Back to Journals » Nature and Science of Sleep » Volume 16
Assessing the Real-World, Long-Term Impact of Lemborexant on Sleep Quality in a Home-Based Clinical Study
Authors Miyata S , Iwamoto K , Okada I , Fujimoto A, Kogo Y, Mori D, Amano M, Matsuyama N, Nishida K, Ando M , Taoka T, Naganawa S, Ozaki N
Received 8 November 2023
Accepted for publication 1 March 2024
Published 19 March 2024 Volume 2024:16 Pages 291—303
DOI https://doi.org/10.2147/NSS.S448871
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Ahmed BaHammam
Seiko Miyata,1,* Kunihiro Iwamoto,1,* Ippei Okada,1,* Akihiro Fujimoto,2 Yuki Kogo,2 Daisuke Mori,1,3,4 Manabu Amano,5 Nao Matsuyama,5 Kazuki Nishida,5 Masahiko Ando,5 Toshiaki Taoka,6 Shinji Naganawa,7 Norio Ozaki1,4
1Department of Psychiatry, Nagoya University, Graduate School of Medicine, Nagoya, Japan; 2Medical Headquarters, Eisai Co., Ltd., Tokyo, Japan; 3Brain and Mind Research Center, Nagoya University, Nagoya, Japan; 4Pathophysiology of Mental Disorders, Nagoya University, Graduate School of Medicine, Nagoya, Japan; 5Department of Advanced Medicine, Nagoya University Hospital, Nagoya, Japan; 6Department of Innovative Biomedical Visualization (Ibmv), Nagoya University, Graduate School of Medicine, Nagoya, Japan; 7Department of Radiology, Nagoya University, Graduate School of Medicine, Nagoya, Japan
*These authors contributed equally to this work
Correspondence: Seiko Miyata, Department of Psychiatry, Nagoya University, Graduate School of Medicine, 65 Tsurumai, Showa, Nagoya, Aichi, 466-8550, Japan, Tel +81 52 744 2282, Fax +81 52 744 2293, Email [email protected]
Purpose: Both subjective and objective evaluations are essential for the treatment of insomnia. Lemborexant has been shown to be effective in the long-term based solely on a subjective basis, and no long-term objective measures have been evaluated under natural sleep conditions. Small, lightweight sleep electroencephalogram (EEG) monitor was used, instead of polysomnography, to objectively evaluate sleep at home 4 and 12 weeks after lemborexant treatment.
Patients and Methods: Adults and elderly subjects with insomnia disorder, per the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, were enrolled in this open-label, single-arm, single-center trial. Objective and subjective measures of sleep were prospectively assessed. Sleep disturbance, excessive sleepiness, and depressive symptoms were assessed using questionnaires.
Results: A total of 45 subjects were screened, of which 33 were enrolled. Paired t-tests were conducted to evaluate changes in sleep variables and compared with the baseline; subjects showed significant improvements in objective sleep efficiency (SE) and subjective sleep parameters at weeks 4 and 12 following treatment with lemborexant. When baseline values were taken into account, a repeated-multivariate analysis of variance (MANOVA) revealed statistically significant changes in the objective measures. Sleep disturbance, excessive sleepiness, and depressive symptoms improved after three months of lemborexant treatment.
Conclusion: Furthermore, lemborexant therapy improved nocturnal sleep, when measured objectively using sleep EEG monitoring at home, and improved daytime sleepiness and depressive symptoms in older adults with insomnia disorder.
