Back to Journals » Patient Preference and Adherence » Volume 18
An Assessment of Individual Preference for a Novel Capillary Blood Collection System
Authors Pourafshar S, Parikh M, Abdallah B, Al Thubian N, Jacobson JW
Received 22 September 2023
Accepted for publication 13 February 2024
Published 1 March 2024 Volume 2024:18 Pages 531—541
DOI https://doi.org/10.2147/PPA.S437969
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Shirin Pourafshar,1 Monisha Parikh,2 Bilal Abdallah,2 Nasrin Al Thubian,3 James W Jacobson4
1Medical Affairs, Becton, Dickinson and Company, Sparks, MD, USA; 2Medical Affairs, Becton, Dickinson and Company, Franklin Lakes, NJ, USA; 3Medical Affairs, Becton, Dickinson and Company, Mississauga, Ontario, Canada; 4Clinical Affairs, Babson Diagnostics, Austin, TX, USA
Correspondence: James W Jacobson, Vice President of Clinical Affairs, Babson Diagnostics, 1321 Rutherford Ln, Bldg 2, Ste 200, Austin, TX, 78753, USA, Tel +1-888-556-8785, Fax +1-512-277-3206, Email [email protected]
Purpose: Typical barriers to venous blood collection for wellness testing include discomfort, time spent, and collection site accessibility. This study assessed individuals’ experience, satisfaction, and preference associated with a FDA-cleared blood-collection device, the BD MiniDraw™ Capillary Blood Collection System (BD MiniDraw), in retail locations.
Patients and Methods: A total of 113 individuals (≥ 18 years) with venous blood collection experience were enrolled; 107 completed the study. A pre-collection survey gathered information on demographics and past experiences with healthcare and venous blood collection settings. BD MiniDraw collection was conducted at three retail sites (two pharmacies and one grocery store) by trained healthcare workers using the Babson BetterWay blood testing service model. A follow up survey was performed two weeks later to determine experience with, and preference for, BD MiniDraw in terms of staff professionalism, blood collection location, blood collection time, and staff trustworthiness.
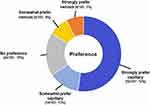
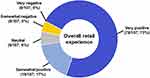
Results: Among the 107 participants, 74 (69%) were female and 33 (31%) were male; the mean age was 49 years (range=18– 71 years). Sixty-six (62%) participants viewed their prior venipuncture experience as “somewhat” or “very” positive. Following capillary collection, 96 (90%) participants expressed a “somewhat” or “very” positive experience with BD MiniDraw at a retail location. In particular, “very satisfied” responses were given for location (87/107; 81%) and collection time (78/1407; 73%). In a subset of respondents (n=89), those reasons (location and time savings) were most frequent for likelihood of future use. Ninety-nine participants (92%) rated the retail blood collection team as “very” or “extremely” trustworthy. Overall, 90 participants (84%) “strongly preferred” (56/107; 52%), “somewhat preferred” (14/107; 13%), or had “no preference” (20/107; 19%) for BD MiniDraw, compared to traditional venous blood collection.
Conclusion: Most participants conveyed a preference for BD MiniDraw, primarily based on the blood collection retail location, perceived time savings, and professionalism and trustworthiness of the staff.
