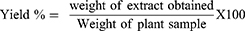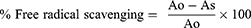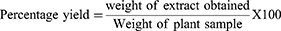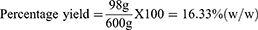Back to Journals » Journal of Experimental Pharmacology » Volume 15
Anti-Diabetic Effects of the 80% Methanolic Extract of Datura Stramonium Linn (Solanaceae) Leaves in Streptozotocin- Induced Diabetic Mice
Authors Baylie T, Kebad A , Ayelgn T , Tiruneh M , Hunie Tesfa K
Received 19 July 2023
Accepted for publication 13 October 2023
Published 18 October 2023 Volume 2023:15 Pages 375—389
DOI https://doi.org/10.2147/JEP.S426925
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Temesgen Baylie,1 Assefa Kebad,2 Tiget Ayelgn,3 Markeshaw Tiruneh,3 Kibur Hunie Tesfa3
1Department of Biomedical Science, School of Medicine, Debre Markos University, Debre Markos, Ethiopia; 2Department of Pharmacology, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 3Department of Biochemistry, School of Medicine, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Temesgen Baylie, Department of Biomedical Science, School of Medicine, Debre Markos University, Debre Markos, Ethiopia, Tel +251 9 38219824, Email [email protected]
Background: Managing diabetes mellitus with currently available drugs is costly, and the chances of side effects are high, leading to further studies for new and better medications from plant sources with the affordable and lower side effects. This study aimed to evaluate the anti-diabetic effects of Datura stramonium Linn (Solanaceae) Leaves Extract in Streptozotocin- Induced Diabetic Mice.
Methods: Male Swiss albino mice were induced into diabetes using 150mg/kg of STZ. Mice were allocated randomly into six groups, five mice per group. Group I was a normal control, Group II was Diabetic negative control, group III was Diabetic positive control, Group IV–VI were Diabetic Mice that treated with extract (100, 200 and 400 mg/kg) for 14 days. The FBG measurements were done on 0, 7th, and 14th days of treatment. After 14th day of treatment the mice were anesthetized with diethyl ether. Then, blood was drawn by cardiac puncture to assess TC, TG, LDL-C, and HDL-C. The antioxidant activity of the extract was determined using a DPPH assay. The data were entered into Epi-Data version 4.6, exported to SPSS version 26.0, and analyzed using a one-way ANOVA followed by a Tukey post hoc test. P < 0.05 was considered statistically significant.
Results: The extract of D. stramonium reduced the FBG level by 19.71%, 30.27%, 40.95%, and 45.67%, respectively, for D. stramonium 100, 200, 400, and GLC 5 mg/kg on the 14th day of treatment. Diabetic mice treated with D. stramonium for 14 days showed a significant decrease in serum TC, LDL, and serum TG and a significant increase in body weight, and HDL level as compared to diabetic negative control. Antioxidant activities of the leaves extract were comparable to ascorbic acid with an IC50 of 172.79 μg/mL.
Conclusion: These findings revealed that the D. stramonium leaves extract possesses significant Anti-diabetic activities.
Keywords: antihyperglycemic, antihyperlipidemic, antioxidant, diabetes mellitus, Datura stramonium
Introduction
Diabetes mellitus is a metabolic disorder characterized by hyperglycaemia with disturbances of carbohydrate, fat and protein metabolism resulting from defects in insulin secretion, insulin action, or both.1 Diabetes mellitus is one of the most prevalent metabolic disorders affect 537 million people (ages 20 to 79) worldwide and is predicted to affect 783 million people by the year 2045.2 In Africa 24 million adults aged 20–79 years are living with diabetes.2 The prevalence of diabetes mellitus in Ethiopia is 2% to 6.5%. It became one of the top 5 listed Sub-Saharan African countries, and 1% of deaths in the country are caused by hyperglycemia and its complication.3 DM is classified into four broad categories: type 1 DM, type 2 DM, GDM, and other specific types.4 Diabetes-related chronic hyperglycemia is linked to long-term damage and dysfunction of various organs, mainly the kidneys, eyes, nerves, heart, and blood vessels.5
The major goals of diabetes management are to lower blood glucose levels, relieve hyperglycemia symptoms, and prevent or delay the onset of diabetic complications.6 Diabetes mellitus is managed with diet, exercise, and pharmaceutical medications such as insulin and oral antihyperglycaemic drugs. Diabetes management without side effects remains a problem. Apart from the currently known therapeutic alternatives for diabetes, such as oral hypoglycemic medications and insulin, both of which have drawbacks like insulin resistance, anorexia nervosa, brain atrophy and fatty liver after chronic treatment using insulin therapy for the management of DM, various medicinal plants have been advocated for diabetic treatment.7 The use of amylin analogues, inhibitors of α- glycosidase, sulphonylureas and biguanides have certain effects like causing hypoglycemia at higher doses, liver problems, lactic acidosis and diarrhoea. Due to the above side effects of currently used drugs, there is a need for safe agents with minimal adverse effects, which can be consumed for long duration.8 The undesirable side effects, high cost and low availability of synthetic drugs have led to a strong preference for hypoglycemic drugs of plant origin, which are believed to be suitable for chronic treatments. Anti-diabetic drug development has switched to medicinal plants in order to provide new promising, efficient treatments with fewer side effects and lower costs. Medicinal plants store many bioactive compounds like antihyperglycemic, and antihyperlipidemic agents.9 In Ethiopia, DM is treated locally using a variety of medicinal plants. One of the plant used to manage diabetes is Datura stramonium roots.10
Datura stramonium belongs to the genus Datura and the family Solanaceae. The plant has been commonly used for antiepileptic, anti-microbial, anti-obesity, anti-viral, anticholinergic, and bronchodilator activities.11 Datura stramonium has traditionally been used to treat diabetes. According to an ethnobotanical survey conducted in East Wollega, Ethiopia, the plant’s root is used orally to treat diabetes.10 Previous studies on the roots and seeds of Datura stramonium demonstrated antihyperglycemic as well antihyperlipidemic activities.12,13 The aqueous crude leaves extract of Datura stramonium revealed α-amylase inhibitory activity, despite a lack of in vivo investigation.14 Hence, this study is aimed to investigate the antioxidant activity in vitro and antihyperglycemic and, antihyperlipidemic effects of Datura stramonium leaves in a diabetic mice model.
