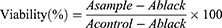Back to Journals » OncoTargets and Therapy » Volume 16
Anti-Glioma Effects of Ligustilide or n-Butylphthalide on Their Own and the Synergistic Effects with Temozolomide via PI3K/Akt Signaling Pathway
Authors Li ZQ, Zhang GS, Liu RQ, Shuai SY, Hu PY , Zheng Q, Xiao SH
Received 24 August 2023
Accepted for publication 17 November 2023
Published 22 November 2023 Volume 2023:16 Pages 983—994
DOI https://doi.org/10.2147/OTT.S432901
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tohru Yamada
Zi-Qi Li,* Guo-Song Zhang,* Ri-Qun Liu, Shu-Yuan Shuai, Peng-Yi Hu, Qin Zheng, Shu-Hua Xiao
Key Laboratory of Modern Preparation of Traditional Chinese Medicine, Ministry of Education, Jiangxi University of Chinese Medicine, Nanchang, 330004, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Peng-Yi Hu; Qin Zheng, Key Laboratory of Modern Preparation of Traditional Chinese Medicine, Ministry of Education, Jiangxi University of Chinese Medicine, Nanchang, 330004, People’s Republic of China, Tel/Fax +86 0791 87118658, Email [email protected]; [email protected]
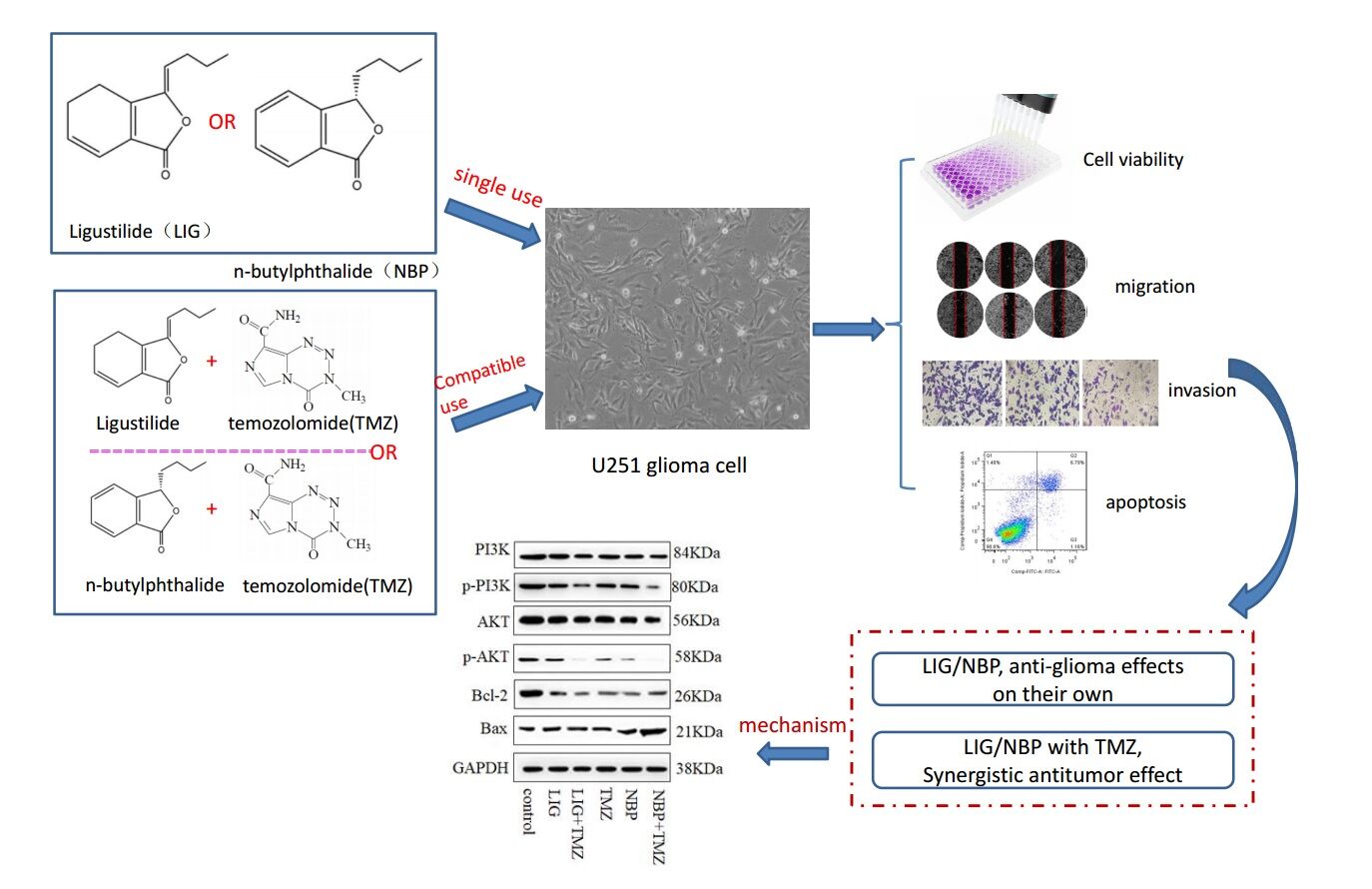
Background: Ligustilide (LIG) and n-butylphthalide (NBP) have neuroprotective effects in cerebral ischemia; however, their roles in gliomas are not well-known.This study aimed to explore the anti-glioma effects of LIG and NBP individually and the synergistic effects of temozolomide (TMZ) via the PI3K/Akt Signaling Pathway.
