Back to Journals » Infection and Drug Resistance » Volume 17
Analysis of the Risk Factors for Plastic Bronchitis in Children with Severe Adenovirus Pneumonia: A Retrospective Study
Authors Xu XH , Cai JR, Fan HF, Shi TT , Yang DY, Huang L, Zhang DW, Lu G
Received 15 December 2023
Accepted for publication 29 February 2024
Published 14 March 2024 Volume 2024:17 Pages 1011—1019
DOI https://doi.org/10.2147/IDR.S452347
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
Xue-hua Xu,1,* Jie-rong Cai,2,* Hui-feng Fan,1,* Ting-ting Shi,1 Di-yuan Yang,1 Li Huang,3 Dong-wei Zhang,1 Gen Lu1
1Department of Respiratory, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, Guangdong, People’s Republic of China; 2Department of Pediatrics, Guangzhou Panyu District Central Hospital, Guangdong, Guangdong, People’s Republic of China; 3Pediatric Intensive Care Unit, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Gen Lu, Department of Respiratory, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, 510120, People’s Republic of China, Tel +86 20 38076135, Fax +86 20 38076626, Email [email protected]
Purpose: Plastic bronchitis (PB), a rare complication of respiratory infection characterized by the formation of casts in the tracheobronchial tree, can lead to airway obstruction and severe condition. Adenovirus is one of the common pathogens of PB caused by infection. This study aimed to evaluate the clinical features and risk factors for PB in children with severe adenovirus pneumonia.
Methods: A retrospective study of children with severe adenovirus pneumonia with bronchoscopy results at Guangzhou Women and Children’s Hospital between January 2018 and January 2020 was performed. Based on bronchoscopy, we divided children with severe adenovirus pneumonia into two groups: PB and non-PB. Binary logistic regression analysis was used to identify independent risk factors for PB in patients with severe adenovirus pneumonia after univariate analysis.
Results: Our study examined 156 patients with severe adenovirus pneumonia with bronchoscopy results in hospital. Among them, 18 developed PB and 138 did not. On multivariate analysis, the independent risk factors of PB in children with severe adenovirus pneumonia were history of allergies (OR 10.147, 95% CI 1.727– 59.612; P=0.010), diminished breath sounds (OR 12.856, 95% CI 3.259– 50.713; P=0.001), and increased proportion of neutrophils (> 70%; OR 8.074, 95% CI 1.991– 32.735; P=0.003).
Conclusion: Children with severe adenovirus pneumonia with a history of allergies, diminished breath sounds, and increased the proportion of neutrophils > 70% may show higher risk of PB.
Keywords: plastic bronchitis, severe adenovirus pneumonia, risk factors, children
Introduction
Plastic bronchitis (PB), also termed fibrinous bronchitis or cast bronchitis, is an acute and serious disease featuring endogenetic foreign bodies form branched mucinous bronchial casts, which partially or extensively block bronchi.1 Due to the different degrees of obstruction, the clinical presentation ranges from mild respiratory symptoms to severe, potentially fatal respiratory failure, which induces the sudden onset of cough, wheezing, progressive dyspnea, and refractory hypoxemia.2 Diagnosis of PB is made based on characteristic bronchial casts through bronchoscopy.
According to the pathology of the bronchial casts, there are two types of PB. Type I is associated with respiratory tract diseases consisting of inflammatory cells, fibrin, and eosinophils. Type II is mainly associated with congenital heart disease consisting of mucus and a few cellular infiltrates.3 Infection is a common cause of type I PB.4 Human adenovirus (HAdV), one of the main pathogens of severe pneumonia in children, can also lead to PB, especially HAdV7.2,5,6 Bronchoscopy combined with bronchoalveolar lavage (BAL) is not only a diagnostic method of PB but also plays an important role in the treatment of adenovirus pneumonia, especially with PB, because it can clamp the blocked mucus plug out of the small bronchi and effectively ease the clinical symptoms of children with pulmonary consolidation and atelectasis.7 Our study aimed to evaluate the clinical features and risk factors for PB in children with severe adenovirus pneumonia.
Methods
Ethics
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Guangzhou Women and Children’s Medical Center, Guangzhou Medical University. All the patients or their guardians provided written informed consent to use clinical and laboratory data from the patients’ medical reports.