Materials and Methods
Drugs, Chemicals, and Instruments
In the study, the drugs, reagents, and instruments used were as follows: streptozotocin (Fisco Research laboratories, India), glibenclamide (Sanofi aventis, France), DPPH (Sigma Aldrich, Germany), ascorbic acid (Blulux Laboratories, India), citric acid (Lab tech chemicals, India), 5% glucose solution (Reyoung Pharmaceuticals, Shandong, China), distilled water, tween 80 (Avishkar Lab Tech chemicals, India), diethyl ether. Filter paper, gauze, oven (Medit-Medizin Technik, Germany), rotary evaporator (Hamato, Japan), lyophilizer (Labfreez, China), centrifuge, digital electronic balance, pH meter, oral gavages, refrigerator, spectrophotometer (Agilent Technologies, Malaysia), caresens glucometer (Seocho-gu, Seoul 06646, Korea), auto lab clinical chemistry analyzer (Beckman coulter, Germany).
Collection and Identification of the Plant
The leaves of Datura stramonium were collected from Gondar town (located in the Central Gondar zone of Amhara region, northwest Ethiopia) in June 2022. During shipment, the plant material was wrapped in plastic sheets and authenticated by Mr. Zelalem Getnet, a botanist at the University of Gondar’s Biology Department. With voucher number 871/11/2022, a specimen was deposited in the Herbarium of Biology Department, College of Natural and Computational Science, University of Gondar.
Preparation of Crude Extract
The leaves of Datura stramonium were washed with distilled water and dried under shade at room temperature. The plant material was coarsely grounded into powder using a mortar and pestle. The coarse powder (600 g) of Datura stramonium leaves was macerated in 80% methanol (1:7 leaves powder to solvent ratio) for 72 hours with mechanical shaking frequently.15 This was repeated 3 times until the extract gave discoloration. Then the plant material was filtered with gauze and then by filter paper. The filtrate of 80% methanolic Datura stramonium leaves extract was concentrated using a rotary evaporator under reduced pressure at a temperature not exceeding 40°C. Then, it was dried by using lyophilizer for 3 days then after it is stored at −40°C. The percentage yield of extraction was calculated by the formula:
It was then powdered, packed into a glass vial, properly labelled, and stored in a desiccator until use.
Qualitative Phytochemical Analysis
Phytochemical screening test was carried out for the 80% methanolic extract of the Datura stramonium leaves using standard procedures to identify the presence of secondary metabolites such as alkaloids, phenol, flavonoids, tannins, glycosides, saponins, steroids, terpenoids, and anthraquinones.16
Determinations of Antioxidant Activity
The free radical scavenging activity of Datura stramonium leaves was examined using a DPPH assay.17 Four mg of DPPH was dissolved in 100 mL methanol in the dark, and 4 mL of a 0.1 mM methanolic solution of DPPH was mixed with a 100 µL methanolic solution of different concentrations (12.5, 25, 50, 100, and 200 µg/mL) of the leaves extract. Ascorbic acid was used as a positive control at the same concentration. It was allowed to mix well and incubated in the dark for 30 minutes at room temperature. A methanolic solution of DPPH without the tested samples was used as a negative control. After 30 minutes, the absorbance of the mixture and the control at 517 nm was measured using a UV spectrophotometer. The test was repeated with the same concentration of each sample in triplicate and the average value was taken. The percentage scavenging of DPPH free radical was calculated using the equation below, and the IC 50 value of each sample, which is the concentration of sample required to inhibit 50% of the DPPH free radical, was obtained from the concentration vs inhibition curve.
Ao is the absorbance of the negative control, and As is the absorbance of the solution in the presence of sample extract or ascorbic acid.
Experimental Animals
A total of 35 mice (30 for the antidiabetic study (males), and 5 for the acute oral toxicity study (females)) were used for this study. Healthy Swiss albino mice (body weight, 25–35 gm; age 6–8 weeks old) were obtained from the animal house of the Department of Pharmacology, School of Pharmacy, and University of Gondar. Female mice were used for acute oral toxicity and male mice for STZ induced diabetic model. Because male mice are more compatible and sensitive than female mice, the researchers utilized healthy male mice in the investigation.18
All the mice were acclimatized to the laboratory condition for one week before commencing the experiments and fed with pellet and tap water ad libitum. The animals were housed in 12 hours light and dark cycle at room temperature. The experiment was performed in the laboratory of the Department of Biochemistry, College of Medicine and Health Sciences, the University of Gondar.
Acute Oral Toxicity Test
An acute oral toxicity test on the leaves extract of Datura stramonium was carried out based on the limit test recommendations of the Organization for Economic Co-operation and Development guideline-425 (OECD).19 Five female mice aged 8–12 weeks old were used for this study. A dose of 2 g/kg was given to the first mouse. Since no death was observed within 24 h, the crude extract of 2 g/kg was administered to 4 additional mice. The mice were kept separately and then observed for behavioural and physical changes with special attention during the first 4 hours. The observation was performed for the general signs and symptoms of toxicity, such as unusual skin and fur color, tremors, convulsions, salivation, diarrhoea, food and water consumption, coma, and mortality. The observation was continued for 14 days.
