Back to Journals » Journal of Multidisciplinary Healthcare » Volume 17
Analysis of Dietary and Nutritional Status of Tuberculosis Patients in Hulunbuir Region
Authors Hao JQ, Zhang L, Yu YQ, Hao MY, Wang AX, Feng FM
Received 15 November 2023
Accepted for publication 16 February 2024
Published 19 March 2024 Volume 2024:17 Pages 1231—1240
DOI https://doi.org/10.2147/JMDH.S450080
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jin-Qi Hao,1,2 Lan Zhang,2 Yan-Qin Yu,2 Ming-Yuan Hao,3 Ai-Xin Wang,3 Fu-Min Feng1
1School of Public Health, North China University of Science and Technology, Tangshan, Hebei Province, 063210, People’s Republic of China; 2School of Public Health, Baotou Medical College, Baotou, Inner Mongolia, 014040, People’s Republic of China; 3The Second People’s Hospital in Hulunbuir, Zaerdong, 162650, People’s Republic of China
Correspondence: Fu-Min Feng, School of Public Health, North China University of Science and Technology, No. 21 Bohai Road, Caofeidian District, Tangshan City, Hebei Province, 063210, People’s Republic of China, Tel +8613931482536, Email [email protected]
Objective: Tuberculosis (TB) is a major public health problem that affects millions of people worldwide. Malnutrition is a common complication of TB and can worsen the disease outcome. The purpose of this study was to investigate the dietary and nutritional status, as well as the dietary structure, of TB patients in Hulunbuir City, Inner Mongolia, China. Additionally, the study aimed to analyze the factors that influence the nutritional status in order to provide a theoretical foundation for the prevention and treatment of TB and related issues.
Methods: A cross-sectional study was conducted on 334 randomly selected TB patients from Hulunbuir City Second Hospital. A questionnaire survey was administered to collect information on demographic characteristics, dietary habits, and food intake. Nutritional status was assessed by body mass index (BMI). Dietary diversity score (DDS) was calculated based on the number of food groups consumed in the previous 24 hours. Statistical analysis was performed using SPSS 20.0 software. Descriptive statistics employed rates and composition ratios, and categorical data was represented using frequencies and percentages. The chi-square test was used to analyze the association between nutritional status and other variables, with a significance level set at α=0.05. Multivariable ordinal logistic regression analysis was performed to identify the independent factors affecting the nutritional status of TB patients.
Results: The univariate analysis revealed statistically significant differences (P < 0.05) in the nutritional status (as measured by BMI) among tuberculosis patients, considering ethnicity, educational level, smoking, meat-based diet, vegetable consumption, and DDS grading. No statistically significant differences were found regarding gender, age, marital status, occupation, sleep duration, alcohol consumption, and consumption of rice and flour dishes. Statistically significant variables from the univariate analysis were included in a multivariable ordinal logistic regression analysis model. The findings highlighted that educational level (high school or below), smoking, meat-based diet, DDS scores of 1– 3, and a primarily vegetable-based diet had independent effects on the nutritional status of tuberculosis patients (all P < 0.05). No significant difference was found in nutritional status between the Han ethnic group and other ethnicities.
Conclusion: The study revealed that the dietary and nutritional status of TB patients in Hulunbuir City was suboptimal and influenced by several factors. Smoking, meat-based diet, and low dietary diversity score were the primary risk factors for malnutrition among TB patients. The study suggests that nutritional education and intervention programs should be implemented for TB patients to improve their dietary quality and nutritional status.
