Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
Application of the Willis Covered Stent in the Treatment of Complex Vascular Diseases of the Internal Carotid Artery and Vertebral Artery: A Retrospective Single-Center Experience
Authors Wu YG, Wang B, Cui H , Zhu H, Gao G
Received 26 June 2023
Accepted for publication 20 September 2023
Published 25 September 2023 Volume 2023:19 Pages 773—782
DOI https://doi.org/10.2147/TCRM.S417803
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Yin-Gang Wu,1,* Bowen Wang,2,* Hao Cui,3,* Hao Zhu,1 Ge Gao1– 3
1Department of Neurosurgery, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, Anhui, 230001, People’s Republic of China; 2Department of Neurosurgery, Wannan Medical College, Wuhu, Anhui, 241001, People’s Republic of China; 3Department of Neurosurgery, Bengbu Medical College, Bengbu, Anhui, 233030, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ge Gao, Department of Neurosurgery, The First Affiliated Hospital of University of Science and Technology of China, Hefei, Anhui, People’s Republic of China, Email [email protected]
Objective: To retrospectively evaluate the efficacy and security of Willis covered stent (WCS) deployment for complex vascular diseases of the internal carotid (ICA) and vertebral (VA) arteries.
Methods: Retrospective analysis was performed on complex vascular disease patients (n=36) treated with WCSs at our center between March 2017 and December 2022, with a 3– 36-months follow-up surveillance and digital subtraction angiography (DSA) examination.
Results: The WCSs were successfully deployed in all the patients. The 36 included lesions were carotid-cavernous sinus fistulas (CCFs; n=10) (27.8%), complex saccular aneurysms (n=10) (27.8%), traumatic pseudoaneurysms (n=7) (19.4%), blood blister-like aneurysms (BBAs; n=5) (13.9%), and iatrogenic carotid or vertebral artery ruptures (n=4) (11.1%). The WCS was released at the communicating segment (n=2) (5.6%), the ophthalmic segment (n=3) (8.3%), the clinoid and cavernous segment (n=28) (77.8%), the petrous segment (n=2) (5.6%) of ICA and the V3 segment (n=1) (2.8%) of VA. Postoperative DSA showed complete lesion occlusion in 26 patients (72.2%) who were immediately treated with WCSs, and endoleaks occurred in 3 patients (8.3%) (endoleaks resolved postadjustment in 7 patients (19.4%)). In patients (n=3) (8.3%) treated with double stents at the break of the ICA, the endoleak remained in 1 CCF patient (2.8%) during the 3-month follow-up, and the residual shunt disappeared after the second stent system was placed 3 months later. No aneurysm, bleeding or infarct recurrence reported, and only 1 patient (2.8%) had mild asymptomatic in-stent stenosis. Deaths and procedural complications did not occur during follow-up.
Conclusion: Treatment with a WCS for intracranial complex vascular diseases resulted in satisfactory clinical outcomes and appeared effective and safe. Controlled, multicenter, large sample sizes and longer follow-up periods studies are necessary.
Keywords: Willis covered stents, complex vascular diseases, endovascular treatment
Introduction
Complex vascular diseases of the internal carotid artery (ICA) and the vertebral artery (VA), including carotid-cavernous sinus fistulas (CCFs), complex saccular aneurysms, traumatic pseudoaneurysms, blood blister-like aneurysms (BBAs) and iatrogenic artery ruptures, are especially tricky for both surgical treatment and routine endovascular procedures. Endovascular therapy was considered to be more suitable because of the bony obstacles and crucial anatomical structures close to the relative arteries, including the posterior fossa cranial nerves, optical apparatus and the cavernous sinus, which pose a very large challenge for microsurgical treatment. Nevertheless, when endovascular treatment was used, only 75.6% of patients achieved complete aneurysm obliteration, while 18.7% of them required further treatment.1
In addition, for recurrent aneurysms, reembolization carries an increased risk of herniation of the coil or migration of the thrombus into the parent vascular, leading to an ischaemic stroke. Recently, Willis covered stents (WCSs; MicroPort, China) have shown promise in treating ICA and VA vascular disease. As a balloon-expandable stent comprising bare metal stent, balloon catheter and expandable polytetrafluoroethylene (ePTFE) membrane, the WCS instantly isolates the aneurysm from the intracranial circulation, restores the artery wall and reduces the mass effect while maintaining the patency of the parent artery.2 Despite these advantages, currently available data are based on small-sized case reports and single-center experiences, as such cases are extremely scarce. In this study, we report our experience and the efficacy and safety of WCS implantation by retrospectively analyzing 36 patients with ICA and VA lesions treated with this stent at our institution.
