Back to Journals » Transplant Research and Risk Management » Volume 14
Amniotic Membrane Transplantation an Experience of a Locally Prepared Tissue
Authors Al-Yousuf N , Alsetri H, Farid E, George SM
Received 6 September 2021
Accepted for publication 12 January 2022
Published 2 February 2022 Volume 2022:14 Pages 7—19
DOI https://doi.org/10.2147/TRRM.S336917
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Qing Yi
Video abstract presented by Nada Al-Yousuf.
Views: 2301
Nada Al-Yousuf,1,2 Hasan Alsetri,3 Eman Farid,4,5 Sara M George4
1Department of Ophthalmology, King Abdullah Medical City, Manama, Kingdom of Bahrain; 2Department of Surgery, College of Medicine, Manama, Kingdom of Bahrain; 3Department of Chemistry and Biochemistry, University of California Los Angeles, Los Angeles, CA, USA; 4Department of Pathology, Salmaniya Medical Complex, Manama, Kingdom of Bahrain; 5Department of Microbiology, Immunology & Infectious diseases, College of medicine, Arabian Gulf University, Manama, Kingdom of Bahrain
Correspondence: Nada Al-Yousuf
Department of Ophthalmology, King Abdullah Medical City, 61, King Abdulaziz Avenue, Manama, Kingdom of Bahrain, Tel +973 77310071, Fax +973 77310001, Email [email protected]
Purpose: To describe the method used locally in amniotic membrane preparation and preservation for ocular surface reconstruction. To report the indications, surgical techniques, outcome and complications of amniotic membrane transplant using the locally prepared tissue. To examine the safety and efficacy of this less commonly studied method in amniotic membrane banking technique.
Patients and Methods: Dimethylsulphoxide (DMSO) was used for the preparation and preservation of the amniotic membrane. A retrospective study was done from 2005 to 2017 to examine the indications of amniotic membrane transplant. The surgical techniques used for different indications are described. Surgical outcome and complications are reported.
Results: The prepared tissue was used for the surgical management of a variety of disorders related to the ocular surface. Over the 12 years period from 2005 to 2017, a total of 135 cases were done. The most common indications for amniotic membrane transplant were pterygium surgery (41%), non-healing corneal ulcer (24%), others (13%), corneal perforation (10%), chemical burn (7%), bullous keratopathy (3%) and conjunctival-corneal scarring (2%). The most common surgical procedures used were inlay, overlay and combination (sandwich) techniques. Success rates for this ocular structure restoration procedure were the highest when treating corneal ulcers (81%), followed by pseudophakic bullous keratopathy (75%), then corneal perforations (70%). The recurrence rate for pterygium with amniotic membrane transplant was 14%. The most common complication was repeat amniotic membrane transplant. There were no complications related to the banking technique.
Conclusion: This method of preparation and preservation of amniotic membranes is safe and effective for ocular surface disorders. Amniotic membrane transplants have high success rates when treating, corneal ulcers, corneal perforations, pseudophakic bullous keratopathy and epidermolysis bullosa.
Keywords: pterygium, bullous keratopathy, corneal perforation, corneal ulcer, descemetocele, epidermolysis bullosa, ocular surface
Introduction
The amniotic membrane (AM) is the innermost layer of the fetal membrane. For several decades it has been used for grafting in general surgery. It was first used in 1910 as a skin graft.1 The use of AM in modern ophthalmology practice was introduced by Kim and Tseng.2 The use of amniotic membrane transplant (AMT) has gained wide popularity with better understanding of its biological properties. The AM has been known for promoting epithelialization, inflammation reduction, anti-scarring, anti-angiogenic properties, antimicrobial properties, and lack of immunogenicity.3–6
The AMT was used with success in chemical and thermal burns, corneal melting and perforation, recurrent corneal erosion, neurotrophic keratopathy and persistent epithelial defect. Moreover, it proved to be effective after surgical release of corneo-conjunctival adhesions, in the management of acute Stevens Johnson syndrome and in toxic epidermal necrolysis. In addition, AMT was found to be advantageous after pterygium surgery.7–16
Although the AM is considered to be safe clinically, complications have been recorded. There is a risk of transmitting infections from donors to recipients.17–19 Possible sources of increased infection risk include if the AM donors were not tested for infectious diseases, if the AM was not processed under aseptic conditions or if there were faults in AM preservation methods.