Keywords: capillary blood collection, phlebotomy, patient preference, blood specimen collection, patient satisfaction
Introduction
Blood testing is often used to facilitate health screening and to diagnose or monitor disease.1–3 Blood tests commonly performed as part of wellness testing include the complete blood count (CBC), and serum chemistry tests, such as a comprehensive metabolic panel, lipid panel, and thyroid function tests. CBC measures the types and numbers of cells in the blood, and helps identify patients at risk for a wide range of conditions, such as anemia, infection, and leukemia.1 The comprehensive metabolic panel (CMP) is used to analyze blood chemistry and can help diagnose and monitor chronic conditions, including diabetes and kidney disease.4 Venipuncture is typically performed to collect blood specimens for CBC, CMP, and other tests. Although common, it requires a skilled phlebotomist or nurse and dedicated healthcare infrastructure (eg, health care provider office, blood collection service center), and is frequently associated with some level of discomfort and bruising (up to 45% of patients) for the patient.5–7
Capillary blood collection is accomplished through a tiny skin puncture and holds several perceived advantages over venipuncture as a blood collection approach.8–10 It is less invasive and has been shown to be less painful;11 it also requires smaller amounts of blood (for CBC and CMP testing), and can be performed relatively quickly. In addition, capillary blood collection could be a viable option for individuals with difficult venous access.12 These include oncology, geriatric, and pediatric patients, or any other patients with hard to access or fragile blood vessels.13–15 In addition, capillary blood collection in ancillary healthcare settings (ie, retail stores) may help improve health care accessibility and reduce the time needed for individual blood collection in rural settings.16 A recent meta-analysis demonstrated generally positive views from primary care physicians regarding the clinical value of diagnostic assays following capillary blood collection to help increase test coverage and improve turn-around-time for results.17
The objective of this study was to determine individual satisfaction with capillary blood collection in retail settings (pharmacy or grocery store) and preference for capillary collection compared to a prior experience with venous blood collection. Enrolled participants completed a pre-collection survey. This was followed by capillary blood collection with the FDA-cleared BD MiniDraw™ Capillary Blood Collection System (BD MiniDraw collection system; Becton, Dickinson and Company; BD Life Sciences—Diagnostic Solutions), which was facilitated by Babson Diagnostics’ BetterWay (Babson Diagnostics, Austin, TX) blood testing service. Approximately two weeks later, participants completed a post-collection survey. In addition to overall experience and preference regarding capillary collection, additional factors were identified, including feelings about staff professionalism, retail location, draw time, and staff trustworthiness.
Materials and Methods
This was a prospective, cross-sectional, single-cohort study involving a pre-collection survey, capillary blood collection at a retail site, and a post-collection survey; it was conducted to gauge preference for capillary blood collection using BD MiniDraw as compared to traditional venous blood collection.
Participants
Ambulatory participants ≥18 years of age were enrolled. Potential participants were recruited from the general population via an Institutional Review Board-approved (Advarra IRB) social media advertisement or directly from local physicians’ offices. All eligible participants had prior venous blood collection experience and provided informed consent and had at least two middle or two ring fingers accessible for capillary blood collection. Exclusion criteria for participants were any self-reported health conditions that would preclude a safe blood collection (eg, fainting disorder); being an employee or affiliate of the study sponsor, or an employee at the retail collection site; having a known allergy to the (plastic-like) materials constituting the BD MiniDraw collection system; and previous participation in a study involving the BD MiniDraw collection system or previous use with the device/system. This study was conducted according to the principles set forth by the Declaration of Helsinki and Good Clinical Practice.
Capillary Blood Collection
Capillary blood collection locations consisted of three sites in Texas that were ancillary healthcare site locations (eg, retail pharmacy or grocery store). Blood was collected using the BD MiniDraw collection system in serum separator tubes (intended for selected serum chemistry tests) and EDTA tubes (intended for hemoglobin and hematocrit [“H&H;” see below] testing). Staff were trained in blood collection using the BD MiniDraw device and did not receive specialized phlebotomy training as part of this study. Training regarding capillary blood collection for technicians for this study consisted of device and protocol description in written and video format, hands-on practice with device components, live training (to success), and access to written instructions and similar material. The BD MiniDraw™ Capillary Blood Collection System consisted of the following components (Figure 1):
- The BD MiniDraw™ Finger Sizing Tool, which helps determine the size of the BD MiniDraw finger sleeve to be utilized during collection.
- The BD MiniDraw™ Finger Sleeve, which includes a port that guides a BD Microtainer© Contact-Activated Lancet (BD Catalog Number 366594, currently marketed by Becton, Dickinson and Company, BD Life Sciences—Diagnostic Solutions, Franklin Lakes, NJ) to the proper lancing position and includes wings that control both squeeze pressure and location in order to minimize hemolysis and optimize collection volume.