Keywords: insomnia, lemborexant, objective sleep evaluation, portable sleep EEG monitoring, subjective sleep evaluation
Introduction
Insomnia is one of the most common sleep disorders encountered in geriatric clinics. The overall prevalences of insomnia symptoms ranges from 20% to 46% in the elderly,1,2 and estimated overall prevalence of insomnia among Japanese was 17.3%.3 Complaints of insomnia among the elderly were 1.3 times higher than among the young.4 Epidemiologic studies have linked insomnia symptoms to the development of other diseases, including type 2 diabetes, dementia, stroke, and chronic kidney disease.5–8 Furthermore, insomnia increases the risk of developing depression,9 industrial and traffic accidents,10,11 impaired working memory,12 and decreased cortical oxygenation during a word fluency task.13
In recent years, cognitive behavioral therapy has been recommended as the first-line treatment for chronic insomnia in adults of any age.14,15 However, pharmacological therapy, including over-the-counter sleep aids, is the most widely used treatment for insomnia.14,15 Older adults may be more vulnerable than younger adults to the adverse events associated with commonly prescribed sedative-hypnotics,16 such as residual morning somnolence, daytime cognitive and psychomotor impairment, falls, and fractures.17,18 In a recently published meta-analysis, the orexin receptor antagonist, lemborexant, showed good efficacy, tolerability, and safety among all hypnotics for adults diagnosed with insomnia disorder.19 Previous research has compared the efficacy of lemborexant with placebo and zolpidem in improving sleep quality, using both objective (polysomnography: PSG) and subjective measures. The results showed that lemborexant therapy significantly improved both latencies to persistent sleep and sleep maintenance assessed objectively via PSG compared with both placebo and zolpidem extended-release therapy.20,21 In addition, a meta-analysis evaluating the effects of orexin receptor antagonists has shown that they promoted REM sleep, had little effect on non-REM sleep, and prolonged sleep duration in patients with insomnia.22 Lemborexant provided significant benefits on sleep onset and sleep maintenance in individuals with insomnia disorder versus placebo over the acute to the long-term period.20,23 A review article24 reported many favorable aspects of safety, including no rebound after discontinuation, adverse events such as headache and upper respiratory tract infections, and little impact on respiratory parameters (apnea-hypopnea index, nocturnal oxygen saturation) and cardiac parameters. Lemborexant has little or no impairment in cognition, driving, subjective sleepiness, or other aspects of daily life, and has also been shown to be effective in subjects with Alzheimer’s disease dementia.25
Laboratory PSG is the gold standard for sleep measurement, which requires an overnight stay in a sleep laboratory with bulky equipment. The artificial nature of the sleep lab can be associated with the first-night effect, and two consecutive nights are preferable in a clinical study paradigm to eliminate this potential issue. Sleep schedules and durations are controlled to keep the testing conditions constant, which is hardly an assessment of the patient’s natural sleep. In addition, repeated PSGs to assess the long-term effects of sleep medication on sleep quality and architecture are burdensome for both patients and healthcare providers.
For long-term evaluation, although subjective sleep assessment is often used to evaluate changes in insomnia symptoms, sleep state misperception, which is the discrepancy between subjective and objective sleep, is often observed in patients with insomnia.26 At-home sleep monitors may help address this problem by allowing participants to sleep in their own beds while maintaining satisfactory agreement with PSG.27,28 A home electroencephalogram (EEG) monitoring device can assess patients’ natural sleep compared with PSG because it uses only one channel and thus imposes a less onerous burden during the night, particularly in patients with insomnia, resulting in various studies incorporating devices as part of their protocol. Daily monitoring of sleep architecture in a population of adolescents (11–17 years old) showed that home sleep monitoring was possible in approximately 95% of cases, and discomfort from wearing the device was rated as minimal to mild.29 A study that monitored sleep architecture and stress for 15 days found that decreased REM sleep and sleep efficiency increased stress the next day.30 In addition, the sleep EEG monitoring over multiple nights revealed that poor sleep quality led to greater variability in blood glucose levels. Thus, home-based sleep EEG monitoring appears to represent a feasible, acceptable method and allows for continuous sleep monitoring in large populations.31 In both research and clinical practice, sleep EEG monitoring has been providing new insights.
The FLUID study was a 12-week trial that prospectively assessed the effect of lemborexant on changes in objective variables using home EEG monitoring and subjective variables using sleep diaries.32 This article presents the results of a primary analysis of the FLUID study, focusing on changes in both objective and subjective sleep quality over the course of the study period.
Materials and Methods
Clinical Trial Oversight
This open-label, single-arm, single-center, FLUID study was conducted at Nagoya University Hospital from June 1, 2021, to September 30, 2022. The study protocol was approved by the Nagoya University Certified Review Board (2021–0079) and was registered in the Japanese Registry of Clinical Trials (jRCT s041210024). The study was conducted in compliance with the Declaration of Helsinki principles and Ministry of Health, Labour and Welfare regulations. Data were collected by site investigators, analyzed by statisticians, and interpreted by the authors. Data analyses were conducted from October 1 to 30, 2022. After explaining the study procedures, risks, and benefits, written informed consent was obtained from each participant.
Participants
The recruited participants were local residents aged ≥ 50 years in Japan. The inclusion criteria were as follows: (1) ability to provide written informed consent before the study began; (2) insomnia disorder based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; (3) a score ≥24 on the Japanese version of the Mini-Mental State Examination. Additional details of key inclusion and exclusion criteria have been described previously.32 Prohibited prescriptions and over-the-counter medications (see the protocol paper32 for details) were not allowed to be concomitantly administered from one week prior to the start of treatment until the end of the continuation phase of treatment. The participants were compensated for their time and travel to the study visits.