Materials and Methods
This work studied the sterilization and storage method described originally by Azuara-Blanco,20 reporting the indications, surgical techniques and outcome of AMT using this method, as an alternative to the routine technique described originally by Kim and Tseng.2
The placenta was obtained from caesarean section delivery. Consent was taken from the mother. Mothers donating the placenta were screened for human immunodeficiency virus, hepatitis B and C viruses, cytomegalovirus, and syphilis. The same screening tests were repeated 6 months after delivery to account for any “window” period of infectious diseases. The AM was not released for ophthalmic use until repeated serological tests were negative. Under a lamellar flow hood, the placenta was washed with a sterile saline solution. (Figure 1) Then, the clear translucent amnion was separated from the vascular chorion by blunt dissection, through the potential space between the two layers. (Figure 2) The amnion was then separated from the chorion manually and pulled away gradually. (Figure 3) Blood clots were removed from the AM using blunt forceps. (Figure 4) The AM was rinsed in 2 l of sterile saline. This was followed by rinsing the AM with DMSO at concentrations of 4%, 8% and 10% phosphate buffered saline successively for 5 min each. (Figure 5) The AM was then cut into 2 × 2 cm pieces (Figure 6) and was observed for absence of button holes. (Figure 7) Then, the AM was flattened onto a nitrocellulose paper, with the epithelium and basement membrane facing up. (Figure 8) The AM pieces were stored in sterile vials containing 10% DMSO medium and preserved at −80°C until use. (Figure 9) At the time of surgery, the container with the AM was thawed at room temperature, and the membrane was rinsed three times in saline.
 |
Figure 1 Under a lamellar flow hood, the placenta washed with sterile saline. |
 |
Figure 2 The amnion is separated from the chorion by blunt dissection. |
 |
Figure 3 The amnion is pulled from the chorion and separated further. |
 |
Figure 4 The blood clots are removed with non-toothed forceps. |
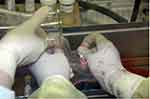 |
Figure 5 The amnion is washed with sterile saline followed by 4%, 8%, and 10% dimethylsulphoxide (DMSO) phosphate buffered saline for 5 minutes each. |
 |
Figure 6 The amniotic membrane is cut into 2×2 cm pieces. |
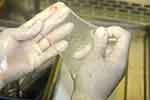 |
Figure 7 The amniotic membrane is observed for integrity and continuity. |
 |
Figure 8 The amniotic membrane is flattened on a nitrocellulose paper with the epithelium and basement membrane facing upwards. |
 |
Figure 9 The amniotic membrane is stored in a sterile vial containing 10% DMSO medium, and preserved at −80°C. |
All patients who underwent AMT from January 2005 to January 2017 were included in the study. Demographics such as age and gender were collected. In addition, the diagnosis for which the AMT was performed was recorded. For categories such as corneal perforation and non-healing corneal ulcers, the underlying diagnosis was documented. Non-healing corneal ulcer was considered if there is no epithelialization after 2 weeks of medical treatment.21 Descemetocele was considered whenever there was a non-healing corneal ulcer associated with herniation of the descemet membrane.14 AMT was performed for corneal perforations whenever the perforation width was 3 mm or less, and the anterior segment structures were not prolapsed. Cases with perforations larger than 3 mm, and iris or lens tissue prolapse required a corneal patch graft.15 A successful AMT was defined by the restoration of the ocular structure integrity. In pterygium surgery, AMT was only used for primary pterygia. Follow up was variable, depending on the disease category and the patients’ availabilities. For pterygium patients and bullous keratopathy patients, follow-up results were available at 12 months. For patients with corneal ulcers, follow-up was variable ranging from 4 to 12 weeks. Patients with chemical burns were expatriates who were only available for 2 to 4 weeks, then lost follow-up. The patient with dystrophic recessive epidermolysis bullosa had 4 years follow-up results.
Approval was obtained from the Secondary Health Care Research Committee (reference number 107290821), which is the official ethical body responsible for research at Salmaniya Medical Complex. The study complied with the declaration of Helsinki. Consent from the mother donating the placenta was taken. Patients’ consent to study the indications of AMT was not required since it was a retrospective study. Patients’ confidentiality was maintained.
Data were entered and analyzed using Statistical Package for Social Sciences (SPSS) for Windows, version 21 (IBM Corp, Armonk, NY, USA). Descriptive statistics using numbers and percentages were performed to display nominal and categorical data.