Materials and Methods: Cytotoxicity of LIG and NBP alone and in combination with TMZ in U251 cells was determined using the CCk-8. The effect of compounds alone or in combination on cell migration was detected using the wound healing assay, and the invasion was evaluated by transwell assays, respectively. Cell apoptosis was quantified by flow cytometry and the changed expressions of proteins were detected by Western blotting.
Results: The results showed that LIG and NBP significantly inhibited the growth of U251 cells at concentrations of 4– 10 μg/mL and 1.5– 6 μg/mL in a dose-dependent manner (p< 0.05, p< 0.01). The combination of 20 μg/mL TMZ with LIG in the concentration range of 4– 10 μg/mL or with NBP of 0.5– 6 μg/mlachieved synergistic effect towardsU251 cells. LIG and NBP, alone or in combination with TMZ, markedly inhibited cell invasion (p< 0.001) and enhanced apoptosis (p< 0.05). The combination of TMZ with LIG or NBP markedly inhibited cell migration (p< 0.001). Western blot analysis showed that LIG, NBP, and TMZ, alone and in combination, significantly decreased the expression of Bcl-2, p-PI3K, and p-Akt, and increased the expression of Bax.
Conclusion: Both LIG and NBP exert anti-glioma effects on their own through the PI3K/Akt pathway and enhance TMZ-mediated anti-glioma efficiency via the same pathway.
Keywords: ligustilide, n-butylphthalide, glioma, synergistic effect, PI3K/Akt signaling pathway
Graphical Abstract:
Introduction
Glioblastoma (GBM) is the most common and aggressive primary brain tumor, with a median survival of less than one year.1 In the past 30 years, the incidence of glioma has been increasing at a rate of 1–2% per year.2 Current approaches to therapy include surgery, radiotherapy, and chemotherapy, as well as emerging gene therapy and immunotherapy in recent years; however, the results are unsatisfactory.3 Temozolomide (TMZ) is an oral alkylating agent and has been widely applied as an effective first-line chemotherapeutic agent for the treatment of glioma patients.4 However, TMZ resistance is one of the main reasons for glioma treatment failure. Drug combinations are one of the effective means to alleviate drug resistance and improve therapeutic effect of glioma,5 such as the combination of TMZ with an inhibitor of histone methyltransferase G9a BIX01294,6 and the combination of TMZ with a prototype cyclooxygenase (COX-2) inhibitor NS-398.7 The mechanism of the resistance to TMZ is various within the same tumor, and therefore it is a challenge to select which drug is compatible with TMZ.
Chuanxiong (Ligusticum chuanxiong Hort., Umbelliferae), a commonly used Chinese medicinal herb with hemodynamic and analgesic effects, has also been used in traditional Chinese medicine compound prescriptions to treat brain tumors, such as the Wenyang Tongqiao decoction,8 JiuweiTongqiao decoction,9 Naoliu power,10 XuefuZhuyu decoction.11 The essential oil of chuanxionginhibited glioma angiogenesis, cell migration, and invasion via the EGFR/VEGF-A signaling pathway.12 Our previous research also showed that chuanxiong essential oil could enhanceTMZ-induced anti-cancer efficiency in vivo.13 As the largest proportion of chuanxiong essential oil, phthalide contains a large amount of ligustilide (LIG) and n-butylphthalide (NBP);14 its chemical structure is shown in Figure 1. LIG significantly reduced T98G cells’ migration,15 inhibited the secretion of vascular endothelial growth factor A (VEGFA) from prostate cancer-related fibroblasts.16 Interestingly, LIG showed little cytotoxicity to normal hepatocytes but restrained cell proliferation and migration, and promoted apoptosis of hepatocellular carcinoma cells.17 NBP in the form of a soft capsule and infusion drip was approved for marketing in 2002 to treat strokes.18 Recently, NBP was found to repress the proliferation of lung cancer cells and significantly reduce tumor volume and weight in vivo.19 However, it is unclear whether LIG and NBP have anti-tumor effects on glioma and whether they can play a synergistic role in combination with TMZ.