Keywords: tuberculosis, nutritional status, dietary diversity score, influencing factors
Background
Pulmonary tuberculosis is a severe, chronic infectious disease, significantly affecting global mortality rates.1,2 According to the World Health Organization, there were an estimated 10 million new cases and 1.4 million deaths from tuberculosis in 2019, with 44% of the cases occurring in the WHO Western Pacific Region and the WHO South-East Asia Region.3 China, as one of the 30 high-burden countries for tuberculosis, experienced an increasing incidence rate, reaching 59 per 100,000 in 2019.4 Inner Mongolia has seen improvements due to comprehensive policies such as the “Inner Mongolia Tuberculosis Prevention and Control Action Plan”, resulting in a decline in tuberculosis cases, with an incidence rate of 49.86 per 100,000 in 2019.5 However, the situation remains critical, especially with the emergence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis strains, which pose a serious threat to public health and tuberculosis control.6 Previous studies have shown that certain lineages of Mycobacterium tuberculosis, such as Beijing and East-African Indian, are more prevalent among MDR and XDR strains than among non-MDR strains, suggesting a possible association between genetic diversity and drug resistance.7,8 Various risk factors, including exposure history, environment, lifestyle choices like smoking and alcohol consumption, and health conditions like tumors and malnutrition, influence tuberculosis risk.9 Managing these factors through promoting healthy behaviors is essential.Malnutrition is a common comorbidity of tuberculosis, affecting both the host immune response and the bacterial growth.10 After the onset of tuberculosis, the body experiences excessive consumption and becomes susceptible to malnutrition, which in turn can impair the treatment outcome and increase the risk of mortality.11 Therefore, managing these factors through promoting healthy behaviors and providing adequate nutritional support is essential for tuberculosis patients.
The Inner Mongolia Autonomous Region is situated in a vast, elongated area, covering a considerable range of longitudes. It displays notable variations in climate, household and cultural practices, population composition, as well as the level of amalgamation between the Mongolian and Han populations in different localities.12 The dietary culture of the region is characterized by distinctive regional elements: meat and milk form the fundamental dietary staples, complemented by the inclusion of noodles, rice, and vegetables. The local populace commonly consumes homemade yogurt and smoked meat, and there is a notable prevalence of alcohol consumption and smoking behaviors.These dietary habits may have an impact on the nutritional status and treatment outcome of tuberculosis patients. Moreover, different dietary patterns may reflect different modes of transmission of tuberculosis, such as household contact, community contact, or animal contact, which could provide clues for identifying the sources of infection and preventing further spread.13,14
The aim of this study was to investigate the dietary intake and nutritional status of tuberculosis patients in Inner Mongolia, and to explore the association between dietary factors and BMI. We also compared the dietary patterns of different ethnic groups and regions to identify the potential dietary risk factors for tuberculosis. This study may provide valuable information for developing effective nutritional interventions for tuberculosis patients in Inner Mongolia, as well as for understanding the epidemiology and transmission dynamics of tuberculosis in this region.
Materials and Methods
Research Subjects
We included patients who were diagnosed with tuberculosis and received treatment at Hulunbuir Second Hospital from January to June 2023. This study was approved by the Ethics Committee of Hulunbuir Second Hospital and conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent before participating in the survey. We defined the inclusion and exclusion criteria as follows:
Inclusion criteria: Patients who were confirmed to have tuberculosis by sputum smear microscopy, culture, or molecular tests, and who had a BMI less than 18.5 or greater than 24.0, indicating malnutrition or overweight, respectively.
Exclusion criteria: Patients who had other chronic diseases, such as diabetes, hypertension, or cardiovascular diseases, or who refused to participate in the survey or provide complete information.
Research Contents
The survey questionnaire collected basic information about the subjects, including their gender, age, ethnicity, height, weight, marital status, and educational level, as well as their diet and lifestyle habits, such as smoking, alcohol consumption, sleep duration, and dietary diversity score (DDS). We analyzed the individual characteristics of tuberculosis patients and examined the relationship between different dietary patterns and lifestyle habits, and BMI, which was the outcome variable of the study. Using the chi-square test, we determined whether there was statistical and practical significance in the composition ratios of various characteristics.