Materials and Methods
Study Design and Patients
This single-center, retrospective, observational study was conducted in the Department of Neurosurgery, the First Affiliated Hospital of USTC (University of Science and Technology of China), Hefei, Anhui, China. This trial adhered to the tenets of the Declaration of Helsinki and was approved by our hospital’s Institutional Review Board (IRB) (approval no. 2021-KY-167). Written informed consent was obtained from all patients or their relatives. All individual information was kept strictly confidential and anonymous in the manuscript. We retrospectively analyzed patients with ICA and VA disease admitted to our hospital’s neurosurgery department who were treated with the WCS system from March 2017 to June 2021. Patient demographics, imaging information, endovascular procedural and postprocedural data, follow-up information and angiographic results were investigated.
Endovascular Technique
Depending on the disease state, different protocols were carried out, such as single or double-covered stent deployment or combined therapies (covered stent plus coiling). As a general rule, single stent method would be chosen in the first instance unless absolutely necessary. For example, double-covered stents are used in patients with highly tortuous parent arteries or aneurysms with >12-mm necks to reduce endoleak incidence. No patients were treated with combined therapies in our study. A right femoral approach was used for endovascular treatment under general anaesthesia. A 6-F long sheath (Neuron MAX, Cook, USA) through an 8-F guiding catheter (Radifocus, Terumo, Japan) was placed into the cervical segment of the ICA. A 5-F intracranial support catheter (DA, ENVOY, USA) was used to provide adequate support when the pathway was too tortuous. Guided by the Roadmap, a microcatheter (Echelon-10, EV3, USA) was used to navigate a 300-cm microguidewire (Synchro-14, Stryker, USA) into the distal segment of the parent artery. Then, WCS (MicroPort, China) was delivered with the microguidewire to bridge the orifice of aneurysm or the base of the fistula. Stenting should extend a minimum 2mm bilaterally and have a diameter about 0.5mm larger than that of the parent artery. In addition, multi-angle angiography was used to verify stent position and to ensure no significant side branch was occluded. The WCS was inflated at a pressure 5 atm and deflated immediately after proper stent placement and satisfactory fistula closure. The proximal edge of the stent grafts was redilated with a higher pressure than the original one if an endoleak was observed to ensure adequate stent expansion for endoleak repair. If the distal endoleak was not resolved after 2–3 balloon reinflation attempts, the procedure was ceased and angiography was performed 3 months later. A second covered stent may be considered for an endoleak at the proximal end of the stent under the following strategy: first, both covered stents need to overlap by at least 3 mm; second, the second stent must be a minimum of 0.5 mm larger than the previous one; and third, the postprocedural signs and symptoms should be monitored until discharge.
Antithrombotic Regimens
Patients were routinely treated with aspirin and clopidogrel (100 and 75 mg daily) for 3 successive days pre-operatively. Emergency patients were treated with a single dose of aspirin and clopidogrel (300 mg each) via nasogastric tube 2 hours before emergency surgery. Additionally, each patient was given heparin 5000 IU prior to the procedure and then a maintenance dose of 2000 IU/hour. Tirofiban was administered intra-arterially to patients with acute thrombosis or thromboembolism. The patients were prescribed aspirin for at least 6 months and clopidogrel for 6 weeks (100 and 75 mg daily) to avoid stenosis and thrombosis in the stent graft area.
Clinical and Imaging Follow-Up
After the procedure, patients were followed up with clinical and DSA examinations. Discharged patients were instructed to return to the hospital if their neurological symptoms worsened, and a CT or MRI scan was arranged as appropriate. Routine follow-up studies were then conducted at 3 months, 6 months, and 12 months post procedure and annually thereafter. The modified Rankin Scale (mRS) was used to assess the outcome of the treatment during the clinical follow-up period. The mRS scores indicated no or mild impairment (0–2) and moderate-to-severe impairment (3–5). The status of the aneurysms or fistulae was assessed by DSA to rule out the possibility of a residual endoleak or the recurrence of in-stent stenosis requiring a shunt. Angiographic data were reviewed by a panel of three experienced neuroradiologists and recorded immediately after the procedure.