Clinical Trial Procedures
The study design has been previously described in detail.32 Briefly, following the initial screening period, eligible participants were treated with lemborexant (5 mg; LEM5) for 12 weeks, followed by a follow-up period of approximately 4 weeks. If a participant did not feel that their sleep or other symptoms had improved, the dose was increased to 10 mg (LEM10) after consulting with a study physician based on the participant’s complaints. The participants completed an electronic sleep diary in the morning from the screening period through week 12. During the test period, all participants visited the hospital on three pairs of two successive days at baseline and weeks 4 and 12 after treatment (Figure 1). All participants underwent a home-based sleep test using Zmachine Insight+ (General Sleep, Cleveland, Ohio, USA) for the night surrounding the hospital visits. Questionnaires including the Pittsburgh Sleep Quality Index Japanese version (PSQI),33 Epworth Sleepiness Scale (ESS),34 temperament and character inventory harm avoidance (TCI-HA),35 and Beck Depression Inventory (BDI)36 were also administered.
Outcomes
Objective sleep parameters at home were evaluated using Zmachine Insight+,27,28 which is a single-channel EEG that monitors sleep and provides algorithm-based sleep staging. The following four parameters were evaluated: latency to persistent sleep (LPS; time elapsed until the onset of a 12-minute period in which 10 minutes were recorded as sleep), wake after sleep onset (WASO; total awake minutes following LPS), total sleep time (TST; accumulation of all epochs determined to represent sleep during the time in bed), and sleep efficacy (SE; proportion of time spent asleep per total recording time; TRT, calculated as TST/TRT×100) scores. Subjective sleep parameters were derived from electronic sleep diaries, and subjective sleep onset latency (sSOL), subjective WASO (sWASO), subjective TST (sTST), and subjective SE (sSE) scores were evaluated. Each sleep diary parameter was calculated as the mean of the seven nights of baseline and weeks 4 and 12 of treatment. The primary and secondary outcomes were the change in objective (LPS, WASO, TST, and SE) and subjective (sSOL, sWASO, sTST, and sSE, as derived from sleep diary data) sleep parameters, respectively, from baseline to weeks 4 and 12.
Safety Assessments
Safety and tolerability were monitored based on repeated assessments of adverse events, clinical examination, and clinical laboratory results at every hospital visit.
Statistical Analysis
Basic statistics were calculated for each score at each evaluation point. A paired t-test was used to compare the objective and subjective sleep parameters at baseline with those at weeks 4 and 12, as previously specified in the statistical analysis plan.32 Since this was a pilot trial, multiple comparisons were not corrected. As a post hoc analysis, a mixed-effects linear regression analysis was performed using the baseline data as a fixed effect to examine the impact of baseline data on objective sleep parameters with lemborexant. Further, a repeated-multivariable analysis of variance (MANOVA) was performed to account for the differences between baseline objective sleep parameters and the cut-off points from PSQI (30 min for LPS, 360 min for TST, and 85% for SE) and the prior trial (45 min for WASO).20 Statistical significance was defined as p < 0.05. All statistical analyses were performed using JMP pro ver.16 and SAS ver.9.4 (SAS Institute, Inc., Cary, NC, USA).
Safety analyses were summarized using descriptive statistics for all participants.
Results
Participant Characteristics
Of the 45 participants screened, 31 began the LEM5 therapy. Subsequently, 10 subjects were titrated to the LEM10 (Figure 2) after the week 4 visit. Among the participants who started LEM5 therapy, 16 (51.6%) were women, with a mean age of 58.5 years (range, 50–72 years) (Table 1). The details of comorbid disease, concomitant medications during the trial period, and insomnia symptoms are presented in Supplement Tables 1–3. Among the participants included, 29 (87.8%) completed the study, while very few people discontinued the study, with one participant discontinuing due to adverse events (3.3%). Among the 28 (90.3%) participants with poor sleep quality (PSQI >5), 58.0% had excessive daytime sleepiness (ESS >10), and four (12.9%) exhibited moderate depressive symptoms (BDI >19).
 |
Table 1 Characteristics of Participants |
 |
Figure 2 Participants through the trial. Abbreviation: MRI, magnetic resonance imaging. |
Objective Sleep Change
Home sleep EEG monitoring with no missing data from bedtime to awakening were available for 27 patients at baseline, 22 patients at week 4, and 23 patients at week 12 (Table 2). SE at week 4 significantly increased from baseline (mean changes 3.0±5.5%; p = 0.032). Similarly, a significantly greater increase from baseline in mean SE was observed at week 12 (mean changes 4.6±9.5%; p = 0.033). TST at weeks 4 and 12 tended to increase from baseline; however, it did not reach a significant level (at week 4, 23.5±64.3 min; p = 0.140; at week 12, 27.5±62.2 min; p = 0.050). For TRT, there were no significant differences in the changes from baseline for weeks 4 and 12. Mean changes from baseline at weeks 4 and 12 for LPS and WASO did not decrease significantly (LPS at week 4, 7.5±26.0 min; p = 0.237 and at week 12, –5.9±31.2 min; p = 0.385 and WASO at week 4, –9.4±41.7 min; p = 0.352 and at week 12, −4.6±35.9 min; p = 0.555) (Table 2).