Data Collection
We selected random samples of tuberculosis patients who visited the Second Hospital of Hulunbuir City in Inner Mongolia from January to June 2023. A survey questionnaire was administered to gather information regarding their general conditions, diet, smoking, drinking, and other lifestyle habits.The questionnaires were distributed, collected, and reviewed on-site by a team of investigators who had undergone standardized training. The survey team promptly addressed any concerns and completed any missing items on the questionnaires. After cross-checking for accuracy, the data was entered twice.A total of 400 questionnaires were distributed to the eligible patients, and 360 questionnaires were returned, with a response rate of 90%. After excluding 26 questionnaires with incomplete or inconsistent information, 334 questionnaires were included in the final analysis, with an effective rate of 83.5%.
Statistical Analysis
The survey data were entered and organized in Excel 2019. Descriptive statistics were computed using SPSS 20.0 software. Quantitative variables with normal distributions were summarized as mean ± standard deviation. Categorical variables were reported as frequency and percentage. The participants were stratified by BMI, and the chi-square test was used to compare the categorical variables between the two groups. Ordered logistic regression analysis was performed on the statistically significant independent variables to identify the influencing factors. A P-value of less than 0.05 was considered statistically significant.
Related Concepts
The Body Mass Index (BMI) is a standard measurement calculated as weight (kg) divided by height (m)2. It is used to assess obesity levels, determine a person’s physical health, and evaluate their nutritional status. The criteria for classification are defined by the American Society for Parenteral and Enteral Nutrition (ASPEN) clinical guidelines: a BMI less than 18.5 indicates underweight, a BMI between 18.50 and 24.0 is considered normal, and a BMI of 24.00 or higher indicates overweight.15
The Dietary Diversity Score (DDS) is commonly employed as a tool to evaluate the diversity of food intake. It utilizes a 9-category method to evaluate the diversity of the foods consumed. This involves calculating the number of different food categories consumed by participants in a survey over a one-week period. The calculation is based on the information gathered through a questionnaire. The calculated DDS is represented by numerical score, where each of the nine food categories contributes to the total score, reflecting the overall dietary diversity.16
Results
Basic Information
A total of 334 tuberculosis patients were included in this study, with their gender, age, ethnicity, educational level, marital status, BMI level, and basic occupational information presented in Table 1.
 |
Table 1 Characteristics and Lifestyle Habits of Tuberculosis Patients |
Analysis of Patient’s Living Habits
The living habits of the patients, including smoking, alcohol consumption, and sleep duration, are shown in Table 1. There were 108 patients who reported smoking, constituting 32.3% of the total sample size. Among the patients, 134 individuals consumed white liquor, representing 40.1% of the total. The number of patients who drink beer is 169, accounting for 50.6%. Among the participants, 291 individuals reported having a sleep duration of over 6 hours, constituting 87.1% of the sample (Table 1).
Analysis of the Patient’s Dietary Situation
Dietary Intake Among Tuberculosis Patients
The daily food intake was assessed by using a food frequency questionnaire (FFQ) based on the Food Guide Pagoda, which was developed by the Chinese Nutrition Society.17 The FFQ included nine food categories: cereals and potatoes, vegetables, fruits, poultry and meat, fish and shrimp, eggs, dairy products, soybeans and their products, and fats and oils. The dietary intake of the patients is shown in Table 2. The patients mainly consumed cereals and potatoes as their staple food, and supplemented with vegetables, fruits, poultry, meat, and eggs. In addition, due to the traditional dietary habits and unique food characteristics of the Inner Mongolia region, the patients also had a higher consumption of dairy products.
 |
Table 2 Dietary Intake of Tuberculosis Patients |
In terms of dietary preferences, men predominantly consume meat and rice-based dishes, while women tend to favor vegetables. The findings demonstrate statistically significant variations in three domains: meat consumption, vegetable intake, and consumption of rice and flour dishes, as illustrated in Table 3.
