Back to Journals » Journal of Inflammation Research » Volume 17
Are Changes in Serum IgG Glycosylation Related to the Severe Course of SARS-CoV-2 Infection and Recovery Process? In Search of New Diagnostic and Prognostic Biomarkers
Authors Sołkiewicz K , Kokot I , Dymicka-Piekarska V , Dorf J , Kratz EM
Received 30 October 2023
Accepted for publication 16 February 2024
Published 2 March 2024 Volume 2024:17 Pages 1413—1427
DOI https://doi.org/10.2147/JIR.S439005
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Katarzyna Sołkiewicz,1 Izabela Kokot,1 Violetta Dymicka-Piekarska,2 Justyna Dorf,2 Ewa Maria Kratz1
1Department of Laboratory Diagnostics, Division of Laboratory Diagnostics, Faculty of Pharmacy, Wroclaw Medical University, Wroclaw, Poland; 2Department of Clinical Laboratory Diagnostics, Medical University of Bialystok, Bialystok, Poland
Correspondence: Ewa Maria Kratz; Katarzyna Sołkiewicz, Email [email protected]; [email protected]
Introduction: Immunoglobulin G (IgG) glycosylation affects its effector functions and is essential in many steps of the inflammatory cascade. Therefore, it may be an important parameter for assessing the body’s immune response during the course of COVID-19 (Coronavirus disease 2019).
Methods: The N- and O-glycosylation of serum IgG in severe COVID-19 patients (n=87), convalescents (n=50), and healthy subjects (n=65) were examined using a modified lectin-ELISA method with specific biotinylated lectins. The obtained data were analyzed using STATISTICA 13.3PL software.
Results: We showed significantly higher expression of Lewisx oligosaccharide structures in severe COVID-19 patients than in the other two groups. Moreover, significantly lower expression of Lewisy sugar structures in IgG glycans was observed in the convalescents when compared with COVID-19 patients and healthy subjects. The lowest expression of highly branched N-glycans in cases of severe COVID-19 indicates that the development of the disease is associated with the presence of typical IgG biantennary N-glycans. The lack of significant differences in the expression of Tn antigen in IgG between studied groups and the significantly lower expression of T antigen in convalescents compared to the patients with severe COVID-19 and healthy subjects indicates a decrease in the content of the T antigen in IgG O-glycans in subjects recovered from COVID-19. Substantially higher reactivities of IgG O-glycans with Jacalin observed in COVID-19 patients and convalescents in comparison to the control group were most probably caused by increased expression of core 3 O-glycans in IgG.
Conclusion: Severe COVID-19 is accompanied by the expression in serum IgG of sialylated biantennary and highly branched N-glycans, decorated by fucose of Lewisx and Lewisy structures. The higher reactivity of IgG O-glycans with Jacalin in severe COVID-19 patients and convalescents indicates that the disease development and the recovery process are most probably accompanied by increased expression of the core 3 O-glycans.
Keywords: serum IgG, glycosylation, SARS-CoV-2, COVID-19, inflammation
Introduction
Coronaviruses most often cause mild to moderate upper-respiratory tract illnesses in humans. Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the SARS-CoV-2 virus (Severe Acute Respiratory Syndrome Coronavirus 2). Like other coronaviruses, SARS-CoV-2 has four structural proteins, known as the S (spike), E (envelope), M (membrane), and N (nucleocapsid) proteins. The N protein holds the RNA genome; together, the S, E, and M proteins create the viral envelope.1 Patients with SARS-CoV-2 infection can experience a range of clinical manifestations, from no symptoms, a mild infection similar to a cold, through flu-like symptoms, to pneumonia requiring hospitalization until death.2 In the first few days after infection with SARS-CoV-2, most patients are asymptomatic and may develop mild upper respiratory symptoms and/or systemic flu-like illness. Severe COVID-19 usually develops at least one week after infection, which may suggest a greater role for a dysregulated immune response than a direct viral cytopathic effect.3 Immunoglobulin G (IgG) constitutes about 75% of human blood serum immunoglobulins, mediating key cellular functions during viral and bacterial infections. In addition to their neutralizing effect, IgG antibodies have the ability to bind to foreign antigens, forming immune complexes (ICs), which can influence the pathogenesis of disease, especially those with an inflammatory background. This is characteristic of some autoimmune and infectious diseases, where the ICs produced cause a hyper-inflammatory reaction that damages host tissues.4 Glycoproteins’ glycosylation plays an essential role in regulating inflammation and immune response in infectious diseases. IgG in its constant domain (Fc) has one biantennary N-linked glycan, which is attached to asparagine 297 (Asn 297),5,6 composed of a constant core structure containing three mannose residues and four N-acetylglucosamine (GlcNAc) residues, and may contain additional core fucose, as well as bisecting GlcNAc. The branching arms (α-6 and α-3) may vary in their glycosylation patterns consisting of terminal sialic acid (SA), galactose, and fucose.5–7 The second N-glycosylation site is found in the VH and VL (heavy and light chain of variable regions, respectively) and has been detected in 15–25% of all serum IgG. Glycans present in the IgG Fab region increase the stability of the antibody and modulate the binding of IgG to antigens.8 The presence or absence of one sugar residue in the oligosaccharide structure of N-glycan may result in stimulation or suppression of the immune response. Changes in IgG Fc glycosylation are known to be involved in the pathogenesis of rheumatoid arthritis (RA), Crohn’s disease, and lupus erythematosus, where reduced galactosylation and sialylation of IgG activates effector cells and initiates the inflammatory response.9 It has been documented for serum IgG that galactosylation of conserved N-glycans (Asn-297) in the heavy chain CH2 domains is reduced in patients with rheumatoid arthritis, and that IgG agalactosylation level is proportional to disease severity.10 It has