Induction of Diabetes Mellitus in Experimental Mice
Diabetes mellitus was induced in fasting mice (16 hours) by a single IP injection of STZ at a concentration of 150 mg/kg body weight in 0.1 M citrate buffer (pH 4.5). It was then given to each mouse immediately. To prevent death from hypoglycemic shock, the mice were given free access to a 5% glucose solution for the next 24 hours after 6 hours. Blood samples were taken from the tails of mice three days following STZ injection and measured using a CareSens glucometer. Diabetic mice were included in this study if their FBG levels were greater than 200 mg/dL.20
Grouping and Dosing of Animals
These experimental mice were randomly divided into six groups consisting of 5 male mice in each group. The first group was normal mice, and the next five were diabetic mice.
- Group I (Normal control) was given only 2% tween 80, 10 mL/kg distilled water.
- Group II STZ-induced diabetic mice that served as diabetic negative control was given 2% tween 80, 10 mL/kg distilled water.
- Group III STZ-induced diabetic mice were treated with 100 mg/kg of Datura stramonium Leaves Extract.
- Group IV STZ-induced diabetic mice were treated with 200 mg/kg of Datura stramonium Leaves Extract.
- Group V STZ-induced diabetic mice were treated with 400 mg/kg of Datura stramonium Leaves Extract.
- Group VI STZ-induced diabetic mice that served as diabetic positive control were treated with 5 mg/kg of glibenclamide.
Dose selection was based on oral acute toxicity test. The middle dose was one-tenth of the limit dose, the lower dose was calculated as half of the middle dose, and the higher dose was twice the middle dose. Mice were treated orally with 2% tween 80 10 mL/kg DW, plant extract (100mg/kg, 200mg/kg, and400mg/kg), and glibenclamide (5mg/kg) by using 3mL oral gavage according to their respective grouping once daily for 14 days.
Blood Sample Collection
Blood sample was collected from the tail vein of the mice using insulin syringe, and fasting blood glucose was estimated using a CareSens glucometer after overnight fasting just before starting the treatment (3rd days after STZ injection) as baseline (0) and then on the 7th and 14th day of treatment.
At the end of the experiment, on the 14th day, mice were fasted overnight and anesthetized with diethyl ether by inhalations. Then, 1 mL of blood was drawn by cardiac puncture to assess total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL) and high-density lipoprotein cholesterol (HDL). To prepare serum, the blood sample was transferred into a serum separator tube (SST) and left to clot at room temperature for 30 min immediately following collection. Subsequently, the clotted blood sample was centrifuged at 3000 rpm for 10 min. Finally, the serum was transferred into a Nunc tube and stored in a deep freezer until the analyses were performed.
Data Analysis
Data were entered into Epi-data software version 4.6 and then exported to SPSS version 26.0 software for analysis and Microsoft Excel. Results were presented by tables and figures and expressed as mean ± standard error of the mean (SEM). Statistical data analyses were done using one-way ANOVA followed by the Tukey post hoc test. P < 0.05 was considered statistically significant.
Results
Percentage Yield of Crude Extract
The crude extract obtained from 600 gram coarse Powder D. stramonium leaves was 98 gram. Therefore, the percentage yield of these extracts by using 80% methanol (80/20 v/v) was calculated and given as follows:
Therefore, 16.33% w/w of crude extract was obtained from dried powder.
General Observations
Thirty-seven mice received STZ, of which 29 had fasting blood sugar levels >200 mg/dl which is 78.37% of mice. Out of the 29 diabetic mice, 25 were selected randomly for five groups and 5 mice for a single group. Out of the 8 mice rejected from the experiment, 5 were non-diabetic, and 3 were above the detection limits of the glucometer (BGL > 600 mg/dl). Twenty-three out of these 25 diabetic mice survived the whole experimental period. Two diabetic mice died at 1st and 3rd treatment days (one from diabetic negative control and one from DSLE 400) may be due to personal error during oral gavage; these mice were substituted according to protocol. Therefore, only the data of 25 overtly diabetic male mice, 5 normal control male mice, and 5 female mice for acute oral toxicity study were included in this study.
Acute Toxicity Test
Acute toxicity test results revealed no mortality at a dose of 2000 mg/kg in female mice within 14 days of observation. The mice did not show any toxic effects like changes in physical and behavioural activities, such as unusual skin and fur color, tremors, convulsions, salivation, diarrhea, and decreased food and water consumption.
Phytochemicals Screening Test
Phytochemical screening tests showed in this study comprise many secondary metabolites (see Table 1). The result revealed that the D. stramonium leaves extract are good source of tannins, saponins, flavonoids, alkaloids, phenols, glycosides, steroids, and Terpenoids. However, anthraquinone was absent.
 |
Table 1 Phytochemical Screening of Datura Stramonium Leaves |
Anti-Oxidant Activity of Datura Stramonium Leaves
The IC50 values were obtained from the equations represented by a function of Y (D.S) and Y (A.A) for extract and ascorbic acid, respectively (Figure 1). The result from Figure 1 indicates that the IC50 of D. stramonium Leaves is 172.79 μg/mL and ascorbic acid is 76.33 μg/mL. The percentage inhibition between extract and ascorbic acid did not produce a significant difference. As the concentration of the sample increased, the percentage inhibition of DPPH radical also increased.