- The BD MiniDraw™ SST™ Capillary Blood Collection Tube is FDA-cleared (notification date 12/01/2023) for collection of capillary blood specimens from individuals, ages ≥18 years. It is intended to collect 435μL (minimum volume for analysis) to 635μL (maximum volume of collection tube) of whole blood. It contains a silica-based clot activator and polymer gel to separate blood cells and serum upon centrifugation for serum samples, and is intended for separation, transportation, and storage with subsequent measurement of alkaline phosphatase, alanine aminotransferase, sodium, chloride, albumin, blood urea nitrogen, calcium, creatinine, total bilirubin, total protein, high density lipoprotein, low density lipoprotein, total cholesterol, and triglycerides.
- The BD MiniDraw™ Hemoglobin & Hematocrit (H&H) Capillary Blood Collection Tube with K2EDTA (FDA approved on 11/27/23) is intended to collect 225μL (minimum volume for analysis) to 425μL (maximum volume of collection tube) of anticoagulated whole blood from individuals, ages ≥18 years. It is intended for specimen collection for measurement of hemoglobin and hematocrit with subsequent analysis on the Sysmex XN — Series™ systems. A collector connects to the finger port and directs blood to the collection tube.
The BD MiniDraw capillary collection was performed using Babson’s BetterWay blood testing service model. Once blood was collected, the collection tube was placed in the Babson Sample Preparation Device (Babson Diagnostics, Austin, TX), which automates sample processing, including centrifugation and cold storage, preparing the sample for transport to Babson Diagnostics for analysis (Figure S1). For the purposes of this preference study, no blood specimens were analyzed, and handling of BD MiniDraw tubes ended once collection tubes were stored in the sample preparation device. Thus, the full workflow from blood collection to testing, and results turnaround, was not compared between capillary collection and venipuncture in this study.
Surveys
Participant surveys were conducted at study enrollment (demographics and healthcare experience as part of a pre-collection survey) by Babson clinical staff and after the capillary blood collection (preference as part of a post-collection survey) by a third party. Responses were evaluated on a seven-point Likert scale. The pre-collection survey included questions about participant demographics including age, gender, education, employment, etc., and about medical insurance, preexisting health conditions, medical history, and venous blood collection experience. Responses to the post-collection survey items were used for main outcome analyses in this study; the survey items for the main analyses were (Table S1):
- How do you feel about this finger blood draw compared to a blood draw from the arm (venipuncture)?
- How would you rate your overall experience with the finger blood draw you received for the study?
- How would you rate your satisfaction with the professionalism of the staff who performed the finger blood draw you received during the study? [Professionalism, here, refers to the skill and competency of the person conducting the service.]
- How would you rate your satisfaction with the location of the finger blood draw you received for the study? [By location I mean the pharmacy where it was conducted].
- How would you rate your satisfaction with the amount of time you spent getting the finger blood draw you received for the study?
- In your opinion, how trustworthy was the pharmacy team member that performed the finger blood draw during the study? [Trust/trustworthiness, here, refers to your level of confidence in the person conducting the service.]
- When thinking about getting blood drawn from your finger like you did during the clinical study, how much do you trust a pharmacy compared to a hospital or doctor’s office for this purpose?
- When thinking about getting blood drawn from your finger like you did during the clinical study, how much do you trust a pharmacy compared to a service center like LabCorp or Quest for this purpose?
- Which of the following best describes your feelings about having your blood drawn the traditional way from your arm, for testing purposes?
Secondary outcomes for this study included likelihood of future use, reasons for future use, purposes of future use, and feelings about traditional blood draw. The complete set of survey questions is listed in the Supplementary Methodology.