Case Definition and Identification
This study enrolled 156 patients with severe adenovirus pneumonia who were admitted to Guangzhou Women and Children’s Medical Center between January 2018 and January 2020.
The inclusion criteria were:
The guidelines of the American Thoracic Society for the management of community-acquired pneumonia are as follows.8 The criteria for severe pneumonia were having one or more major or two or more minor criteria.
The exclusion criteria were:
Study Design and Data Collection
This retrospective observational study was conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines. We collected and analyzed the bronchoscopy results of children with adenovirus pneumonia. All of the patients with PB were diagnosed with severe adenovirus pneumonia. Then, based on the bronchoscopy results, severe cases were divided into PB (n=18) and non-PB (n=138) groups (see Figure 1). Clinical data were collected from the patients’ medical records, including demographic characteristics (e.g., age and sex), clinical symptoms and signs (e.g., fever duration, cough, wheezing, shortness of breath, hypoxia, digestive symptoms, neurological symptoms, diminished breath sounds, and moist crackles), laboratory findings (e.g., blood cell counts, inflammatory markers, and organ functions), coinfection pathogens (other viruses, bacteria, and Mycoplasma), chest radiological manifestations (pulmonary consolidation and pleural effusion), treatments and complications. PCR testing, cultures of nasopharyngeal secretions, and BAL during hospital stay were used to identify infection with other pathogens in all patients. Those with a wide range of lesions found on chest radiography underwent high-resolution computed tomography (HRCT).
 |
Figure 1 Study flowchart. |
Statistical Analysis
Statistical analysis was performed using SPSS 22.0. Discrete data are expressed as numbers and percentages, and continuous data are presented as medians with interquartile ranges. Fisher’s exact tests were used to evaluate the differences in categorical variables and the nonparametric Mann–Whitney test in continuous variables. Univariate analyses were performed to determine the risk factors significantly associated with PB in children with severe adenovirus pneumonia. To determine the independent contribution of each factor to the outcomes, multiple logistic regression analysis was performed. Statistical significance was set at P<0.05.
Results
Baseline Characteristics
There were 156 children with severe adenovirus pneumonia who had received bronchoscopy in hospital in our study. Based on the bronchoscopy results, 18 patients had PB and 138 did not. The incidence of PB in children with severe adenovirus pneumonia was 11.54%. We analyzed the clinical features of PB caused by adenovirus by comparing the demographics, clinical symptoms and signs, laboratory indices and radiological findings between the PB and non-PB groups. There were 13 males in the PB group (72.22%) and 87 in the non-PB group (63.04%), a nonsignificant difference (P>0.05). The median age in the PB and non-PB groups showed no statistically significant difference either (P>0.05). About a third patients in the PB group had an allergy history (six of 18, 33.3%), while few patients in the non-PB did (nine of 138, 6.52%), a significant difference (P<0.05; Table 1).
 |
Table 1 Demographic characteristics and clinical features of PB and non-PB children with severe adenovirus pneumonia at admission |
As for clinical symptoms and signs, there were no significant differences in fever, cough, shortness of breath, wheeze, gastrointestinal symptoms (vomit and diarrhea), moist rales, hepatomegaly, or splenomegaly between the two groups (P>0.05). However, about half the children with PB (10 of 18, 55.56%) and about a third of those without PB (41 of 138, 29.71%) had hypoxia, a significant difference (P<0.05). About a quarter of the children in the PB group (five of 18, 27.78%) had neurological symptoms (e.g., hypnosia, dysphoria and convulsions), while just one in 10 in the non-PB group did (14 of 138, 10.14%), a significant difference (P<0.05). What is more, more than half the children with PB (14 of 18, 77.78%) showed diminished breath sounds, while about a quarter of those without PB did (33 of 138, 23.91%), also a significant difference (P<0.05; Table 1).