Statistical Analysis
Version 23.0 of SPSS (IBM, USA) was utilized to analyze the statistical data. Continuous data are presented in mean±SD, while categorical variables were expressed in terms of percentage or frequency.
Results
Patient Demographics
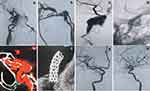
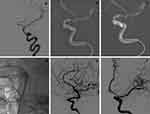
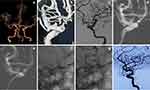
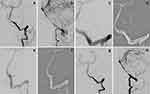
The demographic information of the 36 patients is depicted in Table 1. Of all the patients, 17 (47.2%) were male and 19 (52.8%) were female; the mean age was 45.8 ± 10.7 years (range, 15–75 years). In terms of diagnosis, 10 cases (27.8%) were CCF (representative images: Figure 1), 10 cases (27.8%) were complex saccular aneurysm (representative images: Figure 2), 7 cases (19.4%) were traumatic pseudoaneurysm (representative images: Figure 3), 5 cases (13.9%) were BBA (representative images: Figure 4), and 4 cases (11.1%) were iatrogenic cerebral artery rupture (representative images: Figure 5). Twenty-eight patients (77.8%) had hemorrhagic symptoms, such as subarachnoid hemorrhage (SAH), intracranial hemorrhage, epistaxis and sinus hematoma. Eight patients (22.2%) manifested nonemergency symptoms, including dizziness, headaches, seizures, diplopia, compressive effects and neurological deficits.
 |
Table 1 Baseline Characteristics of the Patients |
Lesion Site and Treatment Characteristics
The characteristics of complex vascular disease patients who were treated with a WCS are listed in Table 2. Lesions were found in the cavernous segment (C4) or the clinoid segment (C5) in 27 patients (75.0%), the ophthalmic segment (C6) in 3 patients (8.3%), the communicating segment (C7) in 2 patients (5.6%), the petrous segment (C2) in 2 patients (5.6%) and the V3 segment of VA in 1 patient (2.7%). A total of 39 covered stents were implanted into 36 target arteries, including 33 and 3 patients who received 1 and 2 stents per lesion, respectively.
 |
Table 2 Lesion Site and Treatment Characteristics |
Primary Procedural Outcomes and Follow-Up results
Details of the endovascular procedure as well as the clinical and DSA findings following the procedure are summarized in Table 3. Stent navigation and deployment was successfully achieved in all the patients and did not cause ruptured aneurysm, perforated artery or stent migration/collapse. The procedure succeeded at the first attempt in 26 of 36 patients. Minor endoleak was detected in 10 patients (27.8%) instantly after the initial deployment of the stents. The situation was resolved by balloon reinflation in 7 patients (19.4%). The left 3 patients (8.3%) had stubborn proximal end endoleak, which was resolved by the second stent deployment. Procedure-related complications occurred in 13 patients (36.1%), including acute thrombosis (2 patients) (15.4%), cerebrovascular spasm (8 patients) (61.5%) and late in-stent stenosis (3 patients) (23.1%). For acute thrombosis, 2.4 mL of tirofiban hydrochloride (Lunan Beta, China) was immediately given via the arterial routes, and the involved vessel was re-canalized with no permanent damage. Immediate administration of papaverine through the arterial pathway alleviated most vasospasms and achieved a satisfactory result. In this program, angiography post-procedure detected occlusion of ophthalmic arteries in 3 patients (8.3%). In total, 1 patient (2.7%) with visual abnormalities had a direct damage to optic nerve, while 2 patients (5.6%) revealed no vision loss.
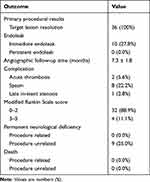 |
Table 3 Primary Procedural and Follow-Up Results |
All patients were routinely followed up with DSA. The average duration was 7.3 ± 1.8 months (range 3 to 12 months). The angiographic follow-up showed no lesions and no evidence of persistent endoleak. In total, 35 patients (97.2%) had good parent artery patency, and 1 patient (2.8%) had minor symptomless in-stent stenosis. During the period of follow-up, no procedure-related morbidity or mortality was observed. Good and poor neurologic status (mRS score 0–2 and 3–5) at follow-up was in 32 patients (88.9%) and 4 patients (11.1%), respectively.