Statistical Analysis
Cross tabulation and chi-square testing was performed using Minitab® statistical software (version 21.2) to determine any association (either direct or indirect) of staff professionalism (Question 3), retail location (Question 4), time for collection (Question 5), trustworthiness (Questions 6–8), and venipuncture experience (Question 9) with capillary preference (Question 1) or overall retail experience (Question 2). Comparisons included Q1 versus each, separate question (Q3, Q4, Q5, Q6, Q7, Q8, and Q9) and for Q2 versus each separate question (Q3-Q9). P-values (significance threshold = p<0.05) were utilized to determine whether the null hypothesis (no association between results of survey questions) was accepted or rejected. The Pearson Chi-Square test was used, and Fisher’s Exact Test was applied, to determine p-values to account for any observed low number of survey question response results.
Results
Participants and Previous Venous Blood Collection Experience
Of the 113 participants enrolled, 107 (94.7%) responded to both surveys (six did not complete the post-collection survey and were excluded from the analyses). More than half were age 45 or older, with a mean age of 49 years (range 18–71 years). Thirty-three (33; 31%) respondents were male and 74 (69%) were female. By race/ethnicity the population was largely Caucasian (71; 66%); 20 (19%) were Hispanic and 11 (10%) were African American/Black. Fifty-two (52; 48%) resided in a suburban setting and 51 (48%) lived in an urban setting; the remaining participants lived in rural areas (Table S2). Prior to undergoing study-related activities, 66 (62%) respondents (from the pre-study survey) expressed either a “somewhat” or “very positive” experience regarding their most recent venipuncture (Figure S2).
Capillary Collection Preference and Overall Retail Experience
The post-collection survey results for overall capillary collection preference showed that compared to their most recent venipuncture, 70 (65%) respondents preferred capillary collection whereas only 17 (15%) respondents preferred venous collection (Figure 2). Ninety-six (96; 90%) respondents gave positive feedback regarding the overall retail capillary collection experience compared to 5 (5%) respondents that reported negative feedback (Figure 3).
Detailed Collection Experience
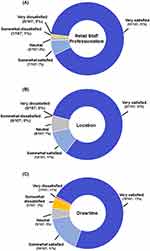
Satisfaction with the retail collection experience was also examined in terms of staff professionalism, collection location, and collection time (Figure 4). The “very satisfied” response represented the greatest proportion of responses for staff professionalism (97 responses; 91%), collection location (87 responses; 81%), and collection time (78 responses; 73%). “Somewhat satisfied” was the second most frequent response for all three questions. Relatively few responses included a dissatisfied (“somewhat” or “very dissatisfied”) response; the collection time category had the most dissatisfied responses (6 responses; 6%) of the three questions. The respondents were also asked about their perception of trustworthiness for the retail capillary blood collection team. All respondents stated that the team was either extremely, very, or somewhat trustworthy. The respondents were also asked about the retail capillary blood collection team’s trustworthiness as it compared to the respondent’s feelings on trustworthiness of personnel at sites similar to a healthcare providers office or a service center. The majority of respondents expressed equal feelings of trustworthiness, for either the physician’s office or service center sites, when compared to the retail collection site (Figure 5).
The chi-square statistic was utilized to evaluate any statistical differences between expected and actual responses for capillary preference and positive overall retail experience, based on responses regarding staff professionalism, collection location, collection time, retail staff trustworthiness, trust in retail staff over healthcare provider/service center for capillary collection, and the respondent’s view on venipuncture. As shown in Table 1, the proportions with positive responses for time (66/96; 68.8%; p-value=0.0453) and for trust in retail (compared to an HCP or service center; 28/34; 82.4%; p-value=0.0297) were greater than expected for respondents that also preferred capillary collection. The proportions for positive responses regarding professionalism (95/104; 91.4%; p-value=0.0274) and time (89/96; 92.7%; p-value=0.0142) were greater than expected for overall retail experience (Table 2).
 |
Table 1 Association Between Preferences for Professionalism, Location, Time, and Trust with Preference for Capillary Collection |
 |
Table 2 Association Between Preferences for Professionalism, Location, Time, and Trust with a Positive Overall Retail Experience |
Future Blood Collections with a Capillary Collection Device
A total of 89 participants (83%) had “sufficient trust in”/“a sufficiently positive experience” with the capillary collection process to indicate they would use it in the future. Reasons selected for future use of retail capillary blood collection included logistics (collection time, location), results accuracy, and comfort (Figure S3). The most common answers included time savings (stated by 62 [70%] respondents), availability in retail locations (stated by 62 [70%] respondents), and time convenience (stated by 60 [67%] respondents).