Results
AMT Indications
A total of 135 cases of AMT were done from 2005 to 2017. Females formed 55% of the total cases. All patients were adults. The age range was from 22 to 85 years. The average age was 48 years. The commonest indication was pterygium surgery (41%) followed by non-healing corneal ulcers (24%), others (13%), corneal perforation (10%), chemical burns (7%), bullous keratopathy (3%) and conjunctival-corneal scarring (2%) (Figure 10). The “others” group included one leaking bleb, one exposed glaucoma drainage tube, one Sjogren's syndrome case, two band keratopathies, two shield ulcers, one recurrent corneal erosions and 10 cases with missing diagnosis. The conjunctival-corneal scarring group included one case of ocular cicatricial pemphigoid (OCP), one case of symblephara and one case of dystrophic recessive epidermolysis bullosa. Studying the AMT indications across gender revealed that chemical burns were found among males exclusively. Corneal ulcers and corneal perforations were more common in females, while pterygium surgery was more common in males (Figure 11).
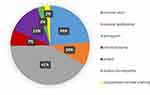 |
Figure 10 Indications of amniotic membrane transplant using locally prepared tissue. |
 |
Figure 11 Gender distribution of amniotic membrane transplant indications. |
Surgical Techniques and Outcome
Pterygium was the most common indication for AMT, with a total of 56 (41%) cases. Under local anesthesia, the pterygium was excised and dissected from the conjunctival part towards the corneal part. A diamond burr was used to level the corneal end. Hemostasis was performed. The bare sclera was measured using a caliper. The AM was cut and fashioned to cover the bare sclera. It was oriented with the epithelium side up. The AM was sutured to the episclera using 8/0 vicryl suture. (Figure 12) Out of the 56 cases, 8 had recurrence of pterygium at 12 months follow-up. The recurrence rate of primary pterygium with AMT at 1 year follow-up was 14%.
 |
Figure 12 Patch graft of the amniotic membrane on the denuded sclera after pterygium excision. |
Non-healing corneal ulcer was the second most common indication for AMT with a total of 32 (24%) cases. This category included 15 cases of Descemetocele. The underlying diseases of non-healing corneal ulcers included peripheral corneal ulcerations with and without rheumatic disorders, eyelid malpositions, thyroid ophthalmopathy, post-keratitis ulceration and neurotrophic keratopathy. Corneal ulcers with descemetocele were considered an ocular emergency because of the risk of perforation. In these situations, AMT was performed as soon as possible. The surgical technique used was the “sandwich” technique, which is a combination of graft (inlay) and patch (overlay) techniques. Under local or general anesthesia, the denuded stroma and any epithelium around the edge of the epithelial defect were debrided. The amniotic membrane was fashioned and fitted to cover the ulcer with the epithelial side facing up (graft) and sutured to the edge of the defect using 10/0 nylon (Figure 13). Another layer, acting as a biological dressing, was used to cover the graft with the epithelial layer facing up. It was spread from limbus to limbus and sutured with 8/0 vicryl to the episclera. Out of the 32 cases performed 26 healed, 4 needed repeat AMT, 2 patients progressed and needed penetrating keratoplasty (PKP). The success rate was 81%.
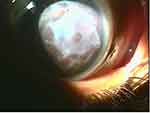 |
Figure 13 Inlay (patch) graft for descemetocele. |
There were 13 (10%) cases of corneal perforations that underwent AMT. The underlying disease of this category included rheumatoid arthritis, systemic lupus erythematosus, neurotrophic ulcers, Mooren ulcer and Sjogren’s syndrome. Corneal perforations were considered an ocular emergency. Under local or general anesthesia, the bed of the perforation was debrided, the amniotic membrane was folded several times depending on the depth of the perforation. The top layer was oriented with the epithelial side up. A patch of AM was then used to cover the perforation area (inlay) and sutured with 10/0 nylon. This is followed by a bigger sheet of AM to cover the cornea and sutured using 8/0 vicryl to the episclera (overlay). Both inlay and overlay techniques were done with the epithelial side of the AM facing up. A bandage contact lens was placed. Out of the 13 cases performed, 9 healed, 2 needed repeat AMT and 2 needed PKP. The success rate was 70%.
There were 9 (10%) cases of acute chemical burns. All patients had occupational injuries caused by alkaline chemical burns. AMT was performed 2 weeks after the initial medical treatment. Under general anesthesia, debridement was done to remove necrotic tissue and to ensure the absence of any retained chemical particles. The AM was used epithelial side up to cover the damaged tissue. It was applied to cover the cornea, limbus, the damaged conjunctival surface, the fornices and the tarsal conjunctiva using 8/0 vicryl. Double armed sutures were used to secure the AM on the palpebral conjunctiva and were supported with bolster on the eyelid skin. Symblepharon ring was inserted. Bandage contact lens was applied. Two patients had severe chemical injury where tenonplasty was combined with AMT. Unfortunately, no long term follow-up data were available.