 |
Figure 1 Percentage inhibition of DPPH free radical by ascorbic acid (AA) and methanolic leaves extract of Datura stramonium (D.S). |
Effect of Datura Stramonium Leaves on Body Weight
The groups had no significant initial body weight difference. The body weight of diabetic negative control mice was significantly reduced compared with the normal control mice on the 0, 7th, and 14th days of treatment. DSLE at doses of 100 mg/kg showed significant (P < 0.01) (29.88 ± 0.95) improvement in body weight of the diabetic mice on the 14th day, whereas DSLE 200 mg/kg (P < 0.05 and P < 0.01) (29.70 ± 0.53 and 30.08 ± 0.60), DSLE 400 mg/kg (30.30 ± 0.41 and 31.30 ± 0.25), and GLC 5 mg/Kg (30.96 ± 0.59 and 32.28 ± 0.58) (P < 0.01 and P < 0.001) showed significant improvement in body weight on 7th and 14th day respectively compared to the diabetes negative control (Table 2). There was no significant difference in body weight change between the GLC and extract treated groups as well as among the three doses of extract treated groups. The body weight of normal control mice was significantly (P < 0.05) increased on the 14th day, while the diabetic control mice significantly (P < 0.001) decreased on the 7th and 14th days compared to baseline body weight in their respective group. Similarly, compared with baseline body weight, the diabetic mice treated with DSLE 400 mg/kg and GLC 5 mg/kg significantly increased on the 7th and 14th days. However, the body weight of diabetic mice treated with DSLE 100 and 200 mg/kg was not significantly changed from the baseline.
 |
Table 2 Effect of Datura Stramonium Leaves Extract on Body Weight in Diabetic Mice |
Effect of Datura Stramonium Leaves on FBG Level in Diabetic Mice
The FBG levels of diabetic negative control mice were significantly (P < 0.001) higher than those of normal control mice on the 0, 7th, and 14th days (Table 3). However, there were no significant differences in baseline FBG levels across all diabetic mice groups. All doses of extract-treated groups had significantly higher FBG levels at all-time points compared to normal control (P < 0.001). On the 7th day, treatment doses of DSLE 200 mg/kg (P < 0.01) (239.80 ± 16.76), DSLE 400 mg/kg (220.40 ± 16.76), and GLC 5 mg/kg (207.00 ± 9.54) (P < 0.001) produced statistically significant reductions in FBG levels as compared to diabetes negative control. Similarly, as compared to diabetes negative control, DSLE 100 mg/kg (P < 0.01) (239.40 ± 13.66) and all other treatment groups (DSLE 200 mg/kg (210.00 ± 16.30)), DSLE 400 mg/kg (182.80 ± 5.90), and GLC 5 mg/kg (165.80 ± 5.31) cause statistically significant (P < 0.001) reductions in FBG levels on the 14th day. There was no significant difference between DSLE 400 mg/kg treated group and the group treated with 5 mg/kg GLC on 0, 7th, and 14th days.
 |
Table 3 Effect of Datura Stramonium Leaves on Fasting Blood Glucose in Diabetic Mice |
In diabetic mice treated with 100 mg/kg, 200 mg/kg and 400 mg/kg DSLE, and 5 mg/kg GLC, FBG level was significantly decreased in the 7th and 14th day of treatment compared to baseline BGL in their respective group. However, neither the diabetes negative control group nor the normal control group showed a significant change in FBG levels as compared to baseline BGL. According to the intra-group analysis, DSLE 100 mg/kg (10.06% and 19.71%), DSLE 200 mg/kg (20.38% and 30.27%), DSLE 400 mg/kg (28.81% and 40.95%), and GLC 5 mg/kg (32.17% and 45.67%) reduction in FBG level on the 7th and 14th days, respectively, from baseline BGL.
Effect of Datura Stramonium Leaves Extract on Serum Lipid Profile in Diabetic Mice
The level of serum lipid profile of experimental mice is shown in (Table 4). Serum total cholesterol, triacylglycerol, and LDL-C increased significantly (P < 0.001) in diabetic negative control mice compared to the normal control mice. On the other hand, the level of HDL-C decreased significantly (P < 0.001) in diabetic negative control mice compared to the normal control mice. All doses of extract (DSLE 100mg/kg, DSLE 200 mg/kg, DSLE 400mg/kg) and GLC 5mg/kg were given for 14 days, and the level of TG, TC and LDL decreased significantly (p < 0.001) compared to diabetes negative control group. Similarly, as compared to the diabetic negative control, HDL level was significantly raised with tested doses of DSLE 100 mg/kg (p < 0.05), DSLE 200 mg/kg, DSLE 400 mg/kg, and GLC 5 mg/kg (p < 0.001) after 14 days of treatment.
 |
Table 4 Effect of Datura Stramonium Leaves Extract on Lipid Profile in Diabetic Mice After 14 Days of Treatment |
Figure 2 shows the effect of DSLE on serum TG levels in normal and diabetic mice. There were significant (P < 0.001) increases in serum TG in the diabetic negative control group as compared to the normal control. However, administration of DSLE at 100 mg/kg (147.00 ± 2.77), 200 mg/kg (134.60), 400 mg/kg (125.00 ± 1.58), and GLC 5 mg/kg (88.40 ± 2.48) reduced significantly (p < 0.001) serum TG level compared to diabetic negative control after 14 days of treatment.
Figure 3 shows the effect of DSLE on serum TC in normal and diabetic mice. There was a significant (p < 0.001) rise in serum TC in the diabetic negative control group compared to the normal control. However, administration of all three doses of DSLE and GLC 5 mg/kg significantly reduced (p < 0.001) serum TC compared to the diabetic negative control group after 14 days of treatment.
Figure 4 illustrates the effect of DSLE on serum HDL in normal and diabetic mice. There was a significant (P < 0.001) decrease in serum HDL in the diabetic negative control compared to the normal control group. However, administration of all three doses of DSLE and GLC 5 mg/kg significantly increased the HDL levels as compared to diabetic negative control after 14 days of treatment.
Figure 5 describes the effect of DSLE on serum LDL in normal and diabetic mice. There was a significant (p < 0.001) rise in serum LDL in the diabetic negative control compared to the normal control group. However, administration of all doses of DSLE at 100mg/kg (96.20 ± 1.64), 200mg/kg (77.48 ± 1.43), 400mg/kg (67.80 ± 1.10), and GLC 5 mg/kg (37.32 ± 1.44) reduced significantly (p < 0.001) serum LDL levels compared to the diabetic negative control group after 14 days of treatment.