Discussion
In this study, individual preference was assessed for a FDA-cleared, novel capillary blood collection device (BD MiniDraw) used in a retail blood collection setting. Results from the post-collection survey revealed that capillary blood collection was preferred over venipuncture by individuals, and that the majority of individuals were satisfied or very satisfied with the retail collection experience. The majority of individuals were also satisfied or very satisfied with the staff professionalism at the retail site, the retail location in which the collection was conducted, and the time spent for the collection. Finally, individuals viewed their capillary blood collection staff as trustworthy, overall, and largely viewed their capillary collection staff as equally trustworthy to staff performing venipuncture in either a doctor’s office setting or a blood collection service center. These results suggest that capillary blood collection may effectively serve a broad range of individuals, including those for whom traditional venous blood collection is challenging.
The use of capillary collection for blood testing as part of routine screenings/wellness exams in adults has not yet been widely adopted. Capillary collection has typically been reserved for situations involving infants or individuals for whom venipuncture is problematic, such as the elderly, the obese, or anyone with fragile or inaccessible veins.9,12,18 For example, over 50% of all procedures occurring in the neonatal unit utilize capillary blood samples.12,19,20 Interestingly, “annual physical blood work” was the most common reason individuals indicated for considering capillary collection in the future (Figure S3). Unlike traditional capillary blood collection, BD MiniDraw capillary collection does not require specialized training, and the results from this work suggest a general trust in retail personnel. The results, here, provide evidence that BD MiniDraw collection would be a preferred approach by patients and represents an option to improve flexibility for those that require blood collection as part of wellness testing. Future studies should be conducted to determine how the capillary method could increase access to blood collection in healthy people and patients with pre-existing conditions.
Barriers/lack of patient satisfaction with traditional blood collection techniques and settings may result in testing non-compliance for individual orders and standing orders/repeat testing in chronic health condition management. This was apparent during the recent COVID-19 pandemic, during which, inefficiencies and deficits attributed to established healthcare workflows were exposed.21–23 Although capillary collection using BD MiniDraw does not cover all of the testing that can be performed on venous specimens, the analytes covered in the BD MiniDraw intended use represent some of the most commonly performed blood tests and could address the needs of individuals that require blood testing to aid diagnoses and monitor chronic conditions. Further, the convenience and ease of capillary blood collection may facilitate preventive care—especially when barriers exist that promote patient non-compliance (Figure S3). Improving coverage for those without recent or regular blood collection, in addition to improving individual patient health, could help reduce the overall public healthcare burden through early diagnosis and monitoring. Finally, capillary blood collection could be impactful in low-resource settings or countries that have shortages of technicians that are qualified for performing venipuncture.
Preference for capillary collection, compared to venipuncture, in a retail location, was established in this study. Chi-square analysis revealed a significant association between time and both overall capillary preference and overall satisfaction with the retail experience, suggesting that the convenience of time is a major driver for positive feelings regarding capillary collection. Staff professionalism also showed a significant association with overall retail experience, again suggesting that positive feelings regarding capillary collection staff may have a major influence on the overall experience. Although the retail pharmacy team was largely seen as trustworthy, a fewer proportion of respondents indicated the top choice (“extremely trustworthy”) as for professionalism, location, and draw time. However, our results indicate that participants who viewed the retail location as more trustworthy than a doctor’s office or service center were more likely to prefer capillary collection over venipuncture. Interestingly, those participants that viewed venipuncture favorably were less likely to view capillary collection favorably. Overall, the professionalism and trustworthiness of the collection staff should help support the convenience of capillary collection in ancillary collection locations.