In terms of laboratory indices, levels of leukocytes, hemoglobin, HsCRP, LDH, liver enzymes, (ALT, AST) and coagulation functioning (PT, APTT, FIB) showed no statistically significant differences between children in the PB and non-PB groups (P>0.05). On the contrary, there were significant differences in thrombocytopenia (six of 18, 33.33%: 19 of 138, 13.76%) and high proportion of neutrophils (10 of 18, 55.56%: 36 of 138, 26.09%) between the two groups (P<0.05). As we all know, adenovirus pneumonia is prone to coinfection with other pathogens. In our study, children with severe adenovirus pneumonia, the most common coinfection was Mycoplasma pneumoniae, follow by bacteria and other viruses. There was no significant difference between the groups (P>0.05; Table 2).
 |
Table 2 Laboratory indices of PB and non-PB children with severe adenovirus pneumonia |
With regard to radiological findings, about four in five of the patients shown lung consolidation in HRCT, with about one in five showing single-lobe consolidation and three in five multilobe consolidation, none of which was significanly different between the groups on HRCT (Figure 2). In addition, about half the children in both groups manifested pleural effusion (P>0.05; Table 3). As is well known, the gold standard for the diagnosis of PB is the detection of bronchial dendritic casts on bronchoscopy. Figure 3 shows an extracted bronchial cast by flexible bronchoscopy in a patient with PB caused by HAdV.
 |
Table 3 Radiological findings for PB and non-PB children with severe adenovirus pneumonia |
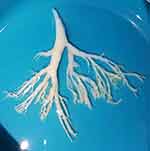 |
Figure 3 Extracted bronchial cast by flexible bronchoscopy in a patient with PB caused by HAdV. |
Most of the children had been treated with intravenous immunoglobulin and about half with systemic corticosteroids, whether in the PB or non-PB group. Even so, more children developed respiratory failure and more need of mechanical ventilation in the PB group than the non-PB group, a significant difference (P<0.05). Of the 18 patients with PB, 16 improved with treatment, while two patients died even after aggressive treatment, for a mortality rate of 11.11%. However, there were no significant differences in hospital stays or mortality between the two groups (P>0.05; Table 4).
 |
Table 4 Treatments and outcomes for PB and non-PB children with severe adenovirus pneumonia |
Risk Factors for PB in Children with Severe Adenovirus Pneumonia
On univariate analysis, sex, age, duration of fever, wheeze, shortness of breath, gastrointestinal symptoms, leucocyte level, anemia, HsCRP level, liver enzymes, LDH level, ALB level, coagulation function, coinfection, multilobe consolidation, and pleural effusion showed no relationship with the risk of PB in children with severe adenovirus pneumonia (all P>0.05). Nevertheless, allergy history, hypoxia, neurological symptoms, diminished breath sounds, thrombocytopenia, and high proportion of neutrophils were associated with the risk of PB in children with severe adenovirus pneumonia (all P<0.05). Furthermore, multivariate analysis revealed that a history of allergies (OR 10.147, 95% CI 1.727–59.612; P=0.010), diminished breath sounds (OR 12.856, 95% CI 3.259–50.713; P=0.001), and increased proportion of neutrophils (>70%; OR 8.074, 95% CI 1.991–32.735; P=0.003) were independent risk factors for PB in children with severe adenovirus pneumonia (Table 5).
 |
Table 5 Logistic regression analysis of risk factors for PB in children with severe adenovirus pneumonia |
Discussion
PB is a rare, highly variable, and potentially fatal complication.9 HAdV plays an important role in severe pneumonia.6 Owing to casts blocking the airway, PB may lead to serious clinical symptoms, acute respiratory failure, and even death.3 Therefore, our study aimed to evaluate the clinical features and risk factors for PB in children with severe adenovirus pneumonia.
In our 18 children with PB, similar to previous studies9,10 the most common symptoms were cough, hyperpyrexia, diminished breath sounds, shortness of breath, and hypoxemia. The proportion of patients with elevated white blood cells was not high, but elevated HsCRP, LDH, and neutrophils were high. Most of their chest imaging showed pulmonary consolidation or atelectasis, and about half had pleural effusion. Reported mortality rates for PB have ranged from 5% to 60%,11,12 and half the patients with PB in our study underwent respiratory failure and needed mechanical ventilation, while two died, for a mortality rate of 11.11%. Furthermore, we found that a history of allergies, diminished breath sounds, and increased proportion of neutrophils (>70%) were independent risk factors for PB in children with severe adenovirus pneumonia.