Discussion
Acute Oral Toxicity Test of the Plant Extract
Administering DSLE at a single dose of 2000 mg/kg did not cause mortality within the first day as well as for the following 14 days of observation. Physical and behavioral observations of the mice also revealed no visible signs of toxicity like unusual skin and fur color, diarrhea, salivation, tremors, convulsions, decreased food and water consumption, and the like. This finding revealed that the median lethal dose (LD50) of the plant extract is greater than 2000 mg/kg, which is similar to previous study done on the same plant leaves.21
Phytochemicals of Datura Stramonium Leaves Extract
Phytochemicals play a major role in antihyperglycemic, antihyperlipidemic, and antioxidant activities.22,23 Bioactive compounds are obtained from various medicinal plant sources, including flavonoids, phenolic, alkaloids, terpenoids, saponins, tannins, glycosides, glycolipids, dietary fibres, carotenoids, anthocyanins have been reported to have potent antidiabetic activity.24 In this study, qualitative phytochemical screening of the DSLE showed the presence of phenols, flavonoids, terpenoids, saponins, tannins, alkaloids, glycosides, and steroids. This is in line with D. stramonium seed extract.13 Similarly, alkaloids, flavonoids, phenols, tannins, saponins, and glycosides have been presented in D. metel and D. innoxia plant extract.25 It is difficult to make a definite suggestion about the main mechanism of action of a specific phytochemical class. Each of these phytochemical classes has diverse mechanisms of action.26
Anti-Oxidant Activity of Datura Stramonium Leaves Extract
Oxidative stress plays a central role in the impairment of insulin action and exacerbating DM complications. Therefore, antioxidants and antidiabetic drugs are frequently recommended to avoid diabetic complications.27 IC50 of the DSLE was found to be 172.79 μg/mL, and IC50 of ascorbic acid was 76.33 μg/mL there was no significant difference between the two in DPPH scavenging activity. DSLE concentration-dependent antioxidant activity in this study, comparable with ascorbic acid. This finding is in line with antioxidant activity of the roots and seeds extract of D. stramonium;12,13 the leaves extract of D. metel and D. innoxia.28 Antioxidant activities of DSLE might be due to the presence of phenolic compounds that can to donate hydrogen atoms or electrons and capture the free radical.29 DSLE might be exerting antioxidant activity through the enhancement of endogenous free radical scavenging enzymes. This is supported by the administration of Leaves and Seeds of D. metel increased levels of superoxide dismutase, glutathione peroxidase, and catalase in Frog’s Heart Failure Model.30
Effect of Datura Stramonium Leaves on Body Weight
The body weight of daily treated diabetic mice was measured because it is the best indicator of good health and an effective metabolic balance. The body weight of the normal control group significantly increased on the 14th day, while the diabetic negative control significantly decreased on the 7th and 14th days compared to the baseline body weight in their respective group. Diabetic negative control group, significant body weight loss was observed compared with normal control. This is consistent with a study of Hydromethanolic Extract of Hagenia abyssinica in diabetics mice.31 In diabetic mice, this loss of body weight may be due to tissue protein breakdown and muscle wasting due to unavailability of carbohydrates as an energy source, catabolism of fats (catabolic effects of insulin deficiency), and volume depletion associated with osmotic diuresis.32
However, DSLE treatment at doses of 100 mg/kg showed significant improvement in body weight of the diabetic mice on the 14th day, whereas DSLE 200 mg/kg, DSLE 400 mg/kg, and GLC 5 mg/Kg showed significant improvement in body weight on 7th and 14th days compared to the diabetes negative control. This finding is similar to hydromethanolic seed and root extract of D. stramonium in diabetics mice.12,13 The protective effect of DSLE on body weight loss may be due to its capability to decrease hyperglycemia which was an indication of proper glucose utilization. Here, the bioactive compounds of DSLE may help suppress the free radicals generated due to hyperglycemia, and control over muscle wasting or sparing protein catabolism resulting from glycemic control in treated diabetic mice ultimately leading to normalizing the level of body weight.
Antihyperglycemic Effect of the Datura Stramonium Leaves Extract in Diabetic Mice
The increase in FBG level is an important characteristic feature of DM. Studies showed that a single IP injection of a high dose of STZ could produce sustained hyperglycemia in mice at least for a period of 8 weeks.33 Likewise, STZ induced continuous hyperglycemia in this study with no substantial change in BGL during the study period of 14 days, as observed in the diabetic negative control. The FBG levels of the diabetic negative control group were significantly higher than those of the normal control. However, all doses of DSLE reduced FBG levels compared to the diabetic negative control. This result agrees with the treatment of photosynthesized gold nanoparticles from D. stramonium seed in diabetes induced mice,34 seeds and roots extract of D. stramonium12,13 and extract of Datura metel in diabetic induced mice.11,35 Hence, when the doses of DSLE increased, the percentage of FBG level reduction also increased. The difference among the three DSLE doses (100, 200 and 400 mg/kg) might be attributed to the latter containing a higher concentration of the active component responsible for more fall of FBG levels than the former.
The glycemic control was no significant difference between GLC 5 mg/kg and 400mg/kg DSLE on 7th and 14th treatment days. Therefore, the increment of the DSLE doses may further provide a similar result as the GLC. This might be due to similar mechanisms of action. Glibenclamide produces its effect via blockage of ATP-sensitive K+ channels in the plasma membrane. This leads to membrane depolarization, activates voltage-gated Ca2+ channels, a rise in cytosolic (Ca2+) and release of endogenous insulin in β-cells of the pancreas.36 This suggests that STZ at 150 mg/Kg IP might not be sufficient for complete destruction of β-cells and/or few cells remaining capable of regenerating and secret insulin.