 |
Table 3 Analysis of Food Types by Gender |
Patient Dietary Diversification Scoring
Each of the one or two types of food in the same category is assigned a score of 1 point. Two categories of food receive a score of 2 points. The highest possible score is 9 points. Scores are not duplicated for the same category of food, and the frequency and quantity of food intake are not considered. The DDS is categorized as insufficient (1–3 points), moderate (4–6 points), or adequate (7–9 points) based on the diversity of the diet structure. The mean DDS score for the tuberculosis patients surveyed was (6.17±2.329). Of the tuberculosis patients surveyed, 9.6%, 41.3%, and 49.1% had insufficient, moderate, and adequate food diversity, respectively (Table 4).
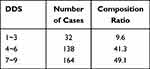 |
Table 4 Diversity Scores of Tuberculosis Patients |
Univariate Analysis of Patient BMI
BMI is a widely used indicator of nutritional status, and it has been shown that low BMI is associated with increased risk of mortality and morbidity in tuberculosis patients.18
The univariate analysis of the influencing factors revealed statistically significant differences (P < 0.05) in the nutritional status BMI of tuberculosis patients based on ethnicity, educational level, smoking status, meat consumption, vegetable consumption, and DDS classification, involving a total of 6 variables. However, no statistically significant differences (P > 0.05) were found regarding gender, age, marital status, occupation, sleep duration, alcohol consumption, consumption of rice and flour dishes, or balanced nutrition. Please refer to Table 5 for further information.
 |
Table 5 Univariate Analysis of BMI in Tuberculosis Patients, n (%) |
Multifactorial Analysis Using Ordered Logistic Regression on Factors Influencing the Nutritional Status of Patients
The variables with statistically significant differences in the univariate analysis were included in the multi-factor ordinal logistic regression analysis model. The analysis indicated that an educational level of high school or below (OR=2.817, 95% CI: 0.357 to 1.714, P=0.003), smoking (OR=0.605, 95% CI: −1.235 to 2.31, P=0.049), a meat-based diet (OR=1.526, 95% CI: −1.045 to 1.891, P=0.037), DDS scores of 1–3 (OR=0.787, 95% CI: −2.259 to 1.781, P=0.031) were independent factors influencing the nutritional status of individuals with pulmonary tuberculosis. No significant difference was found in nutritional status between the Han ethnic group and other ethnicities (OR=0.969, 95% CI: −0.677 to 0.614, P=0.923), as presented in Table 6.
 |
Table 6 Multivariable Ordered Logistic Regression Analysis of Factors Influencing the Nutritional Status of Pulmonary Tuberculosis Patients |
Discussion
The nutritional status of tuberculosis patients is an important factor that affects the disease progression and outcome. Previous studies have shown that malnutrition, especially underweight, is associated with increased risk of tuberculosis infection, severity, mortality, and relapse.19–21 Moreover, dietary factors, such as dietary diversity, intake of micronutrients, and consumption of animal products, may also influence the immune response and the treatment outcome of tuberculosis patients.22–24 Therefore, it is essential to assess the dietary and nutritional status of tuberculosis patients and identify the potential risk factors that may affect their health and recovery.