also been reported that more than 85% of IgG is fucosylated.11 In mammals, core fucosylation (α1,6-fucosylation) involves the attachment of fucose to the innermost GlcNAc at the reducing end of N-glycans by α1,6-fucosyltransferase. The lack of core fucose in IgG1 glycans is directly related to increased binding affinity to FcγRIIIa and FcγRIIIb resulting from glycan–glycan interactions between FcγRIII glycans attached to Asn162 and IgG glycans attached to Asn297, which increase cellular cytotoxicity.12–14 Fucosylated glycans are involved in various physiological processes and/or pathologies, including tissue development, cell adhesion, fertilization, angiogenesis15 as well as cancer metastasis.16 Alterations in glycoprotein glycans’ fucosylation were observed in various inflammatory conditions, including RA,17–21 chronic pancreatitis,22 Crohn’s disease,23 and sclerosing cholangitis.24 Besides N-glycans present in the Fab and Fc regions, the presence of O-linked glycans was also observed in the hinge region of the IgG molecule.25,26 Similarly to N-glycosylation, O-glycosylation is a post-translational modification that occurs after protein synthesis and involves the attachment of a sugar molecule to the oxygen atom of the serine (Ser) or threonine (Thr) residues of the polypeptide chain.27,28 The most common and well-known type of protein O-glycosylation is mucin-type glycosylation (GalNAc type), a diverse form of post-translational modification that can occur in any protein and is initiated by a family of up to 20 GalNAc polypeptide transferases that decorate proteins with GalNAc residues (GalNAcα1-O-Ser/Thr, called the Tn antigen).29,30 Tn antigen formation is the initial step of the O-glycosylation pathway and can be further extended in three different ways: (1) by adding α2,6-linked sialic acid (forming the sialyl-Tn antigen), (2) by adding galactose and forming the oligosaccharide structure called T antigen (core 1), or (3) by adding galactose to N-acetylglucosamine linked directly to Ser/Thr (core 3).30
As IgG expression is closely associated with some viral infections, in our study, we set out to characterize the profile and degree of IgG N- and O-glycosylation in sera of severe COVID-19-infected patients as well as verify whether there are any differences in IgG glycosylation between patients with severe COVID-19, convalescents, and healthy subjects. Our research goals were also focused on searching for sufficiently sensitive and specific diagnostic glyco-biomarkers, usable in the monitoring of disease progression and/or the recovery process.
Materials and Methods
Study Groups
The research was conducted in accordance with the Helsinki-II declaration, and the protocol was approved by the Bioethics Committee of the Medical University of Bialystok (Permission No. APK.002.26.2021, APK-002.171.2023) and Bioethics Human Research Committee of Wroclaw Medical University (Permission No. KB-36/2023, KB-89/2023). Informed consent was obtained from each study participant. The study group consisted of 87 patients with a SARS-CoV-2 infection diagnosis, confirmed using a polymerase chain reaction (PCR) test, who were admitted to the Emergency Department of the University Clinical Hospital in Bialystok between January and November 2021. We enrolled patients in stages 3 (n=75) and 4 (n=12), based on the Modified Early Warning Score (MEWS) classification. They required intensive treatment because of pneumonia, with or without acute respiratory distress syndrome (ARDS), or due to multiple organ dysfunction syndrome (MODS).31 None of the patients from the COVID-19 group were in the 1st or 2nd stage of the disease (exclusion criteria). The MEWS score is recommended by the Polish Society of Epidemiology and Infectious Diseases and relies on the following parameters: blood pressure, heart rate, respiratory rate, body temperature, and neurological symptoms.31 Four stages of COVID-19 progression were described based on the above parameters: 1) asymptomatic and mildly symptomatic infection, 2) symptomatic infection with pneumonia, without symptoms of ARDS, 3) symptomatic infection with pneumonia and symptoms of ARDS, 4) symptomatic infection with MODS (Table S1, Supplementary Materials). The project included SARS-CoV-2 infected patients who were conscious and able to make decisions about participation in the study. Table 1 presents the characteristics of severe COVID-19 patients. The participants of the remaining two study groups included in the project had to meet the criteria described below. The convalescents group consisted of 50 convalescents (19 males/31 females, age 28–75) whose blood was positive for IgG antibodies of SARS-CoV-2, who had suffered from SARS-CoV-2 infection in the last 3–4 weeks before recruitment to the study, did not take any medications, for whom the course of the disease was mild (fatigue, increased temperature, loss of taste and smell, muscle pain and headache). None of the patients required hospitalization, and they were SARS-CoV-2 negative at the time of collecting biological material for the present study. Convalescents qualified for the study did not receive any anti-inflammatory drugs at the time of blood collection for the study. The control group was composed of 65 healthy subjects (27 males/38 females, aged 30–74), who did not suffer from SARS-CoV-2, and whose blood was free from specific IgG antibodies against this virus. Participants with comorbidities, both convalescents and healthy subjects from the control group, were excluded from participation in the study.
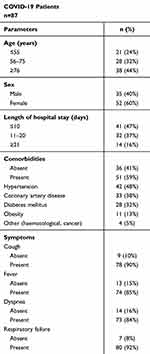 |
Table 1 Characteristics of Patients with Severe COVID-19 |
Material
In all groups of participants, venous blood (5.5 mL) was collected in test tubes without anticoagulants (S-Monovette, SARSTEDT, Germany). Within 30 min after collection, blood was centrifuged for 20 min at 1000×g to obtain serum, which was further stored at −75°C at the Department of Clinical Laboratory Diagnostics Medical University of Bialystok. Until the start of the research, all blood sera were transported from the Medical University of Bialystok and stored at (−86) degrees Celsius at the Wroclaw Medical University Biobank.