Pancreas β-cells are sensitive to damage by free radicals, which can be generated by STZ and hyperglycemia.37 Previous studies revealed that different plant extracts had shown pancreas β cell-protective activity due to their antioxidant activities.22,23 In the same way, DSLE showed anti-oxidant activity in the present study. This suggests that the β-cell protecting effect secondary to the antioxidant activity of DSLE might contribute to its anti-hyperglycemic activity in STZ-induced diabetic mice. Another study suggested the antidiabetic action of D. stramonium leaves through inhibiting pancreatic α-amylase.14
The antihyperglycemic activity of DSLE may be due to phytochemicals like alkaloids, phenolic, glycosides, saponins, tannins, flavonoids, and terpenoids. The majority of active compounds of alkaloids delay carbohydrate digestion and absorption, promote glucose uptake; glycosides promote insulin secretion, glycogen synthesis, increase glycolysis and lower gluconeogenesis; saponins increase regeneration of pancreatic β-cells.26 Flavonoids insulin-mimetic, diminish glucose absorption, increasing expression and translocation of GLUT-4, and promote proliferation of pancreatic β-cells.38 Tannins inducing glucose transport;39 Terpenoids inhibiting gluconeogenesis and glycogenolysis.40 Thus, the blood glucose-lowering effect of the DSLE may be due to the presence of different secondary metabolites with possible independent or synergistic effects.
Antihyperlipidemic Effect of the Datura Stramonium Leaves Extract in Diabetic Mice
One of the major metabolic disorders in uncontrolled DM is the impairment in lipid metabolism.41 The mechanism for this is the activation of HSL during insulin deficiency. Following that increase in FFA mobilization from adipose tissue leads to triglyceride synthesis in the liver.42 Insulin deficiency also causes decreased activity of LPL, which leads to decreased clearance of VLDL and chylomicrons. Additionally, an increased triglyceride level can stimulate the enzymatic activity of CETP, resulting in increased triglyceride content of HDL and LDL. Triglyceride-enriched HDL particles are subjected to increased catabolism, whereas triglyceride-enriched LDL particles undergo subsequent hydrolysis via LPL or hepatic lipase, resulting in LDL particle size.43
In this study, there were significant increases in the TC, TG, and LDL levels, and at the same time HDL cholesterol was decreased in diabetic negative control group compared with the normal control. The elevated TG level in diabetic mice might be due to the consequence of increased synthesis of VLDL in liver and diminished catabolism. Since insulin has a potent inhibitory effect on lipolysis in adipocytes, insulin deficiency is associated with excess lipolysis and an increased influx of free fatty acids to the liver.44 The increased levels of LDL and VLDL in the STZ diabetic mice might also be due to over production of LDL and VLDL by the liver, in turn by the stimulation of hepatic triglyceride synthesis as a result of FFA influx.42 Elevated TG level-rich lipoproteins could result from the reduction of LPL activity.45
However, administration all doses of extract and GLC for 14 days significantly decreased TC, TG, and LDL-C levels and elevation of HDL-C levels compared to the diabetic negative control. Administration of 400mg/kg DSLE was reduced by 29.50% in TC, 25.60% in TG, and 46.00% in LDL, and increased by 47.61% in HDL compared to the diabetic negative control. When the doses of DSLE increased, the percentage of HDL level elevation and TC, TG, and LDL level reduction also increased. This result coincides with the effects of D. stramonium roots, D. metel leaves and seeds extract in diabetic mice.11,12,35 The reduction in TC and TG levels in DSLE-treated groups might be due to the inhibition of endogenous triacylglycerol synthesis in the liver. The presence of a responsible active compound to suppress the activity of HSL in adipose tissue or increased activity of hepatic lipase or LPL.44 The availability of hypocholesterolemic compounds in DSLE may act as an inhibitor for HMG CoA reductase46 or increase the fecal content by inhibiting cholesterol absorption from the intestine.47 Increased levels of HDL-C in DSLE-treated groups could be due to the enhancement of LCAT, which plays a crucial role in incorporating the free cholesterol into HDL, which takes back to the liver.48 LDL-C reducing effect of DSLE is presumably attributed to increased expression of LDL receptor, which enhances LDL particle uptake in the liver from the circulation through the depletion of intracellular cholesterol.49
Conclusion
In this study, DSLE revealed a significant reduction of FBG levels in STZ-induced diabetic mice. Similarly, this extract significantly reduces TC, TG, and LDL and increases HDL cholesterol in diabetic mice. In addition, the in vitro study of DPPH suggested that DSLE works by inhibiting free radicals activity. These effects might be through different mechanisms attributed by secondary metabolites present in the extract either synergistically or independently.
Generally, the overall findings of this study suggested that DSLE has the antihyperglycemic, antihyperlipidemic as well as antioxidant activities.
Abbreviations
BGL, Blood Glucose Level; CE, Cholesterol Ester; DM, Diabetes Mellitus; D.S, Datura stramonium; DSLE, Datura stramonium Leaves Extract; FBG, Fasting Blood Glucose; HDL, High-Density Lipoprotein; IC50, Inhibition Concentrations; LD50, Median lethal dose; LDL, Low Density Lipoprotein; OECD, Organization for Economic Cooperation and Development; STZ, Streptozotocin; TC, Total Cholesterol; TG, Triglyceride; VLDL, Very Low-Density Lipoprotein; WHO, World Health Organization.
Data Sharing Statement
All the data that were used during the process of the experiment are available from the corresponding author and proved upon reasonable request.
Ethical Approval
The research was conducted after getting an ethical approval letter with Reference number: SOM/1793/2022 from the School of Medicine Ethical Review Committee, College of Medicine and Health Sciences, University of Gondar. All procedures on mice were carried out in accordance with Guidelines for Care and Use of Laboratory Animals.50
Acknowledgments
We would like to thank the University of Gondar for enabling them to use their laboratory setup to conduct this study. We also like to appreciate Mr. Zelalem Getnet (botanist), for the identification and authentication of the plant material. We would like to express my deepest gratitude to the laboratory assistant for their unwavering assistance during the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts interest in this work.