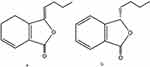 |
Figure 1 The chemical structures of ligustilide (LIG, a) and n-butylphthalide (NBP, b). |
U251 cells are a type of human glioblastoma cell line that are commonly used in research studies related to brain tumors. These cells have several characteristics, including: (1) They are highly invasive and can migrate through tissue barriers. (2) They have a high rate of proliferation, meaning they can rapidly divide and replicate. (3) They have the ability to form tumors in vivo and in vitro. (4) They express several markers associated with glioblastoma, such as GFAP and vimentin. (5) They are resistant to chemotherapy and radiation therapy, which makes them a useful model for studying drug resistance mechanisms.20–22 Overall, U251 cells are a valuable tool for studying the biology of glioblastoma and developing new therapies for this aggressive type of brain cancer. Therefore, in this paper, we adopted U251 cells to study the effects of LIG and NBP on the antitumor effects of TMZ.
The phosphatidylinositol 3-kinase (PI3K) signaling pathway plays an important role in various cell physiological and pathological processes, including cell growth, survival, motility, DNA repair, and metabolism. Aberrant activation of PI3K- protein-serine-threonine kinase (Akt) pathway is closely associated with the occurrence, proliferation, apoptosis, invasion, metastasis, and multi-drug resistance in cancer cells, etc.23 Blocking the PI3K/Akt pathway may induce glioma cell apoptosis and inhibit tumor cell metastasis and proliferation.24,25 Therefore, the aims of this study were to (1) explore the anti-tumor effects of LIG and NBP on the glioma cell lineU251 in vitro, and elucidate the mechanisms related to the PI3K/Akt signaling pathway; (2) investigate the synergistic anti-tumor effect of TMZ in combination with LIG or NBP and elucidate the mechanisms to provide a theoretical basis for clinicians to choose treatment measures.
Materials and Methods
Chemicals and Reagents
Human glioma U251 cells were obtained from ZhongqiaoXinzhou Biotechnology Co., Ltd. (Shanghai, China); D-HanksTrypsin-EDTA, Dimethylsulfoxide (DMSO), 0.1% crystal violet and PBS solution were obtained from Solarbio (Beijing, China); Annexin V conjugated to fluorescein-isothiocyanate (Annexin V-FITC), Cell Counting Kit 8 assayandTissues fixation solution were bought from Dalian Meilun Biotechnology Co., Ltd. (Dalian, China); Dulbecco’s modified Eagle’s medium (DMEM) high-sugar complete medium was purchased from Shanghai ZhongqiaoXinzhou Biotechnology Co. Ltd. (Shanghai, China); LIG was purchased from Chengdu Ruifensi Biotechnology Co. Ltd. (Chengdu, China); NBP was purchased from Chengdu Lemeitian Pharmaceutical Technology Co., Ltd. (Chengdu, China); TMZ was purchased from Shanghai Yuanye Biotechnology Co., LTD (Shanghai, China); bicinchoninic acid (BCA) assay kit was purchased from Solarbio (Beijing, China); primary anti-bodies against Bax, Bcl-2, Akt, phosphorylatedAkt (Ser473, p-Akt), and GAPDH were purchased from Annoron Biotechnology Co., Ltd (Beijing, China); anti-PI3Kp8 5was purchased from Hangzhou HuaAn Biotechnology Co., Ltd (Hangzhou, China); anti-phosphoPI3K (Tyr607, p-PI3K) was purchased from Affinity Biosciences (Beijing, China); and secondary anti-bodies (goat anti-rabbit IgG and goat anti-mouse IgG) were purchased from Cell Signaling Technology (Beverly, MA, USA).
Cell Lines and Cell Culture
U251 cells were cultured in DMEM high-sugar complete medium with 10% fatal bovine, 1% streptomycin (100 μg/mL) and 1% penicillin (100 U/mL), and maintained in a humidified atmosphere of 5% CO2 at 37 °C.
Cell Viability Analysis Detected by CCk-8 Assay
The effects of LIG, NBP, and TMZ on the growth of U251 cells were determined using a CCk-8 assay. LIG, NBP, TMZ, LIG-TMZ, and NBP-TMZ were dissolved and diluted in DMEM supplemented with 1% DMSO. U251 cells were seeded in 96 microtiter plates with a total culture medium of 100μL and a density of 8×104 cells/mL, and then the plates were incubated in a humidified atmosphere of 5% CO2 at 37 °C. After 24 h, the medium was removed and replaced with culture medium containing different compounds. After incubation for 24 hr, the medium was removed, and 100 µL fresh medium supplemented with 10% CCk-8 was added into each well and incubated for 20 min. Absorbance was measured at 490 nm using a Multiskan Go microplate reader (BioTek, Winooski, VT, USA). The absorption of wells containing compounds and cells was recorded as Asample, and the absorption of wells with the same concentration of compounds but without cells was recorded as Ablank. The absorption of wells containing cells but no compounds was recorded as Acontrol. The average absorbance of six measurements for each compound is represented as a percentage of the absorbance of the untreated control and plotted against the complex concentration.