 |
Table 2 Objective Sleep Parameters at Baseline, and 4th and 12th Weeks |
Relationship analysis between baseline and post-lemborexant changes revealed that for LPS, WASO, TST, and SE, poor sleep at baseline was associated with greater improvement in these measures using lemborexant treatment (LPS, estimate = −0.59, standard error = 0.18, t-value = –3.18, p = 0.004; WASO, estimate = –0.76, standard error = 0.20, t-value = –3.68, p = 0.001; TST, estimate = –0.56, standard error = 0.11, t-value = –4.97, p < 0.0001; SE, estimate = –0.39, standard error = 0.11, t-value = –3.25, p = 0.003).
The MANOVA revealed that WASO and TST at weeks 4 and 12 were significantly improved from baseline (WASO at week 4, F(1, 16) = 11.7, p = 0.003, and at week 12, F(1, 20) = 9.20, p = 0.006; TST at week 4, F(1, 16) = 22.1, p < 0.001, and at week 12, F(1, 20) = 8.15, p = 0.009). The improvement in LPS and SE at week 12 was significant compared to baseline (LPS, F(1, 20) = 12.2, p = 0.002 and SE, F(1, 20) = 16.4, p < 0.001), although there were no significant changes in those at week 4 (LPS, F(1, 16) = 1.59, p = 0.224 and SE, F(1, 16) = 1.40, p = 0.252) (Table 2).
Subjective Sleep Change
All subjective sleep parameters significantly changed during lemborexant treatment. In detail, sSOL and sWASO decreased significantly from baseline during 4 and over 12 weeks of treatment (sSOL at week 4, –24.8±27.9 min; p < 0.001, and at week 12, –27.9±28.9min; p < 0.001; sWASO at week 4, –26.2±39.3min; p < 0.001, and at week 12, –31.0±38.0min; p < 0.001). Lemborexant treatment significantly increased both sTST and sSE at week 4 compared with those at baseline, and its effect was maintained across 12 weeks (sTST at week 4, 76.4±66.2 min; p < 0.001, and at week 12, 88.3±78.1 min; p < 0.001 and sSE at week 4, 14.6±16.0%; p < 0.001, and at week 12, 18.9±17.4%; p < 0.001, Table 3).
 |
Table 3 Subjective Sleep Parameters at Baseline, and 4th and 12th Weeks |
Sleep Disturbance, Daytime Sleepiness, Harm Avoidance, and Depressive Symptom
PSQI and BDI did not decrease significantly at week 4, but by week 12, both improved significantly from baseline (PSQI at week 4, –0.5±2.9; p = 0.388; week 12, –1.2±2.4; p = 0.012; BDI at week 4, –1.2±4.5; p = 0.153; week 12, –3.1±5.2; p = 0.003). At week 4, ESS decreased significantly from baseline; at week 12, this decrease was maintained (at week 4, –3.2±4.4; p < 0.001, and at week 12, –4.3±4.7; p < 0.001). TCI HA20 did not show any significant changes between baseline and weeks 4 and 12 (at week 4, –0.2±3.2; p = 0.699, and at week 12, –0.8±3.6; p = 0.245, Table 4).
 |
Table 4 Sleep Disturbance, Daytime Sleepiness, and Depressive Symptoms at Baseline, and 4th and 12th Weeks |
Safety
Somnolence was the most common adverse event (Table 5). Non-serious AEs were mostly mild or moderate in severity. All adverse events were transient and resolved completely. Falls were reported as an adverse event by one participant taking LEM5 therapy; however, this happened before taking lemborexant and was not considered related to the study drug by the investigator. No deaths occurred during the study. No evidence of suicidality was found, and no notable findings were reported in clinical laboratory tests, vital signs, weight, or electrocardiograms.
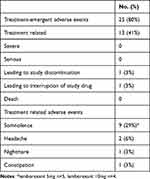 |
Table 5 Safety Summary During Treatment and Follow-Up Periods |
Discussion
This study prospectively investigated the changes in objective and subjective sleep quality during 12 weeks of lemborexant treatment. The findings from the analyses demonstrate the objective improvement of sleep quality using lemborexant treatment across 12 weeks and support the previously reported benefit of lemborexant on nighttime measures of insomnia.20 To the best of our knowledge, this is the first study to show improvement of objective sleep indices when taking into consideration the baseline values for long-term lemborexant treatment in a more natural sleep environment.