Discussion
Complex vascular diseases of the ICA and VA, such as CCF, complex saccular aneurysms, traumatic pseudoaneurysms, BBA and iatrogenic artery ruptures, are usually located deep inside the head, in close proximity to a series of important anatomical structures. Conventional surgery can lead to serious bleeding and even death during the operation.3,4 In the last few years, there has been an increase in the use of interventional therapies for the treatment of these diseases, as these therapies have benefits of minimum injury and good healing efficacy.5 However, they have some serious drawbacks, including a high relapse rate and an obvious mass impact.2,6 The WCS implantation was first reported by Li et al7 and has been considered one of the most promising methods since 2006. The WCS implantation is the perfect treatment for aneurysms or carotid arteriovenous fistulas. The purpose of the procedure is to remold the parent artery and keep the lesion isolated from the circulation. By reconstructing the parent artery lumen, the aneurysm cavity pressure and fistula burden is released, initial hemodynamics are maintained, and clots develop in the aneurysm cavity and fistula until occlusion occurs, which minimizes the risk of procedural rupture, rebleeding and mass effects.8 Compared with traditional surgical procedures, the WCS implantation is simple, quick, safe and associated with fewer wounds. When compared to conventional interventional therapy, the WCS implantation offers many benefits: (1) immediate isolation of the aneurysmal sac and fistula from the bloodstream; (2) no contact with the aneurysm sac or fistula, thus reducing the likelihood of procedure-related rupture; and (3) no mass effects.9
In our study, 39 WCSs were deployed into the parent arteries of 36 patients during interventional therapy. The results of the initial and final angiographic follow-up examinations demonstrated total exclusion of the aneurysm sac and fistula from the bloodstream in all 36 patients, without patency loss of the ICA. The clinical follow-up showed a good functional neurological status in 32 patients (88.9%) and poor functional neurological status in 4 patients (11.1%). This study demonstrated that WCSs are safe and efficient for complex vascular diseases treatment.
Although WCS implantation is a remarkable development in the endovascular treatment of complex vascular diseases, it has some associated limitations. The stiffness of the WCS is higher than that of the naked stent, which leads to poor targeting when traversing the tortuous intracranial vasculature, especially in deep vascular curves. Moreover, the WCS lacks sufficient flexibility to conform to the configuration of the tortuous parent artery, resulting in poor stent apposition to the vascular wall.10 Although the WCS can be easily passed through the tortuous artery via the delivery channel created by the intracranial assist catheter, navigation still requires caution; both target vessel and the stent can be damaged when the stent is painstakingly navigated through the tortuous vascular system. Therefore, the WCS is not recommended for use in deep vascular curves.11–13 Unlike previous studies, in this study, all the stents were successfully deployed to their intended vessels with no overt resistance. This can be attributed to appropriate patient selection and precise stent deployment.
Occlusion of Side Branches
It is also important to consider that the WCS may cover perforating arteries or lateral branches, which restricts its use to certain anatomic sites. The main important branches are the ophthalmic arteries (OA), anterior choroidal arteries (AchA) and posterior communicating arteries (PcomA) arteries. According to some studies, the OA is not always necessary, as blood supply to the central retinal artery can be ensured by the external carotid artery collateral networks, which is essential for vision. Acute visual loss may happen after OA occlusion without sufficient compensation from the ipsilateral external carotid artery.14,15 Therefore, if the OA must be sacrificed, a balloon occlusion test (BOT) should be carried out to assess the lateral branch compensating. The AchA mainly supplies the regions of the inner capsule, optic tract and cerebral peduncle.16 Before stent deployment, the multi-angle angiogram should be carefully evaluated to ensure that the AchA is not adversely affected. The PcomA can be sacrificed if necessary if it is not a fetal posterior cerebral artery and there are no other fetal abnormalities.17
In our study, angiography performed immediately following the procedure showed an occluded OA in 3 patients. One emergency patient with postprocedure vision loss had a direct optic nerve injury, while 2 patients had no abnormal vision. This is consistent with previous research. In my opinion, assessing whether the anastomosis can compensate for OA blood supply during surgery is challenging, it is important to select a WCS of appropriate length to prevent OA occlusion during surgery.