Cell Migration Determined by the Wound Healing Assay
U251 cells were inoculated into 6-well plates at a density of 1×105 cells/mL and cultured until the cell density reached 80%–90%. Sterile of 200μL was used to scratch the plate surface vertically and achieve a similar cell wound width. Fresh medium supplemented with different compounds was added after removing the original culture medium. The scratch area in each group was recorded at 0 and 24 h using an inverted microscope, respectively. The wound-healing area was measured using a Nikon Eclipse TS10 inverted microscope (Nikon, Japan). Each experiment was performed in triplicates.
Cell Invasion Detected by Transwell assays
The substrate and pre-cooled serum-free solutions were uniformly coated on the surface of the upper chamber of transwell at a ratio of 1:9 for 6 h. Then, 200 μL of DMEM was added to hydrate the basement membrane for 2h in a humidified atmosphere of 5% CO2 at 37 °C. After removing the original culture medium, U251 cells treated with different compounds were seeded in the upper chamber with a total culture medium of 200μL and a density of 4×105 cells/mL, and 700 µL of culture medium without cells or compounds was added to the lower chamber. After incubation for 24 h, the U251 cells in the upper chamber were lightly removed and the chamber was fixed with fixative for 20 min. The fixative in chamber was removed, and the membrane was stained with 1% crystal violet for 15 min. The number of cells passing through the matrix glue was photographed and counted under a microscope. Crystal violet stained positive cells that pass through the matrix glue are considered to be invading cells.
Cell apoptosis Determined by Annexin V-FITC/PI Double Staining Assay
An annexin V-FITC/PI cell apoptosis detection kit was used to observe the effects of the compounds on the apoptosis of U251 cells. Cells were inoculated into 6-well plates at a density of 2×105 cells/mL and cultured until the cell density reached 80%–90%. Fresh medium supplemented with different compounds was added after removing the original culture medium. After incubation for 24 h, cells at a density of 2×106 cells/mL in 1×binding buffer (100 μL) were incubated with PI (5 μL) and Annexin V-FITC (5 μL) at room temperature for 15 min. Then, 1×binding buffer (400 μL) was added and the apoptosis level of glioma cells was investigated and analyzed using a BDFACSVerseflow cytometer (BD, USA).
Protein Expressions Detected by Western Blot analysis
Total protein was extracted from U251 glioma cells after 24 h treatment with different compounds, and its concentration was determined with BCA kit. Proteins were extracted from whole cell lysates, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to a polyvinylidene fluoride membrane. The following primary anti-bodies were used: rabbit anti-Bax (1:5000), anti-Bcl-2 (1:5000), anti-PI3K (1:500), anti-p-PI3K (1:500), anti-Akt (1:1000), and anti-p-Akt (1:1000). The membranes were then incubated with horseradish peroxidase-conjugated secondary anti-bodies (1:5000). The transferred proteins were incubated with an enhanced chemiluminescence substrate solution and visualized using ImageJ software (Bio-Rad). Relative expressions of proteins were quantified densitometrically using Quantity One software (Bio-Rad Laboratories, Richmond, CA, USA) and calculated according to the reference band of glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Statistics Analysis
The Viability of cells in the CCk-8 assay was calculated by using the following Equation 1:
Experimentally derived in vitro data were demonstrated as the mean ± standard deviation. Statistical analyses were performed using Prism 7.0 (GraphSoftware, San Diego, CA, USA) or SPSS 26.0 (IBM, Armonk, NY, USA). Data were analyzed by one-way analysis of variance, and the differences among multiple groups compared to the control or TMZ groups were analyzed by the least significant difference test. P<0.05 was considered statistically significant.
Results
Effects of Compounds Alone or in Combination on U251 Cell Death
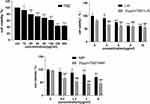
The effects of LIG and NBP alone or in combination with TMZ on the proliferation of U251 cells were evaluated using the CCK-8 assay (Figure 2). The TMZ group significantly inhibited growth of U251 cells in the concentration range of 20–200 µg/mL, and 20 µg/mL of TMZ was used for subsequent compatibility tests. Compared to the control group, both LIG and NBP significantly inhibited the growth of U251 cells in the concentration range of 4–10 µg/mL and 1.5–6 µg/mL in a dose-dependent manner (p<0.05, p<0.01). In combination therapy, LIG or NBP significantly enhanced TMZ inhibition of cell growth when compared with the TMZ alone group (p<0.001). The combination of 20 µg/mL TMZ with LIG in the concentration range of 4–10 µg/mL or with NBP of 0.5–6 µg/mL achieved enhanced effects against U251 cells.