In this study, we showed that SE by sleep EEG monitoring significantly increased at weeks 4 and 12 compared to baseline, with a mean increase of approximately 4%. Previous studies showed that one month of lemborexant therapy significantly improved sleep efficiency compared with both placebo and zolpidem measured objectively with PSG.20,37 In trials evaluating the efficacy of lemborexant,20,37 objective sleep measures were assessed using PSG. The recording time was constant and performed at sleep centers, which is different from the usual sleep environment. In this study, the patients followed their usual sleep schedules at home and recorded their sleep. Although our study protocol was different from the previous one, our results are consistent with these results, and home sleep EEG records showed a persistent effect of lemborexant three months later.
LPS, WASO, and TST at week 12 tended to improve with lemborexant treatment; however, this was not significant in the primary analysis. In the Phase 3 study, baseline PSG excluded participants who did not meet the WASO criteria (WASO average ≥ 60 min on the two consecutive PSGs, with neither night < 45 min), and the effects of lemborexant were assessed by comparison with placebo and zolpidem tartrate extended-release.20 These two points differed from the present study. Thus, the objective sleep parameters at baseline may have influenced the results. According to the mixed-effects linear regression analysis, the baseline data significantly influenced the effect of lemborexant. Moreover, the MANOVA showed that objective sleep parameters significantly improved with treatment when the baseline was considered. In particular, WASO and TST showed consistent and significant improvements from week 4 to week 12 compared to baseline. In insomnia participants with objective short sleep duration (< 6 h), lemborexant consistently showed subjective and objective benefits on SOL, WASO, and TST compared to placebo.38 Together, lemborexant had a modest effect in patients with mild objective insomnia and a significant effect in patients with severe objective insomnia, compared to baseline. This suggests that lemborexant have an ameliorating effect on insomnia in severe cases of insomnia, but not unnecessarily prolong sleep in mild cases. Therefore, it may be a well-tolerated and effective treatment option.
There are several possible reasons for the differences between the previous study20 and this study in terms of LPS and WASO, which include insomnia severity, number of study cases, and study protocol. First, the severity of insomnia among the participants in this study was lower since our study included participants with LPS < 30 min (51.6%) and WASO < 45 min (41.9%); on the other hand, the previous study excluded them. Second, the number of cases that could be objectively evaluated for sleep was smaller than that in the previous study (n=1006). The current study design and procedures differed from previous studies;20,21,23 open-label single-arm and randomized double-blind placebo-controlled trials, one night of home sleep EEG monitoring on a flexible sleep schedule and two nights of PSG on a strict sleep schedule in the sleep laboratory. Open-label studies are considered more susceptible to bias for both researchers and participants than double-blind controlled trials.39,40 Because of individual daily differences in sleep parameters,41 the study design and procedures may hinder the identification of disturbed sleep. Although the impact could be minimal, the above differences between this study and the previous study might have affected the results.
We showed that all subjective sleep parameters, such as sSOL, sWASO, sTST, and sSE, significantly improved from baseline after lemborexant treatment. In Japanese individuals and in subjects ≥55 years of age, lemborexant treatment provided significant benefit versus placebo on patient-reported parameters of sleep onset and sleep maintenance as assessed at the beginning (first seven nights) and at the end of the first six months of treatment, and was well tolerated.20,23,42 Our findings were supported by these studies.
In this study, patient-reported sleep onset latency, sleep maintenance, and subjective sleep quality, as assessed by the PSQI, significantly improved with lemborexant treatment. In addition, home sleep EEG monitoring revealed that SE significantly improved after treatment. The relationship between objective sleep variables and the perception of sleep quality has been studied. Among objective sleep variables, including SE, arousals, spectral frequency, and cyclic alternating pattern, objective SE was most consistently related to sleep quality.43 Therefore, the results of this study suggest that the improvement in SE may have led to an improvement in subjective sleep quality.
Objective and subjective sleep parameters were assessed simultaneously from baseline to weeks 4 and 12. We found that some participants showed discrepancies between objective and subjective sleep assessments. The number of days that sleep was evaluated differed, with one night of objective evaluation and seven nights of subjective evaluation. Thus, the operability of the EEG monitoring device and the discomfort caused by wearing it29 may have had an impact on a few patients. Sleep disorders associated with insomnia include obstructive sleep apnea (OSA) and periodic limb movements (PLMs) /restless legs syndrome (RLS). These were excluded during the screening examination, but should be considered if there is a discrepancy between objective and subjective sleep assessment. Furthermore, sleep state misperception to underestimate TST and overestimate LPS and WASO is common, with a prevalence ranging from 9.2–56% in patients with insomnia.25,44 In the present study, some cases were found to have discrepancies between objective and subjective indices. In future studies, it will be necessary to examine the discrepancy between objective and subjective sleep, and the degree of improvement.