A total of 4 (3%) cases with pseudophakic bullous keratopathy (PBK) were operated on. Under local anesthesia, the loose bullous epithelium was scraped creating an epithelial defect leaving a rim of 2 mm near the limbus. A single layer of AM was applied with the epithelium surface up and sutured with 10/0 nylon to the limbus. One patient had dislocation of the AM that required suturing. At 12 months follow-up, one patient had recurrence of bullae and required bandage contact lens. The success rate was 75%.
The conjunctival corneal scarring group included one patient with ocular cicatricial pemphigoid, one patient with symblephara and one patient with dystrophic recessive epidermolysis bullosa. Under general anesthesia, removal of fibrovascular tissue was done along with hemostasis. The AM was used with epithelial side up and applied to cover the cornea, limbus, the conjunctiva, the fornices and the tarsal conjunctiva using 8/0 vicryl. Double armed sutures were used to secure the AM on the palpebral conjunctiva and were supported with bolster on the eyelid skin. Symblepharon ring was inserted. Bandage contact lens was applied. The patient with OCP had hematoma in the early post-operative period that got absorbed spontaneously. Vision improvement and ocular surface stability were achieved at 4 weeks follow-up. However, at 12 months follow-up, the conjunctival corneal scarring recurred. The patient with symblephara showed improvement and stability in the ocular surface at 1 year follow-up. The AMT was particularly successful in the patient with dystrophic recessive epidermolysis bullosa. The patient was a 23-year-old Bahraini female. She had corneal scarring and vascularization with limitation of ocular motility due to symblephara. Release of symblephara and superficial keratectomy were done. AMT was done with epithelial side up to cover the cornea, conjunctiva and the palpebral conjunctiva. Full ocular motility was achieved immediately in the post-operative period. At 3 months follow-up, she had complete epithelialization of the cornea and improvement in visual acuity from Hand Motion to 0.4. At 4 years follow-up the visual acuity and the ocular surface were stable (Figure 14).
 |
Figure 14 (A) The patient with epidermolysis bullosa 3 weeks post AMT. (B) The same eye 4 years later with improved vision and stable ocular surface. |
Complications
The most common complication was repeat AMT. It was done in six (4%) cases. There was one hematoma formation, and one patient had dislocation of the graft. There were no cases of infection or hypopyon.
Discussion
The AM preparation technique described by Kim and Tseng2 is extensively used and reported in the medical literature.4,15,21–24 It involved washing the AM with antibiotic–antimycotic cocktail to protect against gram positive and gram-negative bacteria and fungi. The cocktail consisted of 100 lg/mL neomycin, 50 lg/mL streptomycin, 50 lg/mL penicillin, and 2.5 lg/mL amphotericin B. The AM pieces were stored in 50% glycerol in Dulbecco’s Modified Eagle Medium (DMEM, Gibco) or TC-199.
The technique we report herewith uses Dimethyl sulfoxide (DMSO) for sterilisation, replacing the antibiotic mixture in the Tseng protocol. DMSO in this method is also used for preservation.20 Jirsova and Jones in their review mentioned that a limiting factor for using DMSO for AM storage is its toxicity.25 The study we report, being a long follow-up series, confirms the safety and efficacy of this method of AM preparation and preservation. It provides clinicians and researchers another reliable option for AM banking technique. It also adds to existing clinical studies confirming the success using this method in ocular surface surgery.20,26
Arora et al used fresh AM for their procedures. They argue that the fresh AM is easier to handle.27 We favor the cryopreserved method, which allows the repeat of serology tests 6 months later, accounting for any window period of infectious diseases. In addition, the use of cryopreserved AM increased the life span up to 3 years, and avoided wasting any unused tissue.