There are two types of PB based on cause and pathology. Type I is usually associated with respiratory diseases and type II with congenital heart disease.2 All of our patients were considered to be type I PB, for all of them were undergoing respiratory infections and none had congenital heart disease, although without pathological results. Pathophysiology of type I PB is constant inflammation/irritation in the bronchial mucosa by allergens or infective agents, leading to induction of mucin hypersecretion by inflammatory cytokines (goblet cell hyperplasia), and finally thick mucinous material casts develop, consisting of fibrin, neutrophils, and eosinophils.13 PB may be associated with allergies, asthma, and allergic bronchopulmonary aspergillosis, among others.14,15 A previous study of 42 children with PB showed that 31% had a history of allergic illnesses.16 In our study, 33.33% children with PB had a history of allergies, including food allergy, drug allergy, and dust allergy. We drew the conclusion that allergy history is an independent risk factor for PB in children with severe adenovirus pneumonia. Allergy is an interrelated syndrome group that develops through skin–digestive tract–respiratory tract sensitization.17 The relationship between airway allergy and respiratory virus infection is interactive. On the one hand, airway allergy may increase the risk and severity of respiratory viral infections.18 On the other hand, viral infection could initiate, maintain, and even overactivate the airway allergy.19 Both infection and allergy can lead to respiratory epithelial damage, inflammatory response, and excessive production of mucus, probably followed by the formation of bronchial casts, but the specific mechanism still needs to be explored.
HAdV can directly invade cells and indirectly induce immunoresponse to damage the respiratory epithelium, leading to cell lysis and eventual death.5 The epithelial cells on the surface of the airway are necrotic and denudated, and lamina propria of bronchi that are congested, edematous, and infiltrated with inflammatory cells, as well as airway mucus hypersecretions, results in occlusion of the bronchial lumen and even PB.6,20 Bronchial blockage can lead to pulmonary consolidation and atelectasis. The flow of air into the alveoli may be reduced or the flow rate slowed, and consequently the auscultation of breath sounds may be diminished. As such, it is not difficult to understand that diminished breath sounds are risk factors for PB.
Routine blood testing is easily conducted in clinical practice, and contributes to early and rapid diagnosis of disease. Neutrophils, important blood cells, can endocytose and kill pathogens, playing an important role in maintaining human immunity. One study identified the neutrophil ratio as one of the significant predictive factors to construct nomograms for predicting PB.21 Similarly, we found that increased proportion of neutrophils >70% is one of the independent risk factors for PB in severe adenovirus pneumonia. Neutrophil infiltration occurs in the early stage of HAdV infection,22 and it is a double-edged sword. On the one hand, it plays an important role in pathogen clearance, and on the other hand, its excessive activation can lead to airway epithelium damage, adding to infiltration of inflammatory cells, and may lead to airway obstruction.23 Our study only examined the level of neutrophils in blood and not in BAL. Future studies could evaluate the relationship between PB and neutrophils in BAL.
There are several limitations in this study. It was a single-center and retrospective study and included a small number of subjects. The other limitation is not examining the influence of the timing of BAL in the illness course. Finally, we did not explore the relationship between the specific serotyping of HAdV and PB in our study.
Conclusion
HAdV pneumonia can cause PB. When children with severe adenovirus pneumonia have a history of allergies, diminished breath sounds, and increased proportion of neutrophils (>70%), HAdV PB should be strongly considered. The timely application of bronchoscopy plays an important role in disease recovery and prognosis.