Methods
IgG concentration values in sera, necessary for the calculation of the amount of IgG for the lectin-ELISA test, were estimated using the turbidimetric method, as described previously.32 The profile and degree of IgG N- and O-glycosylation were determined using a modified solid-phase lectin-ELISA, as previously described in detail.26,33,34 The research methodology used was developed at the Department of Laboratory Diagnostics, under the supervision of Ewa Maria Kratz, during the realization of previous research on IgG glycosylation, the results of which were published in 2021–2022.26,33,34 The lectin-ELISA method used is based on the reactivity of IgG glycans with specific biotinylated lectins (Vector Laboratories Inc., Burlingame, CA, USA). In short: the microtiter plates (Maxisorp, Dako, Denmark) were incubated with 0.01 mg/mL protein G (Abcam, Boston, MA, USA) solution in 10 mM TBS pH=7.4 for 2 h at 37°C, and then at 4°C overnight. In the next step, 50 µL solution with 500 ng of IgG for N-glycans analysis and 800 ng of IgG for O-glycans analysis, diluted with 10 mM TBS-T (TBS with 0.1% Tween20, pH=7.4), was applied to each well of the ELISA plate and incubated for 3 h at 37°C. Then reduction with dithiothreitol (DTT) was carried out for 70 min at 37°C. After washing (10 mM TBS-T, pH=7.4), the plates were incubated for 90 min at 37°C with biotinylated lectins appropriately diluted with 10 mM TBS-T as follows: RCA-I 1:500, GSL-II 1:400, MAA 1:250, SNA 1:2000, AAL and LCA 1:2000, LTA 1:100, UEA and PHA-L 1:250, VVL and MPL 1:1000, and Jacalin 1:5000. Later, the microtiter plates were incubated at 37°C with phosphatase-labelled ExtrAvidin (Sigma Chemical Co., St. Louis, MO, USA). The plate wells were washed extensively using 10 mM TBS-T, pH=7.4 after each incubation step. Next, the phosphatase reaction was developed with a substrate, p-nitrophenyl phosphate. The reaction was stopped with 100 μL of 1 M NaOH per well, and the absorbance was read at 405 nm (reference filter 630 nm) with a Mindray-96A microplate reader (Shenzhen Mindray Bio-Medical Electronics Co., Shenzhen, China). All samples were tested in duplicate. Blank tests were included in each series of determinations in which all reagents were present, but the biological sample was replaced with 10 mM TBS-T. The relative reactivities of IgG glycans with lectins were expressed in absorbance units (AU). For the specificity of lectins, see Table 2.
 |
Table 2 Specificity of the Lectins Used in the Study |
Statistical Analysis
All data were analyzed using STATISTICA 13.3PL software (StatSoft Inc., Tulsa, USA). Due to the lack of confirmation of a normal distribution, as assessed with the Shapiro–Wilk test, nonparametric methods were used (ANOVA one-way analysis of variance with post hoc test and Bonferroni correction). Statistical significance was established at the level of p<0.05. The results of experimental data were presented as means and standard deviations (SD), and the distribution of the values within analyzed groups was presented as box-whisker plots with median and interquartile (25–75th percentile) ranges. The correlations with a 95% confidence interval were estimated according to the Spearman rank test. A two-tailed p-value of less than 0.05 was considered significant.
Results
The concentrations of serum IgG and its relative reactivities with lectins used are presented in Table 3 as mean absorbance values and standard deviations (SD) for each analyzed group. There were no significant differences in the results obtained for each of the parameters we analyzed between COVID-19 patients in stages 3 and 4 of the disease (Table S2, Supplementary Materials), and therefore both groups were combined and analyzed as one in further analyses. The distributions and median values of IgG relative reactivities with lectins tested, measured for COVID-19, convalescents, and control groups, are presented in Figure 1. The Spearman rank correlations, analyzed between relative reactivities of lectins used with IgG glycans, are shown in Table 4. The ROC (receiver’s operating characteristics) curve analysis was made for all examined parameters. The Youden index method was used for the determination of cut-off points for each relative reactivity of IgG glycans with lectins used. The verification of the clinical value of the laboratory test was based on the value of the area under the curve (AUC) and can be defined as zero – 0–0.5, limited – 0.5–0.7, moderate – 0.7–0.9, and high >0.9.46 Table 5 and Figure 2 presented the results of the ROC curve analysis only for parameters for which AUC was higher than 0.700.