Endoleak
Although the WCS immediately isolates the aneurysm and occludes the fistula, the development of an endoleak after deployment must be addressed as it allows continuous blood flow. Potential reasons for endoleaks include inhomogeneous vessel lumina, rupture of the PTFE membrane, incomplete coverage of aneurysmal or fistula sites, retrograde flow from collateral vessels, and graft placement in acute angulation.18 Previous studies evaluating WCS, the instantaneous endoleak rate was between 16% and 32%.9,19 In our study, this occurrence was recorded in 10 of 36 patients (27.8%) after the first balloon inflation. In general, proximal and distal balloon reinflation can resolve most immediate endoleak events. However, a stubborn endoleak at the proximal end of the stent occurred in the other 3 patients and was suppressed by the second stent deployment. As shown in our study, mismatch between the stent and the lesion is the main reason for the occurrence of endoleaks. Therefore, prior to stent deployment, it is essential to conduct multiple-angle angiograms to ensure the correct position and select the appropriate stent size. A previous study found that endoleak can heal spontaneously at a high rate during follow-up.20 According to our experience, once an endoleak occurs, we should be very careful to resolve it. Long-term blood leakage may lead to disastrous consequences.
In-Stent Stenosis
The most common complication observed at the stent graft long-term follow-up was late in-stent stenosis. It might be caused by insufficient stent dilatation, chronic recoil of the stent, neointimal hyperplasia or in-stent thrombosis, most of which are pathologies.16 Inadequate compliance with dual antiplatelet therapy was described as an independent risk factor for the stenosis of the stent.21 Lu et al found that the incidence of in-stent stenosis in the cavernous and clinoid segment of the ICA was relatively higher.22 The possible reason is that this segment of the vessel was too tortuous to restrict the expansion and apposition of the stent. In this study, only 1 patient (2.8%) had mild asymptomatic in-stent stenosis, and the others had good parent artery patency. This may have been due to strict dual anti-platelet therapy in all patients and the selection of appropriate patients.
Limitations
There are several limitations to this research. First, there may have been selection bias with regard to region and race, as all of the patients were recruited from a single institution. Second, this study failed to compare those who received other treatments. Furthermore, our study is a retrospective study, and further validation is needed from studies with larger sample sizes and longer follow-up.
Conclusion
For the treatment of intracranial complex vascular diseases, the implantation of a WCS appears to be both safe and effective. However, large, controlled multicentered studies with longer follow-ups are needed to further validate these findings.
Funding
This study was supported by grants from Anhui Natural Science Foundation (No:2008085MH250), Special Fund for basic scientific research in Central University (No: WK9110000199) and Key Research and Development Program of Anhui Province in 2021 (No: 202104j07020046).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ihn YK, Shin SH, Baik SK, Choi IS. Complications of endovascular treatment for intracranial aneurysms: management and prevention. Interv Neuroradiol. 2018;24(3):237–245. doi:10.1177/1591019918758493
2. Zhu Y, Tan H, Wu Z, et al. Use of covered stents to treat complex cerebrovascular diseases: expert consensus. Front Cardiovasc Med. 2022;9:934496. doi:10.3389/fcvm.2022.934496
3. Molyneux AJ, Birks J, Clarke A, Sneade M, Kerr RS. The durability of endovascular coiling versus neurosurgical clipping of ruptured cerebral aneurysms: 18 year follow-up of the UK cohort of the International Subarachnoid Aneurysm Trial (ISAT). Lancet. 2015;385(9969):691–697. doi:10.1016/S0140-6736(14)60975-2
4. Lindgren A, Vergouwen MD, van der Schaaf I, et al. Endovascular coiling versus neurosurgical clipping for people with aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2018;8(8):CD003085. doi:10.1002/14651858.CD003085.pub3