The Q value method of Zhengjun Jin was used to analyze the synergistic interaction of the combination group,26–28 and it was shown in Table 1. The Q<0.85 and Q≥1.15 indicate antagonism and synergy, respectively, whereas additivity is indicated by 0.85≤Q<1.15. And the formula is Q = E(a+b)/(Ea+Eb-Ea×Eb), where E(a+b), Ea and Eb are average effect of combination treatment, effect of drug A only and effect of drug B only, respectively. The results showed that the Q values of the combination treatment of TMZ with LIG at 4 or 6 µg/mL were 1.53 and 1.19, respectively. The Q values of the combination treatment of TMZ with different concentrations of NBP were all greater than 1.15, suggesting a synergistic effect. Since both LIG and NBP could play a synergistic role with TMZ at 6 µg/mL, this concentration was used in subsequent tests.
 |
Table 1 Q Values of Survival Rate of U251 Cells in Different Concentration Combined Administration Groups (Mean±SD, n = 6) |
Effects of Compounds Alone or in Combination On Cell Migration and Invasion
The effects of LIG and NBP, alone or in combination with TMZ, on the migration of U251 cells were evaluated using wound healing experiments, and the results are shown in Figure 3. Figure 3 shows that TMZ at 20 µg/mL and LIG and NBP at 6 µg/mL did not significantly inhibit U251 cell migration, while compared with the control group (p> 0.05). The combination of 20 µg/mL TMZ with 6 µg/mL LIG or NBP markedly inhibited cell migration when compared with the control group (p< 0.001), indicating synergistic effects on glioma cell migration.
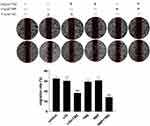 |
Figure 3 Effects of TMZ, LIG and NBP alone or in combination on migration of U251 cells. Values are mean ± standard deviation (n=3). Differs from control group, ***p< 0.001. |
The invasion of U251 cells treated with TMZ, LIG, or NBP alone or in combination for 24 h was determined by transwell assay, and the results are shown in Figure 4. Figure 4 demonstrated that TMZ, LIG, and NBP markedly inhibited the invasion of U251 cells compared with the control group (p< 0.001). The combination of TMZ with LIG or NBP also markedly inhibited cell invasion (p< 0.001), indicating synergistic effects on glioma cell invasion.
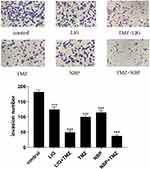 |
Figure 4 Effects of TMZ, LIG and NBP alone or in combination on invasion of U251 cells (×100). Values are mean ± standard deviation (n=3). Differs from control group, ***p< 0.001. |
Effects of Compounds Alone or in Combination on U251 Cell Apoptosis
The effects of LIG/NBP and/or TMZ on U251 cell apoptosis were evaluated using flow cytometric analysis, and the results are shown in Figure 5. Figure 5 shows that TMZ at 20 µg/mL supplemented withLIG orNBP at 6 µg/mL significantly enhanced U251 apoptosis compared with the control group (p> 0.05). The combination of TMZ with NBP markedly enhanced cell apoptosis while compared with the control group or compound alone group (p< 0.001), indicating that NBP in combination with TMZ had synergistic effects on glioma cell apoptosis.
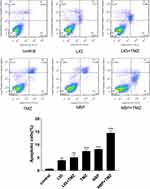 |
Figure 5 Effects of TMZ, LIG and NBP alone or in combination on apoptosis of U251 cells. Values are mean ± standard deviation (n=3). Differs from control group, **p< 0.01, ***p< 0.001. |
Contributions of PI3K/Akt Signaling athways
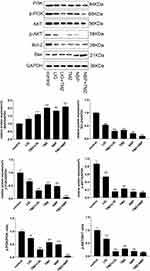
The effects of LIG/NBP and/or TMZ on protein expression were investigated by Western blot analysis, and the results are shown in Figure 6. Figure 6 reveals a band of 21 kDa corresponding to Bax, a band of 26 kDa corresponding to Bcl-2, a band of 56 kDa corresponding to Akt, 58 kDa, 80 kDa corresponding to p-PI3K, and 84 kDa corresponding to PI3K. Compared with the control group, the expression of Bcl-2 in U251 cells was markedly down-regulated by the compounds alone or in combination (p<0.01), while the expression of Bax was markedly up-regulated (p<0.01), which may explain the increase in the apoptosis rate and the decrease in the U251 cell survival rate. Compared with the control group, LIG, NBP, and TMZ alone and in combination significantly decreased the expression of p-PI3K and p-Akt, indicating that the compounds alone and in combination may promote the apoptosis of U251 glioma cells by inhibiting the PI3K/Akt signaling pathway.