 |
Table 3 The Concentrations of IgG in Sera and Relative Reactivities of IgG Glycans with Specific Lectins |
 |
Table 4 The Correlations Between Relative Reactivities of IgG Glycans with Lectins |
 |
Table 5 Summary of the Results of the ROC Curves Analysis of the Relative Reactivities of Serum IgG Glycans with Lectins |
 |
Figure 2 ROC curve analysis of IgG glycans’ relative reactivities with lectins for patients with COVID-19 and convalescents (A–C), convalescents and control (healthy subjects) (D–F), and COVID-19 patients and control (G). For the specificity of lectins’ LTA: Lotus tetragonolobus agglutinin, UEA: Ulex europaeus agglutinin, AAL: Aleuria aurantia lectin Jacalin: Artocarpus integrifolia lectin see Table 2 in the Materials and Methods sections. |
Discussion
Antibodies are extremely important in the protective immune response across many infectious diseases, and their function is mostly determined by their isotype and subclass. The biological activity of the immunoglobulins is tuned at the genomic, subclass, and post-translational level by glycosylation.47 Immunoglobulin G is involved in the pathogenesis and progression of many diseases and can activate a variety of effector mechanisms, such as complement-dependent cytotoxicity, antibody-dependent cellular cytotoxicity, and phagocytosis.48,49 To the best of our knowledge, this is the first study assessing the profile and degree of IgG N- and O-glycosylation using a lectin-based ELISA test in patients with severe COVID-19, convalescents from SARS-CoV-2, and healthy subjects. It has been proven that the complex clinical phenotypes of SARS-CoV-2 infection are related to changes in the degree and profile of IgG N-glycosylation. Decreases in sialylation and galactosylation play a role in COVID-19 pathogenesis via the activation of the lectin-initiated alternative complement pathway. In severe cases, low IgG sialylation contributes to the ADCC-regulated enhancement of inflammatory cytokines.50,51 In the present study, no significant differences between studied groups in the expression of sialic acid, galactose, and N-acetylglucosamine were observed. On the other hand, a high positive correlation was found between IgG glycans reactivities with RCA-I recognizing the non-reducing terminal β1,4-D-galactose (Galβ1,4) and the expression of SNA-reactive α2,6-linked SA. RCA-I has a preference for terminal Galβ1,4 rather than Galβ1,3 or Galβ1,6. However, there seems to be no specific requirement for the sub‐terminal residue, and Galβ1,4GlcNAc, Galβ1,4Glc, and Galβ1,4Man have shown similar reactivities with this lectin. What is important, the presence of SA at the 3-O or 6-O positions modifies the terminal Gal, significantly reducing the binding affinity to RCA-I.52 Taking the above-mentioned information into account, it seems possible that the lack of differences in Galβ1,4 reactivity with lectin may result from the modification of the chemical structure of Galβ1,4 by terminal sialic acid present in IgG glycans, which decreases the availability of this sugar moiety for RCA-I. It should also be noted that there were no differences between the study groups in the expression of agalactosylated N-glycans in IgG, which may indicate that, in this case, the acute inflammation accompanying SARS-CoV-2 infection is not manifested by an increase in the expression of agalactosylated IgG glycans, as may occur in some diseases accompanied by chronic inflammation, eg rheumatoid arthritis.10,53 Approximately 85% of the serum IgG pool is fucosylated, and the degree of IgG fucosylation remains relatively constant throughout most of a person’s life. The mechanisms that control IgG core fucosylation remain unclear. However, it is known that lack of core fucosylation in IgG initiates enhanced antibody-dependent cellular cytotoxicity through increased affinity for the Fc receptor FcRIIIa, which is present on monocytes, macrophages, and NK cells, and can enhance cell activation, pro-inflammatory cytokine production, and cytotoxic effector cell activity.13,54,55 Afucosylated IgG is produced in response to various viral infections, including cytomegalovirus (CMV),56 human immunodeficiency virus (HIV),57 and dengue virus,58 which all target surface-exposed, membrane-embedded proteins. COVID-19 patients with severe symptoms have decreased levels of anti-SARS-CoV-2 IgG fucosylation compared with patients with mild disease.59 No significant differences were observed between the study groups in the relative reactivity of IgG glycans with LCA, a lectin specific to core fucose. On the other hand, we showed that the relative reactivity with another lectin specific to core fucose, AAL, was significantly lower in convalescents than in the group of healthy subjects and patients with COVID-19. The observed discrepancies in the relative reactivities of fucosylated IgG glycans with AAL and LCA may result from differences in the specificity of both lectins, because not only is AAL reactive with α1,6-linked core fucose, but also, unlike LCA, it can also detect α1,3-linked antennary fucose of the Lewisx and/or Lewisy oligosaccharide structures, which most probably have an impact on the results obtained. Fucose, in addition to binding to the first GlcNAc residues in the core part of N-glycan, can also be bound to the terminal Gal residues of the glycan antennas,60 which was documented in the present study via the reactivity of IgG glycans with LTA and UEA, lectins specific to antennary fucose of Lewisx and Lewisy structures, respectively. Larsen et al59 and Chakraborty,61 in their studies using nano-LC-MS and nano-LC-MS/MS for glycosylation analysis, respectively, showed the reduction of core fucose content in IgG in patients with COVID-19. The authors found that severe COVID-19 was associated with an increased abundance of non-neutralizing, afucosylated IgG antibodies that were not present in patients with milder disease. In the present study, we showed a significantly decreased reactivity of IgG glycans with AAL specific to core fucose in the group of convalescents in comparison to patients with severe COVID-19 or healthy subjects, along with no significant differences in IgG concentrations between examined groups. Interestingly, the expression of antennary LTA- and UEA-reactive fucose is also significantly lower in the convalescents than in the other two groups. Additionally, higher reactivity of IgG glycans with LTA significantly differentiates SARS-CoV-2 infected patients from healthy subjects. The high expression of LTA-reactive fucose of Lewisx structure in eg some acute phase glycoproteins is typical for many inflammatory diseases, thus the results obtained here were unsurprising. We found no reports by other authors that could serve as reference points for our research, both in terms of the characteristics of the study groups and the methodology, therefore it is difficult to relate the results of the IgG fucosylation analysis to those obtained by other researchers. From our point of view, the glycosylation analysis using lectin-based methods is especially interesting because it mimics the availability of naturally occurring glycans for endogenic ligands. In the early stage of SARS-CoV-2 infection, IgM antibodies are produced, and react quickly, establishing a short-term response; only later is IgG produced, prolonging the immune response.62 To check the utility of the results obtained for the differentiation of studied groups of participants in a way that reflects their clinical characteristics, using ROC curve analysis, we analyzed the relative reactivities of IgG glycans with used fucose-specific lectins. For the COVID-19 patients and convalescents, the cut-off point for AAL reactivity was 0.361 AU (AUC 0.814) with a sensitivity of 66.7% and specificity of 84.0%, respectively, which indicates that this parameter has a high clinical value for differentiation of above-mentioned groups. The ROC curve analysis also shows that the expression of LTA- and UEA-reactive fucose differentiate COVID-19 patients and convalescents (AUC 0.791, cut-off point 0.473 AU and AUC 0.724, cut-off point 0.251 AU, respectively) with a sensitivity of 70.1% and 59.8% and a specificity of 80.0% and 82.0%, respectively, which indicates that both of these parameters have moderate clinical value. In conclusion, the expression of fucose of Lewisx and Lewisy oligosaccharide structures in IgG may be considered a marker of recovery from severe COVID-19.