Limitations
Several study limitations should be addressed. The study size was small and may not reflect preferences of the US population at large. In addition, due to the current availability of capillary blood collection, data were not sufficient to assess pre-study experience with capillary collection (only 22 participants; at-home testing was excluded from analysis). This was also not strictly a head-to-head comparison between capillary and venous methods; there was no way to compare each method across each location because venipuncture is not conducted in retail settings. Assessment of preference for capillary blood collection was limited to sample collection and did not include test turn-around time, accuracy, and ease of access to test results. Future studies should be conducted in early adopter groups and along further components of the diagnostic workflow, now that the product is cleared. This study did not address issues surrounding performance concerns with capillary collection; however, BD MiniDraw technology is designed to minimize hemolysis and interstitial fluid contamination, with publications on performance forthcoming.24 Finally, accuracy of results interpretation for this study may be diminished due to the reliance (for some outcomes reported here) on individual recall about previous experiences with health care providers and procedures.
Conclusions
Capillary collection in ancillary healthcare locations could help make access to blood collection easier and more convenient for individuals and thereby facilitate integration of blood collection into patients’ everyday lives. As demonstrated here, the high degree of satisfaction with, trust in, and preference for this novel collection method in a retail service center location indicates significant potential for future use as well as broad application in areas of wellness blood testing and medical condition monitoring.
Acknowledgments
The authors would like to thank the following from Becton, Dickinson and Company, BD Life Sciences — Diagnostic Solutions: Valentin Parvu, PhD, for statistical support, and Devin S. Gary, PhD and Dorsey Mills for their input on the content of this manuscript and editorial assistance. We thank Bovitz Inc. (Encino, California) for conducting the survey and providing data analysis. The individuals acknowledged here have no additional funding or additional compensation to disclose.
This work was presented at the 2023 SGIM Annual Meeting and the 2023 Association for Diagnostics & Laboratory Medicine (Formerly AACC) Pre-analytical Conference, and the 2023 APHA Annual Meeting.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was conducted and funded as part of a collaboration between Becton, Dickinson and Company; BD Life Sciences — Diagnostic Solutions and Babson Diagnostics.
Disclosure
MP, BA, and NA-T are employees of Becton, Dickinson and Company, the sponsor of the study. SP was an employee of Becton, Dickinson and Company during the conduct of the study and is now affiliated with Standard Process, Palmyra, WI, USA. JWJ is an employee of Babson Diagnostics. The authors report no other conflicts of interest in this work.
References
1. Nah EH, Kim S, Cho S, Cho HI. Complete blood count reference intervals and patterns of changes across pediatric, adult, and geriatric ages in Korea. Ann Lab Med. 2018;38(6):503–511. doi:10.3343/alm.2018.38.6.503
2. Givler DN, Givler A. Health Screening. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK436014.
3. Quinn JG, Tansey EA, Johnson CD, Roe SM, Montgomery LE. Blood: tests used to assess the physiological and immunological properties of blood. Adv Physiol Educ. 2016;40(2):165–175. doi:10.1152/advan.00079.2015
4. Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA. 2019;322(13):1294–1304. doi:10.1001/jama.2019.14745
5. Kimori K, Konya C, Matsumoto M. Venipuncture-induced hematomas alter skin barrier function in the elderly patients. SAGE Open Nurs. 2018;4:2377960818782050. doi:10.1177/2377960818782050
6. Aykal G, Esen H, Yeğin A, Öz C. The results of a close follow-up of trainees to gain a good blood collection practice. J Med Biochem. 2020;39(3):355–362. doi:10.2478/jomb-2019-0053
7. Stevenson M, Lloyd-Jones M, Morgan MY, et al. Non-invasive diagnostic assessment tools for the detection of liver fibrosis in patients with suspected alcohol-related liver disease: a systematic review and economic evaluation. Southampton (UK): NIHR Journals Library. (Health Technology Assessment, No. 16.4.) Appendix 8, Diagnostic venepuncture: systematic review of adverse events; 2012. Available from: https://www.ncbi.nlm.nih.gov/books/NBK97517/.