Acknowledgments
We are very appreciative of the children and their families.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Xiong L, Rao X, Peng X, et al. Management of plastic bronchitis using α-chymotrypsin: a novel treatment modality. Cureus. 2021;13(2):e13551. doi:10.7759/cureus.13551
2. Yuan L, Huang J, Zhu Q, et al. Plastic bronchitis associated with adenovirus serotype 7 in children. BMC Pediatric. 2020;20(1):268. doi:10.1186/s12887-020-02119-4
3. Maqsood A, Imel L. Plastic Bronchitis. New Engl J Med. 2022;386(8):780. doi:10.1056/NEJMicm2111951
4. Rubin B. Plastic bronchitis. Clinics Chest Med. 2016;37(3):405–408. doi:10.1016/j.ccm.2016.04.003
5. Zhang F, Qin L, Yuan J, et al. Plastic bronchitis due to adenoviral infection: a case report. BMC Pediatric. 2020;20(1):61. doi:10.1186/s12887-020-1954-0
6. Zeng L, Wei J, Tang Y, et al. Clinical characteristics of human adenovirus plastic bronchitis in 10 pediatric cases: a retrospective study of seven years. Virologica Sin. 2021;36(3):550–554. doi:10.1007/s12250-021-00394-8
7. Zhang J, Zhu Y, Zhou Y, et al. Pediatric adenovirus pneumonia: clinical practice and current treatment. Front Med Lausanne. 2023;10:1207568. doi:10.3389/fmed.2023.1207568
8. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the pediatric infectious diseases society and the infectious diseases society of America. Clin Infect Dis. 2011;53(7):e25–e76. doi:10.1093/cid/cir531
9. Patel N, Patel M, Inja R, et al. Plastic bronchitis in adult and pediatric patients: a review of its presentation, diagnosis, and treatment. Missouri Med. 2021;118(4):363–373.
10. Huang J, Yang X, Zhuo Z, et al. Clinical characteristics of plastic bronchitis in children: a retrospective analysis of 43 cases. Respir Res. 2022;23(1):51. doi:10.1186/s12931-022-01975-1
11. Jasinovic T, Kozak F, Moxham J, et al. Casting a look at pediatric plastic bronchitis. Intern j Pediatric Otorhinol. 2015;79(10):1658–1661. doi:10.1016/j.ijporl.2015.07.011
12. Stoddart A, Dincer H, Iber C, et al. Chyloptysis causing plastic bronchitis. Respiratory Med Case Report. 2014;13:4–6. doi:10.1016/j.rmcr.2013.12.001
13. Kumar A, Jat K, Srinivas M, et al. Nebulized N-acetylcysteine for management of plastic bronchitis. Indian Pediatrics. 2018;55(8):701–703. doi:10.1007/s13312-018-1363-8
14. Büyükşahin H N, Emiralioglu N, Sekerel BE, et al. Plastic bronchitis during childhood: diversity of presentation, etiology, treatment, and outcomes. Pediatr Pulmonol. 2023;58(9):2559–2567. doi:10.1002/ppul.26548
15. Li Y, Williams R, Dombrowski N, et al. Current evaluation and management of plastic bronchitis in the pediatric population. Intern j Pediatric Otorhinol. 2020;130:109799. doi:10.1016/j.ijporl.2019.109799
16. Brogan T, Finn L, Pyskaty D, et al. Plastic bronchitis in children: a case series and review of the medical literature. Pediatric Pulm. 2002;34(6):482–487. doi:10.1002/ppul.10179
17. Yang L, Fu J, Zhou Y. Research Progress in Atopic March. Front Immunol. 2020;11:1907. doi:10.3389/fimmu.2020.01907
18. Tantilipikorn P, Auewarakul P. Airway allergy and viral infection. Asian Pac J Allergy Immunol. 2011;29(2):113–119.
19. Hong J, Bentley J, Chung Y, et al. Neonatal rhinovirus induces mucous metaplasia and airways hyperresponsiveness through IL-25 and type 2 innate lymphoid cells. J Allergy Clin Immunol. 2014;134(2):429–439. doi:10.1016/j.jaci.2014.04.020
20. Wang L, Wang W, Sun J, et al. Efficacy of fiberoptic bronchoscopy and bronchoalveolar lavage in childhood-onset, complicated plastic bronchitis. Pediatric Pulm. 2020;55(11):3088–3095. doi:10.1002/ppul.25016
21. Zhao L, Zhang T, Cui X, et al. Development and validation of a nomogram to predict plastic bronchitis in children with refractory Mycoplasma pneumoniae pneumonia. BMC pulm medi. 2022;22(1):253. doi:10.1186/s12890-022-02047-2
22. Xu W, Xu Z, Huang L, et al. Transcriptome sequencing identifies novel immune response genes highly related to the severity of human adenovirus type 55 infection. Front Microbiol. 2019;10:130. doi:10.3389/fmicb.2019.00130
23. Cavallaro E, Liang K, Lawrence M, et al. Neutrophil infiltration and activation in bronchiolitic airways are independent of viral etiology. Pediatric Pulm. 2017;52(2):238–246. doi:10.1002/ppul.23514
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.