For the convalescents and control group of healthy subjects, the cut-off point for ROC curve analysis of relative reactivities of IgG glycans with LTA was 0.347 AU (AUC 0.703, which shows a moderate clinical value) with a sensitivity of 54.0% and specificity of 81.5%. Additionally, the relative reactivity of fucose with UEA also has moderate clinical value (AUC 0.809, cut-off point 0.244 AU, sensitivity 82.0%, specificity 73.8%), which indicates that the observed differences between subjects never infected with SARS-CoV-2 and healthy past COVID-19 patients may be differentiated based on the expression of UEA-reactive fucose in IgG. The expression of highly branched IgG N-glycans reacting with PHA-L was significantly lower in patients with severe COVID-19 in comparison to healthy subjects. This may indicate that the development of the disease is most likely associated with the presence of typical biantennary N-glycans in the IgG molecule. IgG glycan reactivities with PHA-L were also visibly lower in convalescents than in healthy subjects. However, the observed differences were not significant. The observed positive correlations between the relative reactivities of IgG glycans with lectins specific to sialic acid (MAA and SNA) and the expression of antennary fucose of Lewisx and Lewisy structures indicate an increase both in antennary fucosylation of IgG N-glycans and in their sialylation. In addition, the observed positive correlations between the expression of sialic acid and UEA-reactive fucose as well as the relative reactivity of IgG glycans with PHA-L indicate that the expression of highly branched IgG N-glycans may be accompanied by higher expression of sialic acid and α1,2-linked antennary fucose. The elevated expression of highly branched glycans in some glycoproteins, such as eg those directly associated with acute phase response, is associated with the progression of many diseases with accompanying acute inflammation, including cancer.63,64 As far as we know, there is a lack of scientific reports on the expression of highly branched N-glycans in IgG during SARS-CoV-2 infection.
IgG O-glycosylation is a poorly understood process with little information available. Plomp et al25 estimated that, in blood serum, about 10% of IgG3 polyclonal antibodies and about 13% of IgG3 monoclonal antibodies contain O-glycans. In 2022, Sołkiewicz et al26 confirmed the presence of O-glycans in serum IgG in patients with advanced endometriosis and women with other gynaecological diseases. The present study showed no significant differences between studied groups in IgG glycans reactivity with VVL specific to truncated O-glycans (Tn antigen).43,44 On the other hand, the expression of MPL-reactive complete O-glycans (T antigen) in IgG was significantly higher in sera of COVID-19 patients and visibly higher in healthy subjects (although insignificant) when compared with convalescents. In addition, significantly higher relative reactivities of IgG O-glycans with Jacalin were observed in the group of COVID-19 patients and convalescents when compared to healthy subjects, which is most probably caused by increased expression of core 3 O-glycans reacting with Jacalin rather than by the expression of core 1 O-glycans, which also react with MPL but do not enable differentiation between the above mentioned groups. It seems that the increased reactivity of IgG O-glycans with Jacalin is associated with the inflammation development that accompanies severe COVID-19. The open question is whether the (chronic?) inflammatory condition may also accompany the recovery process and, if so, how long it can persist. Ryan et al65 showed persistent changes in the peripheral immune system of SARS-CoV-2 convalescents up to at least 6 months after infection. These changes may have an impact on how patients recovered from SARS-CoV-2 infection respond to other infections encountered during this period. Additional persistent activation of the immune system may also exacerbate other chronic conditions. The results of ROC curve analyses for IgG O-glycan relative reactivities with Jacalin showed that reactivity with this lectin can be used to distinguish a group of severe COVID-19 patients and convalescents from healthy subjects. The cut-off point for IgG O-glycan reactivities with Jacalin was 1.219 AU (AUC 0.770) with a sensitivity of 80.5% and specificity of 70.8% for differentiation COVID-19 patients and healthy subjects, and 1.227 AU (AUC 0.835) with a sensitivity of 88.0% and specificity of 70.8% for differentiation between convalescents and healthy subjects. This parameter has a moderate and high clinical value, respectively, and can be used as a marker of complete recovery after SARS-CoV-2 infection.
Conclusions
COVID-19 remains a global health challenge. To the best of our knowledge, this work is the first to analyse the profile and degree of N- and O-glycosylation of human serum IgG using a selected panel of specific biotinylated lectins in the context of severe SARS-CoV-2 infection. The applied lectin-ELISA method mimics the in vivo interactions between glycoprotein glycans and their endogenous ligands, including the availability of sugar moieties, additionally enabling us to deepen our understanding of the molecular mechanisms of these interactions. Based on the obtained results, we showed that the development of this viral infection is accompanied by the presence of biantennary N-glycans typical for IgG as well as highly branched N-glycans, decorated with LTA- and UEA-reactive fucose and sialylated. The observed higher reactivity of IgG O-glycans with Jacalin in severe COVID-19 patients and convalescents than in the healthy group indicates that the development of the disease and the recovery process are most probably accompanied by increased expression of core 3 O-glycans.
Strength of the Study
- The analysis of IgG glycosylation was provided in three independent groups: patients with severe COVID-19, convalescents, and a group of healthy subjects who had no contact with SARS-CoV-2, as confirmed by the lack of antibodies directed against this virus.
