510. doi:10.1016/j.pop.2017.04.007
17. Polikandrioti M, Koutelekos I, Vasilopoulos G, et al. Anxiety and depression in patients with permanent atrial fibrillation: prevalence and associated factors. Cardiol Res Pract. 2018;2018:7408129. doi:10.1155/2018/7408129
18. von Eisenhart Rothe AF, Goette A, Kirchhof P, et al. Depression in paroxysmal and persistent atrial fibrillation patients: a cross-sectional comparison of patients enroled in two large clinical trials. Europace. 2014;16(6):812–819. doi:10.1093/europace/eut361
19. Thompson TS, Barksdale DJ, Sears SF, Mounsey JP, Pursell I, Gehi AK. The effect of anxiety and depression on symptoms attributed to atrial fibrillation. Pacing Clin Electrophysiol. 2014;37(4):439–446. doi:10.1111/pace.12292
20. McCabe PJ. Psychological distress in patients diagnosed with atrial fibrillation: the state of the science. J Cardiovasc Nurs. 2010;25(1):40–51. doi:10.1097/JCN.0b013e3181b7be36
21. Gobbens RJ, Schols JM, van Assen MA. Exploring the efficiency of the tilburg frailty indicator: a review. Clin Interv Aging. 2017;12:1739–1752. doi:10.2147/CIA.S130686
22. Uchmanowicz I, Jankowska-Polańska B, Uchmanowicz B, Kowalczuk K, Gobbens RJJ. Validity and reliability of the polish version of the Tilburg Frailty Indicator (TFI). J Frailty Aging. 2016;5(1):27–32. doi:10.14283/jfa.2015.66
23. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi:10.1016/0022-3956(82)90033-4
24. Marc LG, Raue PJ, Bruce ML. Screening performance of the Geriatric Depression Scale (GDS-15) in a diverse elderly home care population. Am J Geriatr Psychiatry. 2008;16(11):914–921. doi:10.1097/JGP.0b013e318186bd67
25. Albiński R, Kleszczewska-Albińska A, Bedyńska S. Geriatric Depression Scale (GDS). Validity and reliability of different versions of this tool - research review. Psychiatr Pol. 2011;45(4):555–562.
26. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi:10.1111/j.1600-0447.1983.tb09716.x
27. Mihalca A, Pilecka W. The factorial structure and validity of the Hospital Anxiety and Depression Scale (HADS) in polish adolescents. Psychiatr Pol. 2015;49(5):1071–1088.
28. Mitchell AJ, Meader N, Symonds P. Diagnostic validity of the Hospital Anxiety and Depression Scale (HADS) in cancer and palliative settings: a meta-analysis. J Affect Disord. 2010;126(3):335–348. doi:10.1016/j.jad.2010.01.067
29. Majkowicz M. Practical evaluation of the effectiveness of palliative care - selected research techniques. In: de Walden-gałuszko K, Majkowicz M, editors. Assessing the Quality of Palliative Care in Theory and Practice.
30. World Medical Association. World medical association declaration of helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053.
31. Harrell F. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer-Verlag; 2001. doi:10.1007/978-1-4757-3462-1
32. Wożakowska-Kapłon B, Gorczyca-Michta I, Filipiak KJ, Siebert J. Prevention of thromboembolic complications in the patients with atrial fibrillation - the proposal an algorithm for family doctors. Forum Med Rodz. 2013;7(1):1–15.
33. Madhavan M, Holmes DN, Piccini JP, et al. Association of frailty and cognitive impairment with benefits of oral anticoagulation in patients with atrial fibrillation. Am Heart J. 2019;211:77–89. doi:10.1016/j.ahj.2019.01.005
34. Villani ER, Tummolo AM, Palmer K, et al. Frailty and atrial fibrillation: a systematic review. Eur J Intern Med. 2018;56:33–38. doi:10.1016/j.ejim.2018.04.018
35. Lomper K, Sławuta A, Dudek K, Mazur G, Walfridsson U, Jankowska-Polańska B. Psychometric evaluation of the polish version of the arrhythmia‑specific questionnaire in tachycardia and arrhythmia: a new tool for symptom and health‑related quality of life assessment. Kardiol Pol. 2019;77(5):541–552. doi:10.5603/KP.a2019.0046
36. Thrall G, Lane D, Carroll D, Lip GYH. Quality of life in patients with atrial fibrillation: a systematic review. Am J Med. 2006;119(5):
37. Baumgartner C, Fan D, Fang MC, et al. Anxiety, depression, and adverse clinical outcomes in patients with atrial fibrillation starting warfarin: cardiovascular research network WAVE study. J Am Heart Assoc. 2018;7(8). doi:10.1161/JAHA.117.007814
38. Tavares DMDS, Faria PM, Pegorari MS, Ferreira PCDS, Nascimento JS, Marchiori GF. Frailty syndrome in association with depressive symptoms and functional disability among hospitalized elderly. Issues Ment Health Nurs. 2018;39(5):433–438. doi:10.1080/01612840.2018.1429035
39. Vaughan L, Corbin AL, Goveas JS. Depression and frailty in later life: a systematic review. Clin Interv Aging. 2015;10:1947–1958. doi:10.2147/CIA.S69632
40. Libin E. Coping intelligence: efficient life stress management. Front Psychol. 2017;8. doi:10.3389/fpsyg.2017.00302.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