8. World Health Organization Guidelines on drawing blood: best practices in phlebotomy. Geneva: world Health Organization; 2010. 7, Capillary sampling. Available from: https://www.ncbi.nlm.nih.gov/books/NBK138654/.
9. Tang R, Yang H, Choi JR, et al. Capillary blood for point-of-care testing. Crit Rev Clin Lab Sci. 2017;54(5):294–308. doi:10.1080/10408363.2017.1343796
10. CLSI. Collection of Capillary Blood Specimens.
11. Woods K, Douketis JD, Schnurr T, Kinnon K, Powers P, Crowther MA. Patient preferences for capillary vs. venous INR determination in an anticoagulation clinic: a randomized controlled trial. Thromb ReS. 2004;114(3):161–165. doi:10.1016/j.thromres.2004.05.013
12. Krleza JL, Dorotic A, Grzunov A, Maradin M. Capillary blood sampling: national recommendations on behalf of the croatian society of medical biochemistry and laboratory medicine. Biochem Med. 2015;25(3):335–358. doi:10.11613/bm.2015.034
13. World Health Organization Guidelines on Drawing Blood: best Practices in Phlebotomy. Geneva: world Health Organization; 2010. 6, Paediatric and neonatal blood sampling. Available from: https://www.ncbi.nlm.nih.gov/books/NBK138647/.
14. Gabriel J. Understanding the challenges to vascular access in an ageing population. Br J Nurs. 2017;26(14):S15–s23. doi:10.12968/bjon.2017.26.14.S15
15. Merrill VD, Ward MD, Diaz-McNair J, Pickett EA, Duh SH, Christenson RH. Assessing phlebotomy device preference and specimen quality in an oncology outpatient clinic. J Appl Lab Med. 2022;7(2):532–540. doi:10.1093/jalm/jfab109
16. Camargo MS, Passos LCS, Mistro S, et al. Improving access to the glycated hemoglobin test in rural communities with point-of-care devices: an application study. Front Med. 2021;8:734306. doi:10.3389/fmed.2021.734306
17. Jones CHD, Howick J, Roberts NW, et al. Primary care clinicians’ attitudes towards point-of-care blood testing: a systematic review of qualitative studies. BMC Family Pract. 2013;14(1):117. doi:10.1186/1471-2296-14-117
18. Robison EH, Mondala TS, Williams AR, Head SR, Salomon DR, Kurian SM. Whole genome transcript profiling from fingerstick blood samples: a comparison and feasibility study. BMC Genomics. 2009;10(1):617. doi:10.1186/1471-2164-10-617
19. Barker DP, Rutter N. Exposure to invasive procedures in neonatal intensive care unit admissions. Arch Dis Child Fetal Neonatal Ed. 1995;72(1):F47–8. doi:10.1136/fn.72.1.f47
20. Johnston CC, Collinge JM, Henderson SJ, Anand KJ. A cross-sectional survey of pain and pharmacological analgesia in Canadian neonatal intensive care units. Clin J Pain. 1997;13(4):308–312. doi:10.1097/00002508-199712000-00008
21. Geyman J. COVID-19 has revealed america’s broken health care system: what can we learn? Int J Health Serv. 2021;51(2):188–194. doi:10.1177/0020731420985640
22. Hoffman MSF, McKeage JW, Xu J, Ruddy BP, Nielsen PMF, Taberner AJ. Minimally invasive capillary blood sampling methods. Expert Rev Med Devices. 2023;20(1):5–16. doi:10.1080/17434440.2023.2170783
23. Chudasama YV, Gillies CL, Zaccardi F, et al. Impact of COVID-19 on routine care for chronic diseases: a global survey of views from healthcare professionals. Diabetes Metab Syndr. 2020;14(5):965–967. doi:10.1016/j.dsx.2020.06.042
24. Meites S. Skin-puncture and blood-collecting technique for infants: update and problems. Clin Chem. 1988;34(9):1890–1894. doi:10.1093/clinchem/34.9.1885
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.