- The expression of fucose of Lewisx and Lewisy oligosaccharide structures in IgG N-glycans may be considered a convalescent marker indicative of patients’ recovery from SARS-CoV-2 infection.
- The reactivity of serum IgG with Jacalin and the probable expression of core 3 O-glycans appear effective markers for distinguishing healthy subjects who never were infected with SARS-CoV-2 from patients with severe COVID-19 and convalescents in recovery from infection.
- The use of the lectin-based method for IgG glycan analysis enables in vitro study of the reactions taking place in vivo, which reflect the bioavailability of IgG glycans for their naturally occurring ligands.
Limitations of the Study
- The use for glycoprotein glycosylation analysis of highly specific lectins, with specificity limited to one oligosaccharide structure or sugar residue, would certainly allow for more spectacular conclusions to be drawn. This will hopefully be enabled by the appearance of recombinant lectins in the scientific world.
Institutional Review Board Statement
The study procedures followed in the study were conducted in agreement with the Helsinki-II declaration and the protocol was approved by the Bioethics Committee of Medical University of Bialystok (Permission No. APK.002.26.2021, APK-002.171.2023) and Bioethics Human Research Committee of the Wroclaw Medical University (Permission No. KB-36/2023, KB-89/2023). Written informed consent was obtained from recruited patients.
Data Sharing Statement
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was financed by a subsidy from the Ministry of Health of the Republic of Poland, implemented as part of the subject, according to the entries in the Simple system with the numbers SUBZ.D270.22.047 and SUBZ.D270.23.017.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Yang H, Rao Z. Structural biology of SARS-CoV-2 and implications for therapeutic development. Nat Rev Microbiol. 2021;19(11):685–700. doi:10.1038/s41579-021-00630-8
2. Hu B, Guo H, Zhou P, Shi Z-L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141–154. doi:10.1038/s41579-020-00459-7
3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5
4. Ward PA, Fattahi F, Bosmann M. New insights into molecular mechanisms of immune complex-induced injury in lung. Front Immunol. 2016;7(86). doi:10.3389/fimmu.2016.00086
5. Böhm S, Schwab I, Lux A, Nimmerjahn F. The role of sialic acid as a modulator of the anti-inflammatory activity of IgG. Semin Immunopathol. 2012;34:443–453. doi:10.1007/s00281-012-0308-x
6. Seeling M, Brückner C, Nimmerjahn F. Differential antibody glycosylation in autoimmunity: sweet biomarker or modulator of disease activity? Nat Rev Rheumatol. 2017;13:621–630. doi:10.1038/nrrheum.2017.146
7. Kiyoshi M, Tsumoto K, Ishii-Watabe A, Caaveiro JMM. Glycosylation of IgG-Fc: a molecular perspective. Int Immunol. 2017;29(7):311–317. doi:10.1093/intimm/dxx038
8. Van De Bovenkamp FS, Derksen NIL, Van Breemen MJ, et al. Variable domain N-Linked glycans acquired during antigen-specific immune responses can contribute to immunoglobulin g antibody stability. Front Immunol. 2018;12(9):740. doi:10.3389/fimmu.2018.00740
9. Kozłowska K, Rydlewska M, Ząbczyńska M, Pocheć E. IgG glycosylation in autoimmune diseases. Postepy Hig Med Dosw. 2018;72:975–990. doi:10.5604/01.3001.0012.7351
10. Pasek M, Duk M, Podbielska M, et al. Galactosylation of IgG from rheumatoid arthritis (RA) patients-changes during therapy. Glycoconjugate J. 2006;23(7–8):463–471. doi:10.1007/s10719-006-5409-0
11. Mizuochi T, Taniguchi T, Shimizu A, Kobata A. Structural and numerical variations of the carbohydrate moiety of immunoglobulin G. J Immunol. 1982;129:2016–2020.
12. Shibata-Koyama M, Iida S, Okazaki A, et al. The N-linked oligosaccharide at Fc gamma RIIIa Asn-45: an inhibitory element for high Fc gammaRIIIa binding affinity to IgG glycoforms lacking core fucosylation. Glycobiology. 2009;19:126–134. doi:10.1093/glycob/cwn110
13. Ferrara C, Grau S, Jager C, et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A. 2011;108:12669–12674. doi:10.1073/pnas.1108455108
14. Mizushima T, Yagi H, Takemoto E, et al. Structural basis for improved efficacy of therapeutic antibodies on defucosylation of their Fc glycans. Genes Cells. 2011;16(11):1071–1080. doi:10.1111/j.1365-2443.2011.01552.x
15. Miyoshi E, Moriwaki K, Nakagawa T. Biological function of fucosylation in cancer biology. J Biochem. 2008;143:725–729. doi:10.1093/jb/mvn011