Symptoms associated with insomnia, such as subjective sleep quality, excessive daytime sleepiness, and depressive symptoms, significantly improved after three months of lemborexant treatment. The effects of pharmacologic and non-pharmacologic interventions on insomnia have been reported to improve sleep disturbance, sleepiness, and depressive symptoms.45–47 These symptoms associated with insomnia have a negative impact on daily life. Studies on the short- and long-term effectiveness and safety of lemborexant have reported evidence of their efficacy as well as a favorable safety profile.20,23,37
Study Strengths and Limitations
This FLUID study was the first trial in which sleep EEG monitoring was performed in a natural environment for the long-term use of lemborexant. As the sleep schedule and duration were not controlled, it was possible to assess the patient’s natural sleep. Because the exclusion criteria of this study were set lower than those of the clinical trial, patients with varying symptom severity were included. Therefore, we were able to present data that more closely resembled actual clinical practice. The study included patients with insomnia and a variety of comorbidities and showed that sleep was improved in these patients.
The major limitation was that the study was small and open-label. The single-channel EEG monitor was used, leading to fewer sleep-related data than PSG. Therefore, the influence of sleep disorders affecting insomnia other than OSA and PLMs/RLS assessed in the screening test could not be excluded. The study excluded subjects with clinically significant (unstable) physical and/or mental illnesses as well as subjects with comorbid sleep disorders. These, along with other enrolment criteria, could limit the generalizability of the findings. In addition, sleep EEG monitoring could not be completed for all patients. Poor sensor fitting and recording errors can occur because a medical provider does not perform home testing. Consequently, the number of cases in which objective sleep indices could be evaluated was small. This may have led to a decrease in the statistical power of this study.
Conclusion
Lemborexant therapy significantly increased SE measured objectively using sleep EEG monitoring at home, including significant improvement in excessive daytime sleepiness, subjective sleep quality, and depressive symptoms in older adults with insomnia disorder. When baseline values were included in the analyses, sleep onset and sleep maintenance variables improved significantly with treatment. Despite some limitations, such as the open-label study, the strict criteria for selecting subjects, and the sleep EEG monitoring at home, our findings suggest that lemborexant may significantly improve insomnia in severe cases of insomnia, but not unnecessarily prolong sleep in mild cases. Consequently, it may be a well-tolerated and effective treatment option. Adding home sleep records to patient-reported outcomes would allow for a more accurate evaluation of the effects of sleep medication.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank Tomoko Wada, Keita Kawai, and Minami Kinouchi for their help with the data collection.
Funding
This study was financially supported by Eisai Co., Ltd., the owner and manufacturer of lemborexant.
Disclosure
AF and YK are employees of Eisai Co., Ltd. KI reports personal fees from Eisai, Lundbeck Japan, MSD, Otsuka, Sumitomo Pharma, Takeda, Viatris, Yoshitomi, Meiji Seika Pharma, and Kyowa, outside the submitted work. TT reports grants from Canon Medical Systems, outside the submitted work. NO has received research support or speaker honoraria from, or has served as a joint researcher with, or a consultant to, Otsuka Pharmaceutical Co., Ltd., Sumitomo Pharma Co., Ltd., Viatris Inc., Eisai Co., Ltd., The KAITEKI Institute, Inc., Ricoh Co., Ltd., Mitsubishi Tanabe Pharma Co.; reports personal fees/grants from Eli Lilly, DAIICHI SANKYO, TSUMURA, Takeda, Mochida, Meiji Seika Pharma, EA Pharma, Viatris, Ricoh, Nippon Boehringer Ingelheim, Lundbeck Japan, Nihon Medi-Physics, Nippon Chemiphar, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Endomba FT, Tchebegna PY, Chiabi E, et al. Epidemiology of insomnia disorder in older persons according to the diagnostic and statistical manual of mental disorders: a systematic review and meta-analysis. Eur Geriatr Med. 2023;14(6):1261–1272. doi:10.1007/s41999-023-00862-2
2. Canever JB, Cândido LM, Moreira BS, et al. A nationwide study on sleep complaints and associated factors in older adults: ELSI-Brazil. Cad Saude Publica. 2023;39:e00061923. doi:10.1590/0102-311xen061923
3. Doi Y, Minowa M, Okawa M, Uchiyama M. Prevalence of sleep disturbance and hypnotic medication use in relation to sociodemographic factors in the general Japanese adult population. J Epidemiol. 2000;10(2):79–86. doi:10.2188/jea.10.79
4. Klink ME, Quan SF, Kaltenborn WT, Lebowitz MD. Risk factors associated with complaints of insomnia in a general adult population. Influence of previous complaints of insomnia. Arch Intern Med. 1992;152:1634–1637. doi:10.1001/archinte.1992.00400200070012
5. Lu JL, Freire AX, Molnar MZ, et al. Association of chronic insomnia with mortality and adverse renal outcomes. Mayo Clin Proc. 2018;93(11):1563–1570. doi:10.1016/j.mayocp.2018.05.032
6. LeBlanc ES, Smith NX, Nichols GA, et al. Insomnia is associated with an increased risk of type 2 diabetes in the clinical setting. BMJ Open Diabetes Res Care. 2018;6:e000604.