In this study, we examined the dietary intake and nutritional status of tuberculosis patients in Inner Mongolia, a region notable for its high tuberculosis burden and unique dietary culture. We found significant dietary habits and nutritional status among the patients. A significant proportion of these patients consumed cereals and potatoes, vegetables, fruits, poultry, meat, eggs, and notably, a high intake of dairy products, which is a hallmark of the Inner Mongolian diet. The mean Dietary Diversity Score (DDS) for the patients was 6.17±2.329, indicating a moderate level of dietary diversity.Our results revealed distinct dietary preferences based on gender and ethnicity. Men were more inclined towards meat and rice-based dishes, whereas women showed a preference for dairy products. These findings underscore the influence of gender, ethnicity, and cultural traditions on the dietary patterns of tuberculosis patients in Inner Mongolia.25,26
The study also explored factors affecting the nutritional status of tuberculosis patients. Significant factors included ethnicity, educational level, smoking status, meat-based diet, and DDS classification. Contrary to our initial expectations, Mongolian patients did not demonstrate a higher risk of being underweight compared to Han patients. This outcome might be reflective of the balanced nature of their diet, despite the high consumption of animal products. Patients with lower educational levels (primary school or below) were found to have a higher risk of being overweight, possibly due to limited access to a variety of foods and lower awareness of nutritional balance. Smoking was associated with a lower risk of being underweight, aligning with existing literature that suggests smoking can impact appetite and metabolism.27,28 A meat-based diet, typically high in protein and essential nutrients, was correlated with a lower risk of being underweight. Lastly, the DDS played a crucial role in the nutritional status. A higher DDS was associated with a lower risk of being underweight but did not significantly affect the risk of being overweight, suggesting that a varied diet is beneficial for maintaining an adequate nutritional status, especially in tuberculosis patients.
BMI was chosen as the primary indicator due to its relevance in assessing nutritional status, especially in chronic conditions like tuberculosis.29 Lower BMI is associated with a higher risk of contracting tuberculosis, emphasizing the need for better metabolic health to reduce the disease’s prevalence and mortality.30,31 Dietary patterns, particularly in eastern Inner Mongolia, lean heavily towards meat consumption, influenced by the region’s reliance on animal husbandry and grain production.32 Urban dietary habits are more akin to the Han ethnic group.The majority of survey participants were rural, middle-aged or elderly individuals with limited education. This demographic shows inadequate understanding and protective measures against tuberculosis transmission, necessitating extensive public health education and awareness campaigns, especially in rural areas.Smoking is identified as a critical factor affecting tuberculosis incidence and prognosis, impairing immune function and lung health.33,34 Research consistently shows smoking as a significant risk factor for pulmonary tuberculosis.35
Our study has several limitations that should be acknowledged. Firstly, the sample size of this study was relatively small and limited to specific regions of Hulunbuir City, which may affect the generalizability of the results. Secondly, the study design was cross-sectional, which could not establish causal relationships between dietary factors and BMI. Thirdly, the dietary intake data was collected by using a FFQ, which may be subject to recall bias and measurement errors. Fourthly, the study did not collect data on other potential confounding factors, such as tuberculosis severity, treatment regimen, comorbidities, and socioeconomic status, which may also affect the nutritional status of tuberculosis patients. Therefore, future studies should include larger and more representative samples, use prospective and longitudinal designs, employ more accurate and objective methods to assess dietary intake, and adjust for more covariates to confirm and extend our findings.
Conclusion
In conclusion, this study revealed that the nutritional status and dietary intake of tuberculosis patients in Inner Mongolia were influenced by various factors, such as ethnicity, educational level, smoking, meat-based diet, and dietary diversity. The study also found that underweight and overweight were both prevalent among tuberculosis patients, and that both conditions were associated with increased risk of adverse outcomes. Therefore, it is important to provide individualized and comprehensive nutritional assessment and intervention for tuberculosis patients, taking into account their dietary habits, preferences, and needs. Moreover, it is necessary to improve the health awareness and education of the local population, especially in rural areas, and to promote healthy lifestyle behaviors, such as quitting smoking, reducing alcohol consumption, and increasing physical activity. These measures may help to prevent and control tuberculosis, as well as to improve the quality of life and well-being of the affected individuals.
Abbreviations
DDS, Dietary Diversity Score; BMI, Body Mass Index; AMs, Alveolar Macrophages.
Data Sharing Statement
The data supporting these findings are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of Hulunbuir Second Hospital and conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent before participating in the survey.
Acknowledgments
We would like to thank all the physicians who helped in collecting data from tuberculosis hospitals in Hulunbuir.
Funding
National Natural Science Foundation of China (82260657), Inner Mongolia Natural Science Foundation of China (2023MS08005), Inner Mongolia Autonomous Region Health and Technology Program of China (202201375).