Discussion
Emerging evidence indicates that LIG has anti-tumor effects on Ehrlich solid carcinoma (ESC),29 hepatocellular carcinoma (HCC),17 prostate cancer,16 breast cancer30 and brain tumor,15 whereas NBP exhibits anti-cancer activity in lung cancer.19 There are few reports on the therapeutic effects of LIG and NBP, especially NBP, on gliomas. Notably, LIG promoted apoptosis in ESC by significantly increasing the expression of Bcl2.29 It also dramatically inhibits the viability and migration of HCC and oral cancer cells.31 In this paper, we investigated the functions of LIG and NBP in U251 the proliferation, migration, invasion, and apoptosis. These results indicate that LIG and NBP significantly enhanced U251 glioma cell apoptosis and inhibited U251 cell viability and invasion. Interestingly, both LIG and NBP can inhibit neuronal apoptosis and improve the proliferation induced by cerebral ischemia.32–35
One of the main steps in tumorigenesis and development is to escape apoptosis. And the occurrence and development of tumor is evasion of apoptosis. Therefore, the possible mechanism of apoptosis pathway as LIG and NBP in the treatment of glioma was investigated in this study. This process is regulated by a balance between the pro-apoptotic protein Bax and anti-apoptotic proteins Bcl-2 within cells. Our findings revealed LIG and NBP up-regulated Bax and down-regulated Bcl-2, respectively, leading to imbalance and affecting apoptosis. Bcl-2 is a well-known anti-apoptotic compound, and its deletion or down-regulation has been found in many tumor types.36 In addition, Bax promotes apoptosis by activating cytochrome C and caspase-3.37
The PI3K/AKT signaling pathway plays an important role in regulating cell proliferation and apoptosis, which is often abnormally activated in cancer.19 PI3K is a member of the lipid kinase family, and its activation can catalyze the 3-hydroxyl phosphorylation of PIP2 to generate PIP3, which acts as the second messenger and recruits PDK1 and Akt to the plasma membrane, resulting in Akt activation. Activated Akt then promotes apoptosis by up-regulating Bcl-2 family proteins.38 In this study, we investigated the effects of LIG and NBP on PI3K/Akt pathway. Both LIG and NBP significantly decreased the expression of p-PI3K and p-Akt, indicating that they promote apoptosis of U251 glioma cells by inhibiting the PI3K/Akt signaling pathway. However, the effects of LIG and NBP on the expression of apoptotic executioner proteins, such as cleaved caspase-9 and cleaved caspase-3, were not detected in this study; therefore, further investigation is required.
TMZ is an oral-alkylating drug, whose molecular action is based on the formation of O6-methylguanine, and as a result, O6-methylguanine mispairs with thymine, resulting in cell cycle arrest at G2/M and consequent programmed cell death.39 However, drug resistance limits their antitumor efficacy. The combination of LIG and tamoxifen induces apoptosis in breast cancer cells and S and G2/M phase cell cycle arrest.30 Importantly, LIG restored the sensitivity of breast cancer cells to tamoxifen by reversing the MTA1/IFI16/HDACs complex mediated epigenetic repression of ERɑ, inhibiting autophagy, and accumulating DNA damages.30 Interestingly, LIG and NBP not only have anti-tumor effects but also synergistically inhibit the viability, migration, and invasion of glioma cells when combined with TMZ. Additionally, Western blot analysis indicated that the combined treatment with NBP and TMZ synergistically induced U251 cell apoptosis by inhibiting the PI3K/Akt signaling pathway and disturbing the imbalance of Bcl-2 family expression. These results suggest that both LIG and NBP may be chemo sensitizers that enhance TMZ-induced anti-cancer efficacy against human gliomas.
LIG and NBP have shown synergistic anti-tumor effects with TMZ in vitro cell models; however, further studies are needed to confirm the synergistic effects observed in animal models. In addition, the mechanism of the resistance to TMZ is various within the same tumor, such as DNA damage repair, DNA mismatch repair system, and base excision repair system, etc. In this paper, we confirmed that LIG and NBP have anti-glioma effects in coordination with TMZ through the PI3k-Akt signaling pathway, and whether they can also play synergistic roles through other pathways needs further study.
Conclusion
In summary, the results of the present study demonstrated that both LIG and NBP inhibited proliferation and invasion and induced apoptosis in U251 glioma cells by regulating the PI3K/Akt signaling pathway and that both LIG and NBP have the potential to enhance TMZ-mediated anti-cancer efficiency against human glioma through the PI3K/Akt signaling pathway, which validated that the combination of TMZ with LIG or NBP may be an effective way to treat human glioma. However, this requires further in vivo study.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82060719), the Chinese Medicine Preparation Technology and Equipment Innovation Team of the Jiangxi University of Chinese Medicine (NO.CXTD22006) and the Innovation and Entrepreneurship Project for College Students (NO.X202310412138).
Disclosure
The authors declare that there is no conflict of interest in this work.