7. de Almondes KM, Costa MV, Malloy-Diniz LF, et al. Insomnia and risk of dementia in older adults: systematic review and meta-analysis. J Psychiat Res. 2016;77:109–115. doi:10.1016/j.jpsychires.2016.02.021
8. Wu MP, Lin HJ, Weng SF, et al. Insomnia subtypes and the subsequent risks of stroke: report from a nationally representative cohort. Stroke. 2014;45(5):1349–1354. doi:10.1161/STROKEAHA.113.003675
9. Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(1–3):10–19. doi:10.1016/j.jad.2011.01.011
10. Garbarino S, Magnavita N, Guglielmi O, et al. Insomnia is associated with road accidents. Further evidence from a study on truck drivers. PLoS One. 2017;12:e0187256. doi:10.1371/journal.pone.0187256
11. Kessler RC, Berglund PA, Coulouvrat C, et al. Insomnia and the performance of US workers: results from the America insomnia survey. Sleep. 2011;34(9):1161–1171. doi:10.5665/SLEEP.1230
12. Miyata S, Noda A, Iwamoto K, et al. Poor sleep quality impairs cognitive performance in older adults. J Sleep Res. 2013;22(5):535–541. doi:10.1111/jsr.12054
13. Kato K, Miyata S, Ando M, et al. Influence of sleep duration on cortical oxygenation in elderly individuals. Psychiatry Clin Neurosci. 2017;71(1):44–51. doi:10.1111/pcn.12464
14. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700. doi:10.1111/jsr.12594
15. Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(02):307–349. doi:10.5664/jcsm.6470
16. Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67–76. doi:10.1080/03602530902722679
17. Tsuruta Y, Iwamoto K, Banno M, et al. Effects of hypnotics on prefrontal cortex activity during a verbal fluency task in healthy male subjects: a near-infrared spectroscopy study. Hum Psychopharmacol. 2018;33(6):e2678. doi:10.1002/hup.2678
18. Lou BX, Oks M. Insomnia: pharmacologic treatment. Clin Geriatr Med. 2021;37:401–415. doi:10.1016/j.cger.2021.04.003
19. De Crescenzo F, Gl D, Ostinelli EG, et al. Comparative effects of pharmacological interventions for the acute and long-term management of insomnia disorder in adults: a systematic review and network meta-analysis. Lancet. 2022;400:170–184. doi:10.1016/S0140-6736(22)00878-9
20. Rosenberg R, Murphy P, Zammit G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA network open. 2019;2:e1918254. doi:10.1001/jamanetworkopen.2019.18254
21. Moline M, Zammit G, Cheng JY, et al. Comparison of the effect of lemborexant with placebo and zolpidem tartrate extended release on sleep architecture in older adults with insomnia disorder. J Clin Sleep Med. 2021;17(6):1167–1174. doi:10.5664/jcsm.9150
22. Clark JW, Brian ML, Drummond SPA, Hoyer D, Jacobson LH. Effects of orexin receptor antagonism on human sleep architecture: a systematic review. Sleep Med Rev. 2020;53:101332. doi:10.1016/j.smrv.2020.101332
23. Kärppä M, Yardley J, Pinner K, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2. Sleep. 2020;43:zsaa123. doi:10.1093/sleep/zsaa123
24. Mogavero MP, Silvani A, Lanza G, DelRosso LM, Ferini-Strambi L, Ferri R. Targeting orexin receptors for the treatment of insomnia: from physiological mechanisms to current clinical evidence and recommendations. Nat Sci Sleep. 2023;15:17–38. doi:10.2147/NSS.S201994
25. Moline M, Thein S, Bsharat M, et al. Safety and efficacy of lemborexant in patients with irregular sleep-wake rhythm disorder and Alzheimer’s disease dementia: results from a Phase 2 randomized clinical trial. J Prev Alzheimers Dis. 2021;8:7–18. doi:10.14283/jpad.2020.69
26. Kawai K, Iwamoto K, Miyata S, et al. A study of factors causing sleep state misperception in patients with depression. Nat Sci Sleep. 2022;14:1273–1283. doi:10.2147/NSS.S366774
27. Wang Y, Loparo KA, Kelly MR, et al. Evaluation of an automated single-channel sleep staging algorithm. Nat Sci Sleep. 2015;7:101–111. doi:10.2147/NSS.S77888
28. Miyata S, Iwamoto K, Banno M, et al. Performance of an ambulatory electroencephalogram sleep monitor in patients with psychiatric disorders. J Sleep Res. 2021;30:e13273.