5. de Winkel J, Roozenbeek B, Dijkland SA, et al. Endovascular versus neurosurgical aneurysm treatment: study protocol for the development and validation of a clinical prediction tool for individualised decision making. BMJ Open. 2022;12(12):e065903. doi:10.1136/bmjopen-2022-065903
6. Han YF, Jiang P, Tian ZB, et al. Risk factors for repeated recurrence of cerebral aneurysms treated with endovascular embolization. Front Neurol. 2022;13:938333. doi:10.3389/fneur.2022.938333
7. Li MH, Li YD, Gao BL, et al. A new covered stent designed for intracranial vasculature: application in the management of pseudoaneurysms of the cranial internal carotid artery. AJNR Am J Neuroradiol. 2007;28:1579–1585. doi:10.3174/ajnr.A0668
8. Tan HQ, Li MH, Li YD, et al. Endovascular reconstruction with the Willis covered stent for the treatment of large or giant intracranial aneurysms. Cerebrovasc Dis. 2011;31:154–162. doi:10.1159/000321735
9. Liu Y, Yang HF, Xiong ZY, et al. Efficacy and safety of Willis covered stent for treatment of complex vascular diseases of the internal carotid artery. Ann Vasc Surg. 2019;61:203–211. doi:10.1016/j.avsg.2019.05.027
10. Song J, Oh S, Kim MJ, et al. Endovascular treatment of ruptured blood blister-like aneurysms with multiple (≥3) overlapping enterprise stents and coiling. Acta Neurochir. 2016;158(4):803–809. doi:10.1007/s00701-016-2721-8
11. Zhao Y, Liu Z, Sun R, et al. The clinical efficacy analysis of treatment with a Willis covered stent in traumatic pseudoaneurysm of the internal carotid artery. Front Neurol. 2021;12:739222. doi:10.3389/fneur.2021.739222
12. Fang W, Yu J, Liu Y, et al. Application of the Willis covered stent in the treatment of blood blister-like aneurysms: a single-center experience. Front Neurol. 2022;13:882880. doi:10.3389/fneur.2022.882880
13. Jeong SH, Lee JH, Choi HJ, Kim BC, Yu SH, Lee JI. First line treatment of traumatic carotid cavernous fistulas using covered stents at level 1 regional trauma center. Journal of Korean Neurosurgical Society. 2021;64(5):818–826. doi:10.3340/jkns.2020.0345
14. Salaud C, Decante C, Ploteau S, Hamel A. Implication of the inferolateral trunk of the cavernous internal CAROTID artery in cranial nerve blood supply: anatomical study and review of the literature. Ann Anat. 2019;226:23–28. doi:10.1016/j.aanat.2019.07.004
15. Akdemir Aktaş H, Ergun KM, İ T, Arat A, Hayran KM. Evaluation of the anastomoses between the ophthalmic artery and the middle meningeal artery by superselective angiography. Surg Radiol Anat. 2020;42(11):1355–1361. doi:10.1007/s00276-020-02546-z
16. Hoffmann R, Mintz GS, Dussaillant GR, et al. Patterns and mechanisms of in-stent restenosis. A serial intravascular ultrasound study. Circulation. 1996;94:1247–1254. doi:10.1161/01.cir.94.6.1247
17. Amuluru K, Ho JP, Al-Mufti F, Solander S, Romero CE. Endovascular intervention of acute ischemic stroke due to occlusion of fetal posterior cerebral artery. Interv Neuroradiol. 2019;25(2):202–207. doi:10.1177/1591019918801285
18. Zhu YQ, Li MH, Lin F, et al. Frequency and predictors of endoleaks and long-term patency after covered stent placement for the treatment of intracranial aneurysms: a prospective, non-randomised multicentre experience. Eur Radiol. 2013;23:287–297. doi:10.1007/s00330-012-2581-4
19. Yan P, Zhang Y, Ma C, Liang F, Zhu H, Jiang C. Application of the Willis covered stent in the treatment of intracranial unruptured aneurysms in internal carotid artery: a retrospective single-center experience. J Clin Neurosci. 2020;78:222–227. doi:10.1016/j.jocn.2020.04.045
20. Wang Y, Yu J. Endovascular treatment of aneurysms of the paraophthalmic segment of the internal carotid artery: current status. Front Neurol. 2022;13:913704. doi:10.3389/fneur.2022.913704
21. Ma L, Feng H, Yan S, Xu JC, Tan HQ, Fang C. Endovascular treatment of complex vascular diseases of the internal carotid artery using the Willis covered stent: preliminary experience and technical considerations. Front Neurol. 2020;11:554988. doi:10.3389/fneur.2020.554988
22. Lu D, Ma T, Zhu G, et al. Willis covered stent for treating intracranial pseudoaneurysms of the internal carotid artery: a multi-institutional study. Neuropsychiatr Dis Treat. 2022;18:125–135. doi:10.2147/ndt.s345163
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.