References
1. Tan AC, Ashley DM, López GY, et al. Management of glioblastoma: state of the art and future directions. CA Cancer J Clini. 2020;70(4):299–312. doi:10.3322/caac.21613
2. Chen LH, Pan C, Diplas BH, et al. The integrated genomic and epigenomic landscape of brainstem glioma. Nat commun. 2020;11(1):3077. doi:10.1038/s41467-020-16682-y
3. Wen PY, Weller M, Lee EQ, et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22(8):1073–1113.
4. Pinto F, Costa ÂM, Andrade RP, Reis RM. Brachyury is associated with glioma differentiation and Response to temozolomide. Neurotherapeutics. 2020;17(4):2015–2027. doi:10.1007/s13311-020-00911-9
5. Duan M, Xing Y, Guo J, et al. Borneol increases blood-tumour barrier permeability by regulating the expression levels of tight junction-associated proteins. Pharmal Biol. 2016;54(12):3009–3018. doi:10.1080/13880209.2016.1199044
6. Ciechomska IA, Marciniak MP, Jackl J, et al. Pre-treatment or post-treatment of human glioma cells with BIX01294, the inhibitor of histone methyltransferase G9a, sensitizes cells to temozolomide. Front Pharmacol. 2018;9:1271. doi:10.3389/fphar.2018.01271
7. Jalota A, Kumar M, Das BC, et al. A drug combination targeting hypoxia induced chemoresistance and stemness in glioma cells. Oncotarget. 2018;9(26):18351–18366. doi:10.18632/oncotarget.24839
8. Li WC, Shen HS, Bai X, et al. In vivo study of traditional Chinese medicine Wenyang Tongqiao effect on rat Glioma mechanism. J Tianjin Univ Trad Chin Med. 2015;03(34):156–159. in Chinese.
9. Liu CQ, Zhang XFan HW. In vitro pharmacodynamics and prescription analysis on JiuweiTongqiao Decoction in human glioma U251 cell. Chin J Exper Tradit Med Form. 2017;07(23):148–153. in Chinese.
10. Zhou J, Liu HZhao MR. Clinical research on Naoliu (Brain tumor) powder in treating recurrent brain gliomas. Tianjin J Trad Chin Med. 2008;04:277–280. in Chinese.
11. Liu JM, Huang LW, Zhu XH, et al. Effects of 3 kinds of serum containing blood-activating and stasis-eliminating TCM compound formulas on JAK/STAT signal pathway of glioma U251 cells. China Pharmacy. 2017;16(28):2176–2179. in Chinese.
12. Han R, Sun Y, Ma R, et al. Inhibitory effect of volatile oil from RhizomaLigustici Chuanxiong on glioma angiogenesis based on EGFR/VEGF-A signaling pathway. Mod J Integ Trad Chin West Med. 2023;05(32):633–679. in Chinese.
13. Wu HX. Effects and mechanism of Ligusticum chuanxiong volatile oil in combination with temozolomide in treatment of glioma by regulating P-gp protein. Chin Trad Herb Drug. 2019;22(50):5492–5498. in Chinese.
14. Ren WG, Guo LL, Zhang CY. Research progress and predictive analysis of quality markers in rhizoma ligustici chuanxiong. Moder Trad Chin Med Mat Med World Sci Technol. 2021;09(23):3307–3314. in Chinese.
15. Yin J, Wang C, Mody A, et al. The Effect of Z-ligustilide on the mobility of human glioblastoma T98G cells. PLoS One. 2013;8(6):e66598. doi:10.1371/journal.pone.0066598
16. Ma J, Chen X, Chen Y, et al. Ligustilide inhibits tumor angiogenesis by downregulating VEGFA secretion from cancer-associated fibroblasts in prostate cancer via TLR4. Cancers. 2022;14(10):2406. doi:10.3390/cancers14102406
17. Yang JXing Z, Xing Z. Ligustilide counteracts carcinogenesis and hepatocellular carcinoma cell-evoked macrophage M2 polarization by regulating yes-associated protein-mediated interleukin-6 secretion. Exp. Biol. Med. 2021;246(17):1928–1937. doi:10.1177/15353702211010420
18. Diao X, Deng P, Xie C, et al. Metabolism and pharmacokinetics of 3-n-butylphthalide (NBP) in humans: the role of cytochrome P450s and alcohol dehydrogenase in biotransformation. Drug Metab. Dispos. 2013;41(2):430–444. doi:10.1124/dmd.112.049684
19. Jiang Q, Zhang N, Li X, et al. Dl-3-N-butylphthalide presents anti-cancer activity in lung cancer by targeting PD-1/PD-L1 Signaling. Can Manage Res. 2021;13:8513–8524. doi:10.2147/CMAR.S333416
20. Candolfi M, Curtin JF, Nichols WS, et al. Intracranial glioblastoma models in preclinical neuro-oncology: neuropathological characterization and tumor progression. J Neurooncol. 2007;85(2):133–148. doi:10.1007/s11060-007-9400-9
21. Maqsood MI, Matin MM, Bahrami AR, et al. Immortality of cell lines: challenges and advantages of establishment. Cell Biol. Int. 2013;3:1–8.