29. Lunsford-Avery JR, Keller C, Kollins SH, Krystal AD, Jackson L, Engelhard MM. Feasibility and acceptability of wearable sleep electroencephalogram device use in adolescents: observational study. JMIR Mhealth Uhealth. 2020;8(10):e20590. doi:10.2196/20590
30. Yap Y, Yan Chi Tung N, Collins J, Phillips A, Bei B, Wiley JF. Daily relations between stress and electroencephalography-assessed sleep: a 15-day intensive longitudinal design with ecological momentary assessments. Ann Behav Med. 2022;56(11):1144–1156. doi:10.1093/abm/kaac017
31. Brandt R, Park M, Wroblewski K, et al. Sleep quality and glycaemic variability in a real-life setting in adults with type 1 diabetes. Diabetologia. 2021;64(10):2159–2169. doi:10.1007/s00125-021-05500-9
32. Okada I, Iwamoto K, Miyata S, et al. FLUID study: study protocol for an open-label, single-centre pilot study to investigate the efFect of Lemborexant on sleep management in Japanese subjects aged 50 years and older with insomnia disorder. BMJ open. 2021;11(11):e054885. doi:10.1136/bmjopen-2021-054885
33. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:10.1016/0165-1781(89)90047-4
34. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi:10.1093/sleep/14.6.540
35. Cloninger CR, Przybeck TR, Svrakic DM, Wetzel RD. The Temperament and Character Inventory (TCI): A Guide to Its Development and Use. St Louis: Center for Psychobiology of Personality; 1994.
36. Beck AT, Steer RA, Ball R, Ranieri W. Comparison of beck depression inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi:10.1207/s15327752jpa6703_13
37. Murphy P, Moline M, Mayleben D, et al. Lemborexant, a dual orexin receptor antagonist (DORA) for the treatment of insomnia disorder: results from a bayesian, adaptive, randomized, double-blind, placebo-controlled study. J Clin Sleep Med. 2017;13(11):1289–1299. doi:10.5664/jcsm.6800
38. Schulz KF, Grimes DA. Blinding in randomised trials: hiding who got what. Lancet. 2002;359(9307):696–700. doi:10.1016/S0140-6736(02)07816-9
39. Katz M. Study Design and Statistical Analysis: A Practical Guide for Clinicians. Cambridge: Cambridge University Press; 2006.
40. Molzof HE, Emert SE, Tutek J, et al. Intraindividual sleep variability and its association with insomnia identity and poor sleep. Sleep Med. 2018;52:58–66. doi:10.1016/j.sleep.2018.08.014
41. Inoue Y, Nishida M, Kubota N, et al. Comparison of the treatment effectiveness between lemborexant and zolpidem tartrate extended release for insomnia disorder subtypes defined based on polysomnographic findings. J Clin Sleep Med. 2023;19(3):519–528. doi:10.5664/jcsm.10378
42. Inoue Y, Watanabe T, Takashima S, et al. Efficacy and safety of lemborexant in adults with insomnia: comparing Japanese and non-Japanese subgroups from the global, phase 3, randomized, double-blind, placebo-controlled SUNRISE 2 study. J Clin Sleep Med. 2021;17(5):1067–1074. doi:10.5664/jcsm.9148
43. Cudney LE, Frey BN, McCabe RE, et al. Investigating the relationship between objective measures of sleep and self-report sleep quality in healthy adults: a review. J Clin Sleep Med. 2022;18(3):927–936. doi:10.5664/jcsm.9708
44. Rezaie L, Fobian AD, McCall WV, et al. Paradoxical insomnia and subjective-objective sleep discrepancy: a review. Sleep Med Rev. 2018;40:196–202. doi:10.1016/j.smrv.2018.01.002
45. Xu H, Zhang C, Qian Y, et al. Efficacy of melatonin for sleep disturbance in middle-aged primary insomnia: a double-blind, randomised clinical trial. Sleep Med. 2020;76:113–119. doi:10.1016/j.sleep.2020.10.018
46. Sadler P, McLaren S, Klein B, et al. Cognitive behavior therapy for older adults with insomnia and depression: a randomized controlled trial in community mental health services. Sleep. 2018;41(8):zsy104. doi:10.1093/sleep/zsy104
47. Guthrie KA, Larson JC, Ensrud KE, et al. Effects of pharmacologic and nonpharmacologic interventions on insomnia symptoms and self-reported sleep quality in women with hot flashes: a pooled analysis of individual participant data from four MsFLASH trials. Sleep. 2018;41(1):zsx190. doi:10.1093/sleep/zsx190
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