Disclosure
All authors declare that they have no conflicts of interest in this work.
References
1. World Health Organization. Global Tuberculosis Report 2022. Geneva: World Health Organization. 2022:1–3.
2. Sudarsanam TD, John J, Kang G, et al. Pilot randomized trial of nutritional supplementation in patients with tuberculosis and HIV-tuberculosis coinfection receiving directly observed short-course chemotherapy for tuberculosis. Trop Med Int Health. 2011;16(6):699–706. doi:10.1111/j.1365-3156.2011.02761.x
3. World Health Organization. Tuberculosis Country Profiles: China. Geneva: World Health Organization; 2020.
4. National Health Commission of the People’s Republic of China. Report on the prevention and control of tuberculosis in China (2019). Beijing:National Health Commission of the People’s Republic of China; 2020.
5. Chen T, Guo WR, Li HC, et al. Correlation Analysis of TB Outpatients’ Nutritional Status with Their Diet. Clinical Med & Eng. 2013;20(12):1599–1601.
6. Zhao Y, Xu S, Wang L, et al. National survey of drug-resistant tuberculosis in China. N Engl J Med. 2012;366(23):2161–2170. doi:10.1056/NEJMoa1108789
7. Zhang H, Li D, Zhao L, et al. Genome sequencing of 161 Mycobacterium tuberculosis isolates from China identifies genes and intergenic regions associated with drug resistance. Nat Genet. 2013;45(10):1255–1260. doi:10.1038/ng.2735
8. Zhang Y, Yew WW, Barer MR. Targeting persisters for tuberculosis control. Antimicrob Agents Chemother. 2012;56(5):2223–2230. doi:10.1128/AAC.06288-11
9. Ramachandran G, Hemanth Kumar AK, Bhavani PK, et al. Age, nutritional status and INH acetylator status affect pharmacokinetics of anti-tuberculosis drugs in children. Int J Tuberc Lung Dis. 2013;17(6):800–806. doi:10.5588/ijtld.12.0628
10. Lönnroth K, Williams BG, Stadlin S, Jaramillo E, Dye C. Alcohol use as a risk factor for tuberculosis - a systematic review. BMC Public Health. 2008;8:289. doi:10.1186/1471-2458-8-289
11. McMurray DN. Impact of nutritional deficiencies on resistance to experimental pulmonary tuberculosis. Nutr Rev. 1998;56(1 Pt 2):S147–S152. doi:10.1111/j.1753-4887.1998.tb01633.x
12. Miyata S, Tanaka M, Ihaku D. The prognostic significance of nutritional status using malnutrition universal screening tool in patients with pulmonary tuberculosis. Nutr J. 2013;12:42. doi:10.1186/1475-2891-12-42
13. Lienhardt C, Fielding K, Sillah JS, et al. Investigation of the risk factors for tuberculosis: a case-control study in three countries in West Africa. Int J Epidemiol. 2005;34(4):914–923. doi:10.1093/ije/dyi100
14. Tschopp R, Schelling E, Hattendorf J, Aseffa A, Zinsstag J. Risk factors of bovine tuberculosis in cattle in rural livestock production systems of Ethiopia. Prev Vet Med. 2009;89(3–4):205–211. doi:10.1016/j.prevetmed.2009.02.006
15. Zhang YN, Zheng H, Liang J, et al. Relationship Between Aged People Body Mass Index and Blood Pressure and Prevalence Rate of Hypertension. Chin J Gerontol. 2021;41(20):4333–4335.