References
1. World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation. World Health Organization; 2006.
2. Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi:10.1016/j.diabres.2021.109119
3. Bishu KG, Jenkins C, Yebyo HG, Atsbha M, Wubayehu T, Gebregziabher M. Diabetes in Ethiopia: a systematic review of prevalence, risk factors, complications, and cost. Obes Med. 2019;15:100132. doi:10.1016/j.obmed.2019.100132
4. Care D. Standards of medical care in diabetes 2019. Diabetes Care. 2019;42(Suppl 1):S124–S38.
5. Papatheodorou K, Banach M, Bekiari E, Rizzo M, Edmonds M. Complications of Diabetes 2017. Hindawi; 2018.
6. Silver B, Ramaiya K, Andrew SB, et al. EADSG guidelines: insulin therapy in diabetes. Diabetes Ther. 2018;9(2):449–492. doi:10.1007/s13300-018-0384-6
7. Brutsaert EF. Diabetes mellitus (DM). Merck Manual; 2017.
8. Alamgir A. Therapeutic Use of Medicinal Plants and Their Extracts. Vol. 1. Springer; 2017.
9. Shanmugam KR, Shanmugam B, Subbaiah GV, Ravi S, Reddy KS. Medicinal plants and bioactive compounds for diabetes management: important advances in drug discovery. Curr Pharm Des. 2021;27(6):763–774. doi:10.2174/1381612826666200928160357
10. Suleman S, Alemu T. A survey on utilization of ethnomedicinal plants in Nekemte town, East Wellega (Oromia), Ethiopia. J Herbs Spices Med Plants. 2012;18(1):34–57. doi:10.1080/10496475.2011.645188
11. Al-Snafi AE. Medical importance of Datura fastuosa (syn: datura metel) and Datura stramonium-A review. IOSR J Pharm. 2017;7(2):43–58.
12. Belayneh YM, Birhanu Z, Birru EM, Getenet G. Evaluation of in vivo antidiabetic, antidyslipidemic, and in vitro antioxidant activities of hydromethanolic root extract of Datura stramonium L.(Solanaceae). J Exp Pharmacol. 2019;11:29. doi:10.2147/JEP.S192264
13. Melaku BC, Amare GG. Evaluation of antidiabetic and antioxidant potential of hydromethanolic seed extract of Datura stramonium Linn (Solanaceae). J Exp Pharmacol. 2020;12:181. doi:10.2147/JEP.S258522
14. Shobha G, Soumya C, Shashidhara K, Moses V. Phytochemical profile, antibacterial and antidiabetic effects of crude aqueous leaf extract of Datura stramonium. Pharmacophore. 2014;5(2):273–278.
15. Geresu GD, Umer S, Arayaselassie M, Ashebir G, Makonnen E, Fan Y-C. Hepatoprotective Effects of Crude Stem Bark Extracts and Solvent Fractions of Cordia africana against Acetaminophen-Induced Liver Injury in Rats. Can J Gastroenterol Hepatol. 2022;2022. doi:10.1155/2022/1449286
16. Pandey A, Tripathi S. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J Pharmacogn Phytochem. 2014;2(5).1.
17. Hara K, Someya T, Sano K, Sagane Y, Watanabe T, Wijesekara R. Antioxidant activities of traditional plants in Sri Lanka by DPPH free radical-scavenging assay. Data Br. 2018;17:870–875. doi:10.1016/j.dib.2018.02.013
18. Deeds M, Anderson J, Armstrong A, et al. Single dose streptozotocin-induced diabetes: considerations for study design in islet transplantation models. Lab Anim. 2011;45(3):131–140. doi:10.1258/la.2010.010090
19. Toxicity–Up AO. OECD guideline for testing of chemicals; 2001.
20. Tegegne BA, Mekuria AB, Birru EM. Evaluation of anti-diabetic and anti-hyperlipidemic activities of hydro-alcoholic crude extract of the shoot tips of crinum abyssinicum Hochst. ex A. Rich (Amaryllidaceae) in Mice. J Exp Pharmacol. 2022;14:27. doi:10.2147/JEP.S335650
21. Nasir B, Khan AU, Baig MW, Althobaiti YS, Faheem M, Haq I-U. Datura stramonium leaf extract exhibits anti-inflammatory activity in CCL4-Induced hepatic injury model by modulating oxidative stress markers and iNOS/Nrf2 Expression. Biomed Res Int. 2022;2022:1–20. doi:10.1155/2022/1382878
22. Toma A, Makonnen E, Mekonnen Y, Debella A, Adisakwattana S. Antidiabetic activities of aqueous ethanol and n-butanol fraction of Moringa stenopetala leaves in streptozotocin-induced diabetic rats. BMC Complement Altern Med. 2015;15(1):1–8. doi:10.1186/s12906-015-0779-0
23. Dadi DW, Emire SA, Hagos AD, et al. Antihyperglycemic, vasodilator, and diuretic activities of microencapsulated bioactive product from moringa stenopetala leaves extract. J Food Qual. 2020;2020:1–8. doi:10.1155/2020/8882042
24. Karthikeyan R Evaluation and characterization of herbal extract of Halodule uninervis and its anti diabetic activity: KM College of Pharmacy, Madurai; 2018.