16. Ma B, Simala-Grant JL, Taylor DE. Fucosylation in prokaryotes and eukaryotes. Glycobiology. 2006;16:158–184. doi:10.1093/glycob/cwl040
17. Thompson S, Kelly CA, Griffiths ID, Turner GA. Abnormally-fucosylated serum haptoglobins in patients with inflammatory joint disease. Clin Chim Acta. 1989;184:251–258. doi:10.1016/0009-8981(89)90058-2
18. Brinkman-van der Linden EC, de Haan PF, Havenaar EC, van Dijk W. Inflammation-induced expression of sialyl LewisX is not restricted to alpha1-acid glycoprotein but also occurs to a lesser extent on alpha1-antichymotrypsin and haptoglobin. Glycoconjugate J. 1998;15:177–182. doi:10.1023/a:1006972307166
19. Goodarzi MT, Axford JS, Varanasi SS, et al. Sialyl Lewis(x) expression on IgG in rheumatoid arthritis and other arthritic conditions: a preliminary study. Glycoconjugate J. 1998;15:1149–1154. doi:10.1023/a:1006920007227
20. Li J, Hsu HC, Ding Y, et al. Inhibition of fucosylation reshapes inflammatory macrophages and suppresses type II collagen-induced arthritis. Arthritis Rheumatol. 2014;66:2368–2379. doi:10.1002/art.38711
21. Ryden I, Pahlsson P, Lundblad A, Skogh T. Fucosylation of α1-acid glycoprotein (orosomucoid) compared with traditional biochemical markers of inflammation in recent onset rheumatoid arthritis. Clin Chim Acta. 2002;317:221–229. doi:10.1016/s0009-8981(01)00803-8
22. Sarrats A, Saldova R, Pla E, et al. Glycosylation of liver acute phase proteins in pancreatic cancer and chronic pancreatitis. Proteomics Clin Appl. 2010;4:432–448. doi:10.1002/prca.200900150
23. Miyoshi J, Yajima T, Okamoto S, et al. Ectopic expression of blood type antigens in inflamed mucosa with higher incidence of FUT2 secretor status in colonic Crohn’s disease. J Gastroenterol. 2011;46:1056–1063. doi:10.1007/s00535-011-0425-7
24. Maroni L, van de Graaf SFJ, Hohenester SD, et al. Fucosyltransferase 2: a genetic risk factor for primary sclerosing cholangitis and Crohn’s disease–a comprehensive review. Clin Rev Allergy Immunol. 2015;48:182–191. doi:10.1007/s12016-014-8423-1
25. Plomp R, Dekkers G, Rombouts Y, et al. Hinge-Region O-Glycosylation of Human Immunoglobulin G3 (IgG3). Mol Cell Proteom. 2015;14:1373–1384. doi:10.1074/mcp.M114.047381
26. Sołkiewicz K, Kacperczyk M, Krotkiewski H, Jędryka M, Kratz EM. O-glycosylation changes in serum immunoglobulin G are associated with inflammation development in advanced endometriosis. Int J Mol Sci. 2022;23(15):8087. doi:10.3390/ijms23158087
27. Hounsell EF, Davies MJ, Renouf DV. O-linked protein glycosylation structure and function. Glycoconjugate J. 1996;13:19–26. doi:10.1007/BF01049675
28. Van den Steen P, Rudd PM, Dwek RA, Opdenakker G. Concepts and Principles of O-Linked Glycosylation. Crit Rev Biochem Mol Biol. 1998;33:151–208. doi:10.1080/10409239891204198
29. Inoue M, Yamashina I, Nakada H. Glycosylation of the tandem repeat unit of the MUC2 polypeptide leading to the synthesis of the Tn Antigen. Biochem Biophys Res Commun. 1998;245:23–27. doi:10.1006/bbrc.1998.8369
30. Brockhausen I. Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions. EMBO Rep. 2006;7:599–604.
31. Flisiak R, Horban A, Jaroszewicz J, et al. Management of SARS-CoV-2 infection: recommendations of the Polish Association of Epidemiologists and Infectiologists as of March 31, 2020. Pol Arch Intern Med. 2020;130:352–355. doi:10.20452/pamw.15270
32. Kokot I, Piwowar A, Jędryka M, Sołkiewicz K, Kratz EM. Diagnostic significance of selected serum inflammatory markers in women with advanced endometriosis. Int J Mol Sci. 2021;22(5):2295. doi:10.3390/ijms22052295
33. Sołkiewicz K, Krotkiewski H, Jędryka M, Kratz EM. Variability of serum IgG sialylation and galactosylation degree in women with advanced endometriosis. Sci Rep. 2021;11(1):5586. doi:10.1038/s41598-021-85200-x
34. Sołkiewicz K, Krotkiewski H, Jędryka M, Czekański A, Kratz EM. The alterations of serum IgG fucosylation as a potential additional new diagnostic marker in advanced endometriosis. J Inflamm Res. 2022;15:251–266. eCollection 2022. doi:10.2147/JIR.S341906
35. Lastovickova M, Strouhalova D, Bobalova J. Use of lectin-based affinity techniques in breast cancer glycoproteomics: a review. J Proteome Res. 2020;19:1885–1899. doi:10.1021/acs.jproteome.9b00818
36. Wu AM, Song SC, Sugii S, Herp A. Differential binding properties of Gal/GalNAc specific lectins available for characterization of glycoreceptors. Indian J Biochem Biophys. 1997;34(1–2):61–71.