16. Sun YX. Analysis of Clinical Characteristics and Influencing Factors of Adolescent Pulmonary Tuberculosis Patients in Linyi. Qingdao University. 2019. doi:10.27262/d.cnki.gqdau.2019.000325
17. Zang J, Luo B, Chang S, et al. Validity and reliability of a food frequency questionnaire for assessing dietary intake among Shanghai residents. Nutr J. 2019;18(1):30. doi:10.1186/s12937-019-0454-2
18. Budzyński J, Szukay B. BMI as a biomarker in patients’ nutritional assessment. In: Zalewski BM editor. Biomarkers in Nutrition. Springer International Publishing; 2022:597–629. doi:10.1007/978-3-031-07389-2_36.
19. Lönnroth K, Castro KG, Chakaya JM, et al. Tuberculosis control and elimination 2010-50: cure, care, and social development. Lancet. 2010;375(9728):1814–1829. doi:10.1016/S0140-6736(10)60483-7
20. Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis. 2004;8(3):286–298.
21. Baker MA, Harries AD, Jeon CY, et al. The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med. 2011;9:81. doi:10.1186/1741-7015-9-81
22. Karyadi E, West CE, Schultink W, et al. A double-blind, placebo-controlled study of vitamin A and zinc supplementation in persons with tuberculosis in Indonesia: effects on clinical response and nutritional status. Am J Clin Nutr. 2002;75(4):720–727. doi:10.1093/ajcn/75.4.720
23. van Lettow M, Harries AD, Kumwenda JJ, et al. Micronutrient malnutrition and wasting in adults with pulmonary tuberculosis with and without HIV co-infection in Malawi. BMC Infect Dis. 2004;4(1):61. doi:10.1186/1471-2334-4-61
24. McQuaid CF, Sinha P, Bhargava M, Weerasuriya C, Houben RMGJ, Bhargava A. Tuberculosis and nutrition: what gets measured gets managed. Lancet Respir Med. 2023;11(4):308–310. doi:10.1016/S2213-2600(23)00009-7
25. Zhang JG, Wang ZH, Wang HJ, et al. Dietary patterns and their associations with general obesity and abdominal obesity among young Chinese women. Eur J Clin Nutr. 2015;69(9):1009–1014. doi:10.1038/ejcn.2015.8
26. Zhang J, Wang H, Wang Y, et al. Dietary patterns and their associations with childhood obesity in China. Br J Nutr. 2015;113(12):1978–1984. doi:10.1017/S0007114515001154
27. Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87(4):801–809. doi:10.1093/ajcn/87.4.801
28. Froom P, Melamed S, Benbassat J. Smoking cessation and weight gain. J Fam Pract. 1998;46(6):460–464.
29. Shi GS. Association of Dietary Intake and Underweight in Patients with Pulmonary Tuberculosis. Peking Union Medical Coll. 2017:548.
30. Zhang CY, Zhao F, Xia YY, et al. Prevalence and risk factors of active pulmonary tuberculosis among elderly people in China: a population-based cross-sectional study. Infect Dis Poverty. 2019;8(1):7.
31. Restrepo BI, Fisher-Hoch SP, Crespo JG, et al. Type 2 diabetes and tuberculosis in a dynamic bi-national border population. Epidemiol Infect. 2007;135(3):483–491. doi:10.1017/S0950268806006935
32. Zhang YT. Research on Inner Mongolia’s Food Culture: a Case Study of the Alxa Region. Comp Stud Cult Innov. 2019;3(28):57–58.
33. Evans PA. Smoking and health. Summary of a report of the Royal College of Physicians of London on smoking in relation to cancer of the lung and other diseases. Cent Afr J Med. 1962;8:234–236.
34. Alrouji M, Manouchehrinia A, Gran B, Constantinescu CS. Effects of cigarette smoke on immunity, neuroinflammation and multiple sclerosis. J Neuroimmunol. 2019;329:24–34. doi:10.1016/j.jneuroim.2018.10.004
35. Jayes L, Haslam PL, Gratziou CG, et al. SmokeHaz: systematic Reviews and Meta-analyses of the Effects of Smoking on Respiratory Health. Chest. 2016;150(1):164–179. doi:10.1016/j.chest.2016.03.060
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