22. Wu H, Yang L, Liu H, et al. Exploring the efficacy of tumor electric field therapy against glioblastoma: an in vivo and in vitro study. CNS Neurosci Ther. 2021;27(12):1587–1604. doi:10.1111/cns.13750
23. Jiang N, Dai Q, Su X, et al. Role of PI3K/AKT pathway in cancer: the framework of malignant behavior. Mol Biol Report. 2020;47(6):4587–4629. doi:10.1007/s11033-020-05435-1
24. Jin Q, Zhao J, Zhao Z, et al. CAMK1D inhibits glioma through the PI3K/AKT/mTOR signaling pathway. Front Oncol. 2022;12:845036. doi:10.3389/fonc.2022.845036
25. Li W, Du Q, Li X, et al. Eriodictyol inhibits proliferation, metastasis and induces apoptosis of glioma cells via PI3K/Akt/NF-κB signaling pathway. Front Pharmacol. 2020;11:114. doi:10.3389/fphar.2020.00114
26. Yang Y, Lv QJ, Du QY, Yang BH, Lin RX, Wang SQ. Combined effects of Cantide and chemotherapeutic drugs on inhibition of tumor cells’ growth in vitro and in vivo. World J Gastroenterol. 2005;11(16):2491–2496. doi:10.3748/wjg.v11.i16.2491
27. Yu J, Yang K, Zheng J, et al. Activation of FXR and inhibition of EZH2 synergistically inhibit colorectal cancer through cooperatively accelerating FXR nuclear location and upregulating CDX2 expression. Cell Death Dis. 2022;13(4):388. doi:10.1038/s41419-022-04745-5
28. Duarte D, Vale N. Evaluation of synergism in drug combinations and reference models for future orientations in oncology. Curr Res Pharmacol Drug Discov. 2022;3:100110. doi:10.1016/j.crphar.2022.100110
29. Alshehri AF, KhodierAEAl-Gayyar MM, Al-Gayyar MM. Antitumor activity of ligustilide against Ehrlich solid carcinoma in rats via inhibition of proliferation and activation of autophagy. Cureus. 2023;15(6):e40499. doi:10.7759/cureus.40499
30. Ma H, Li L, Dou G, et al. Z-ligustilide restores tamoxifen sensitivity of ERa negative breast cancer cells by reversing MTA1/IFI16/HDACs complex mediated epigenetic repression of ERa. Oncotarget. 2017;8(17):29328–29345. doi:10.18632/oncotarget.16440
31. Hsu RJ, Peng KY, Hsu WL, et al. Z-ligustilide induces c-Myc-dependent apoptosis via activation of ER-stress signaling in hypoxic oral cancer cells. Front Oncol. 2022;12:824043. doi:10.3389/fonc.2022.824043
32. Bu X, Xia W, Wang X, et al. Butylphthalide inhibits nerve cell apoptosis in cerebral infarction rats via the JNK/p38 MAPK signaling pathway. Exper Ther Med. 2021;21(6):565. doi:10.3892/etm.2021.9997
33. Xu S, Li X, Li Y, et al. Neuroprotective effect of Dl-3-n-butylphthalide against ischemia-reperfusion injury is mediated by ferroptosis regulation via the SLC7A11/GSH/GPX4 pathway and the attenuation of blood-brain barrier disruption. Front Aging Neurosci. 2023;15:1028178.
34. Zhan L, Pang Y, Jiang H, et al. Butylphthalide inhibits TLR4/NF-κB pathway by upregulation of miR-21 to have the neuroprotective effect. J Healthcare Eng. 2022;2022:4687349. doi:10.1155/2022/4687349
35. Zhao DY, Yu DD, Ren L, et al. Ligustilide protects PC12 cells from oxygen-glucose deprivation/reoxygenation-induced apoptosis via the LKB1-AMPK-mTOR signaling pathway. Neul Regen Res. 2020;15(3):473–481. doi:10.4103/1673-5374.266059
36. Hafezi SRahmani M. Targeting BCL-2 in cancer: advances, challenges, and perspectives. Cancers. 2021;13:6.
37. Sharawi ZW. Therapeutic effect of Arthrocnemummachrostachyum methanolic extract on Ehrlich solid tumor in mice. BMC Compl Med Ther. 2020;20(1):153. doi:10.1186/s12906-020-02947-y
38. Rahmani F, Ziaeemehr A, Shahidsales S, et al. Role of regulatory miRNAs of the PI3K/AKT/mTOR signaling in the pathogenesis of hepatocellular carcinoma. J Cell Physiol. 2020;235(5):4146–4152. doi:10.1002/jcp.29333
39. Zając A, Sumorek-Wiadro J, Langner E, et al. Involvement of PI3K pathway in glioma cell resistance to temozolomide treatment. Int J Mole Sci. 2021;22(10):5155. doi:10.3390/ijms22105155
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.