37. Yamashita K, Kochibe N, Ohkura T, Ueda I, Kobata A. Fractionation of L-fucose-containing oligosaccharides on immobilized Aleuria aurantia lectin. J Biol Chem. 1985;260:4688–4693. doi:10.1016/S0021-9258(18)891256
38. Tateno H, Nakamura-Tsuruta S, Hirabayashi J. Comparative analysis of core-fucose-binding lectins from Lens culinaris and Pisum sativum using frontal affinity chromatography. Glycobiology. 2009;19:527–536. doi:10.1093/glycob/cwp016
39. Yan L, Wilkins PP, Alvarez-Manilla G, Do SI, Smith DF, Cummings RD. Immobilized Lotus tetragonolobus agglutinin binds oligosaccharides containing the Le(x) determinant. Glycoconjugate J. 1997;14:45–55. doi:10.1023/a:1018508914551
40. Loris R, De Greve H, Dao-Thi MH, Messens J, Imberty A, Wyns L. Structural basis of carbohydrate recognition by lectin II from Ulex europaeus, a protein with a promiscuous carbohydrate binding site. J Mol Biol. 2000;301:987–1002. doi:10.1006/jmbi.2000.4016
41. Li WP, Zuber C, Roth J. Use of Phaseolus vulgaris leukoagglutinating lectin in histochemical and blotting techniques: a comparison of digoxigenin- and biotin-labelled lectins. Histochem Cell Biol. 1993;100:347–356. doi:10.1007/BF00268933
42. Wu AM. Polyvalent GalNAcα1→Ser/Thr (Tn) and Galβ1→3GalNAcα1→Ser/Thr (T α) as the most potent recognition factors involved in Maclura pomifera agglutinin–glycan interactions. J Biomed Sci. 2005;12(1):135–152. doi:10.1007/s11373-004-8178-4
43. Tollefsen S, Kornfeld R. Vicia villosa lectins. Methods Enzymol. 1987;138:536–544. doi:10.1016/0076-6879(87)38048-6
44. Puri KD, Gopalakrishnan B, Surolia A. Carbohydrate binding specificity of the Tn-antigen binding lectin from Vicia villosa seeds (VVLB4). FEBS Lett. 1992;312:208–212. doi:10.1016/0014-5793(92)80937-c
45. Tachibana K, Nakamura S, Wang H, et al. Elucidation of binding specificity of Jacalin toward O-glycosylated peptides: quantitative analysis by frontal affinity chromatography. Glycobiology. 2006;16:46–53. doi:10.1093/glycob/cwj038
46. Bossuyt X. Clinical performance characteristics of a laboratory test. A practical approach in the autoimmune laboratory. Autoimmune Rev. 2009;8:543–548.
47. Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25(1):21–50. doi:10.1146/annurev.immunol.25.022106.141702
48. Quast I, Lünemann JD. Fc glycan-modulated immunoglobulin G effector functions. J Clin Immunol. 2014;34(Suppl 1):S51–S55. doi:10.1007/s10875-014-0018-3
49. Schroeder HW, Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010;25:41–52. doi:10.1016/j.jaci.2009.09.046
50. Petrović T, Alves I, Bugada D, et al. Composition of the immunoglobulin g glycome associates with the severity of COVID-19. Glycobiology. 2020;31(4):372–377. doi:10.1093/glycob/cwaa102
51. Hou H, Yang H, Liu P, et al. Profile of immunoglobulin G N-glycome in COVID-19 patients: a case-control study. Front Immunol. 2021;12:748566. doi:10.3389/fimmu.2021.748566
52. Wang Y, Yu G, Han Z, et al. Specificities of Ricinus communis agglutinin 120 interaction with sulfated galactose. FEBS Lett. 2011;585(24):3927–3934. doi:10.1016/j.febslet.2011.10.035
53. Liljeblad M, Lundblad A, Pahlsson P. Analysis of agalacto-IgG in rheumatoid arthritis using surface plasmon resonance. Glycoconjugate J. 2000;17(5):323–329. doi:10.1023/a:1007169621518
54. Bournazos S, Wang TT, Ravetch JV. The role and function of fcgamma receptors on myeloid cells. Microbiol Spectr. 2016;4(6). doi:10.1128/microbiolspec.MCHD-0045-2016
55. Li J, Hsu HC, Mountz JD, Allen JG. Unmasking fucosylation: from cell adhesion to immune system regulation and diseases. Cell Chem Biol. 2018;25(5):499–512. doi:10.1016/j.chembiol.2018.02.005
56. Oosterhoff JJ, Larsen MD, van der Schoot CE, Vidarsson G. Afucosylated IgG responses in humans-structural clues to the regulation of humoral immunity. Trends Immunol. 2022;43(10):800–814. doi:10.1016/j.it.2022.08.001
57. Ackerman ME, Crispin M, Yu X, et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J Clin Invest. 2013;123:2183–2192. doi:10.1172/JCI65708
58. Wang TT, Sewatanon J, Memoli MJ, et al. IgG antibodies to dengue enhanced for FcγRIIIA binding determine disease severity. Science. 2017;355(6323):395–398. doi:10.1126/science.aai8128
59. Larsen MD, De Graaf EL, Sonneveld ME, et al. Afucosylated IgG characterizes enveloped viral responses and correlates with COVID-19 severity. Science. 2020;371(6532). doi:10.1126/science.abc8378
60. Zauner G, Selman MH, Bondt A, et al. Glycoproteomic analysis of antibodies. Mol Cell Proteomics. 2013;12:856–865. doi:10.1074/mcp.R112.026005
61. Chakraborty S, Gonzales JC, Sievers BL, et al. Early non-neutralizing, afucosylated antibody responses are associated with COVID-19 severity. Sci Trans Med. 2022;14:635. doi:10.1126/scitranslmed.abm7853
62. Kutsuna S, Asai Y, Matsunaga A, et al. Factors associated with anti-SARS-CoV-2 IgG antibody production in patients convalescing from COVID-19. J Infect Chemother. 2021;27(6):808–813. doi:10.1016/j.jiac.2021.01.006
63. Kajiyama H, Suzuki S, Yoshihara M, et al. Endometriosis and cancer. Free Radic Biol Med. 2019;133:186–192. doi:10.1016/j.freeradbiomed.2018.12.015
64. Hanzawa K, Tanaka-Okamoto M, Murakami H, et al. Increased levels of acidic free-N-glycans, including multi-antennary and fucosylated structures, in the urine of cancer patients. PLoS One. 2022;17(4):e0266927. doi:10.1371/journal.pone.0266927
65. Rayan FJ, Hope CM, Masavuli MG, et al. Long-term perturbation of the peripheral immune system months after SARS-CoV-2 infection. BMC Med. 2022;20(1):26. doi:10.1186/s12916-021-02228-6
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