25. Sharma M, Dhaliwal I, Rana K, Delta AK, Kaushik P. Phytochemistry, pharmacology, and toxicology of datura species—a review. Antioxidants. 2021;10(8):1291. doi:10.3390/antiox10081291
26. Bharti SK, Krishnan S, Kumar A, Kumar A. Antidiabetic phytoconstituents and their mode of action on metabolic pathways. Ther Adv Endocrinol Metab. 2018;9(3):81–100. doi:10.1177/2042018818755019
27. Iqbal S, Sivaraj C, Gunasekaran K. Antioxidant and Anticancer activities of methanol extract of seeds of datura stramonium l. Free Radic Antioxid. 2017;7(2):184–189. doi:10.5530/fra.2017.2.28
28. Bhardwaj K, Kumar S, Ojha S. Antioxidant activity and FT-IR analysis of datura innoxia and datura metel leaf and seed methanolic extracts. Afr J Tradit Complement Altern Med. 2016;13(5):7–16. doi:10.21010/ajtcam.v13i5.2
29. Santos-Sánchez NF, Salas-Coronado R, Villanueva-Cañongo C, Hernández-Carlos B. Antioxidant compounds and their antioxidant mechanism. Antioxidants. 2019;10:1–29.
30. Mbida H, Tsala D, Aboubakar S, et al. Antioxidant activity of aqueous extract of leaves and seeds of datura metel (Solanaceae) in frog’s heart failure model. Evid Based Complement Alternat Med. 2022;2022:1–8. doi:10.1155/2022/5318117
31. Kifle ZD, Belayneh YM. Antidiabetic and anti-hyperlipidemic effects of the crude hydromethanol extract of Hagenia abyssinica (Rosaceae) leaves in streptozotocin-induced diabetic mice. Diabetes Metab Syndr Obes. 2020;13:4085. doi:10.2147/DMSO.S279475
32. Sundaram R, Nandhakumar E, Haseena Banu H. Hesperidin, a citrus flavonoid ameliorates hyperglycemia by regulating key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Toxicol Mech Methods. 2019;29(9):644–653. doi:10.1080/15376516.2019.1646370
33. Tian H-L, Wei L, Xu Z, Zhao R, Jin D, Gao J. Correlations between blood glucose level and diabetes signs in streptozotocin-induced diabetic mice. Glob J Pharmacol. 2010;4(3):111–116.
34. Oladipo I, Lateef A, Azeez M, et al. Antidiabetic properties of phytosynthesized gold nanoparticles (AuNPs) from Datura stramonium seed.
35. Arowora KA, Imo C, Ezeonu CS, Muhammad ZI. Effects of ethanolic extracts of Datura metel on blood lipid profile of male albino rats. Int J. 2016;2(10):248.
36. Jadna Silva Frederico M, Jhonatan Gomes Castro A, Menegaz D, et al. Mechanism of action of novel glibenclamide derivatives on potassium and calcium channels for insulin secretion. Curr Drug Targets. 2017;18(6):641–650. doi:10.2174/1389450117666160615084752
37. Al Nahdi AM, John A, Raza H. Elucidation of molecular mechanisms of streptozotocin-induced oxidative stress, apoptosis, and mitochondrial dysfunction in Rin-5F Pancreatic β -Cells. Oxid Med Cell Longev. 2017;2017:1–15. doi:10.1155/2017/7054272
38. Gaikwad B, Krishna Mohan S, Sandhya Rani M. Phytochemicals for diabetes management. Pharm Crops. 2014;5(1):11–28. doi:10.2174/2210290601405010011
39. Babby A, Elanchezhiyan C, Suhasini S, Chandirasegaran G. Antihyperglycemic effect of tannic acid in streptozotocin induced diabetic rats. Int J Curr Res. 2014;6(3):5396–5398.
40. Ramírez-Espinosa JJ, Rios MY, López-Martínez S, et al. Antidiabetic activity of some pentacyclic acid triterpenoids, role of PTP–1B: in vitro, in silico, and in vivo approaches. Eur J Med Chem. 2011;46(6):2243–2251. doi:10.1016/j.ejmech.2011.03.005
41. Kansal S, Kamble T. Lipid profile in prediabetes. J Assoc Physicians India. 2016;64(3):18–21.
42. Vergès B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia. 2015;58(5):886–899. doi:10.1007/s00125-015-3525-8
43. Sosale A, Saboo B, Sosale B. Saroglitazar for the treatment of hypertrig-lyceridemia in patients with type 2 diabetes: current evidence. Diabetes Metab Syndr Obes. 2015;8:189. doi:10.2147/DMSO.S49592
44. Borle AL, Chhari N, Gupta G, Bathma V. Study of prevalence and pattern of dyslipidaemia in type 2 diabetes mellitus patients attending rural health training centre of medical college in Bhopal, Madhya Pradesh, India. Int J Community Med Public Health. 2016;3(1):140–144. doi:10.18203/2394-6040.ijcmph20151549
45. Taskinen M-R, Borén J. New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis. 2015;239(2):483–495. doi:10.1016/j.atherosclerosis.2015.01.039
46. Jiang S-Y, Li H, Tang -J-J, et al. Discovery of a potent HMG-CoA reductase degrader that eliminates statin-induced reductase accumulation and lowers cholesterol. Nat Commun. 2018;9(1):1–13. doi:10.1038/s41467-018-07590-3
47. Chang Y, Robidoux J. Dyslipidemia management update. Curr Opin Pharmacol. 2017;33:47–55. doi:10.1016/j.coph.2017.04.005
48. Rousset X, Vaisman B, Amar M, Sethi AA, Remaley AT. Lecithin: cholesterol acyltransferase: from biochemistry to role in cardiovascular disease. Curr Opin Endocrinol Diabetes Obes. 2009;16(2):163. doi:10.1097/MED.0b013e328329233b
49. Young SG, Fong LG. Lowering plasma cholesterol by raising LDL receptors—revisited. N Engl J Med. 2012;366(12):1154. doi:10.1056/NEJMe1202168
50. Albus U. Guide for the Care and Use of Laboratory Animals.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.