Pterygium was the commonest indication for AMT in our study. This was related to the high incidence of this disease in hot climate countries.28,29 This number of pterygium surgeries is an underestimate of the actual number because pterygium cases in our institution were also managed with conjunctival autografts. Different surgical modalities for pterygium excision were reported in the literature. The base sclera technique was abandoned due to the high recurrence rate.30 Pterygium excision with mitomycin has been complicated by scleral necrosis.31 The procedure of choice should have low complications and low recurrence rate. The recurrence rate of pterygium surgery with AMT in the current study at 1 year follow-up was 14%. This is comparable to what was reported in the literature.30 Although conjunctival grafts are known to have lower recurrence rates than AMT in pterygium surgeries, AMT still has an important role, especially in procedures with extensive conjunctival involvement, or when sparing the conjunctiva is required for future glaucoma surgeries. Studying the different indications across gender, we found that pterygium was more common in males. This could be related to gender differences in job-related outdoor activities, with resultant UV light exposure. Our finding is consistent with other findings in the region.28
In corneal ulcers, we used the inlay-overlay combined method in all cases. Lacronaza et al, in their clinical series of AMT for corneal ulcers have adopted the same technique.21 They varied the number of layers depending on the severity of the ulcer. Their success rate was 74.4%. Schuerch et al in their study of AMT in corneal ulcers, had variable success rates depending on the underlying disease. It ranged from 52.5% for ulcers related to rheumatic diseases to 93% for neurotrophic ulcers.32 In our series, the success rate for non-healing corneal ulcers was 81%. Nevertheless, it is difficult to compare results because of the variability of the underlying disease, and differences in adjuvant treatments. Our relatively high success rate in this category is probably because we used the “sandwich” technique for all cases. Lui et al conducted a meta-analysis looking at the outcome of different AMT techniques for non-healing corneal ulcers. They found that the “sandwich” technique had the highest rate of healing.33
Our inclusion criteria and surgical technique for AMT to treat small corneal perforations were similar to Krysic and co-workers. They performed AMT as a first-line treatment for perforations without prolapse of anterior segment structures. Their success rate was 86%.15 This is similar to our finding, as we achieved 82% of anatomical preservation. We found the availability of amniotic membranes in local banks particularly convenient. This is because donor corneas were not readily available locally, which made AMT an effective alternative. This also allowed time to obtain a donor cornea after the ocular inflammation settled, which provided a better environment for the corneal graft success.
Examining the different indications across gender, it was found that corneal ulcers and corneal perforations were more common in females. This is probably because of the close association of these conditions with autoimmune disorders.
To treat chemical burns we used the overlay method to cover the cornea, limbus and the palpebral conjunctiva. Arora et al, in their review on AMT to treat chemical burns, their methods included transplanting the AM as a circular patch covering the cornea and limbus.27 We advocate an overlay technique that can be extended to include the palpebral conjunctiva. This has the advantage of reducing the chances of symblephara formation. Unfortunately, long term follow-up results were not available. All our chemical injury patients were expatriates who were lost to follow up. Arora et al, in their review, found that all patients had pain relief.27 This is consistent with our finding. In two cases with severe chemical burns we used tenonplasty, the AM in this surgical modality was used as a patch to cover the cornea, and as a graft to cover the bare sclera after removing the tenon layer.7 Different surgical applications of AMT for chemical burns have been studied. This included self retained cryopreserved AM, modified ocular surface rings and AM extracts.34
Examining the different indications across gender. We found that all the chemical burn patients were male. This is because this injury was occupation related and was found exclusively among male laborers.
Bullous keratopathy in our series was reserved for symptomatic patients with no visual potential. A recent study conducted by one of the authors found that PBK was on the rise in recent years.35 Siu et al used in-lay technique for AMT in PBK. They had follow-up that exceeded 12 months and had success rate of 94% in terms of pain relief.36 We used the overlay technique for PBK. Our results showed 75% success rate at 12 months follow-up. Nonetheless, comparing results is challenging, not only because of non-standardized treatment strategy, but also due to the small number of patients.
AMT was particularly successful in recessive dystrophic epidermolysis bullosa. Our findings are consistent with similar cases reported in the literature.37,38
The use of AMT to treat ocular surfaces has been developed and modified in recent years. In addition to self retained sutureless AM,39 there is growing evidence for the promise of AM extracts in the form of drops and gels with the potential to improve the efficiency of AM in ocular surface disorders.40 Further studies are recommended to shed light on these novel modalities.
Conclusion
The method described herewith for the preparation and preservation of amniotic membranes is safe and effective for a variety of ocular surface disorders. AMT showed significant success rate in corneal ulcers, corneal perforations, PBK and epidermolysis bullosa. It is recommended for hospitals with cornea service to have amniotic membrane tissue ready to use, particularly in emergency situations.
Disclosure
The authors report no conflicts of interest and no financial interest in this work.
References
1. Davis JW. Skin transplantation with a review of 550 cases at the Johns Hopkins Hospital. Johns Hopkins Med J. 1910;15:307.
2. Kim JC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995;14(5):473–484. doi:10.1097/00003226-199509000-00006
3. Chen Z, Lao HY, Liang L. Update on the application of amniotic membrane in immune-related ocular surface diseases. Taiwan J Ophthalmol. 2021;11(2):132–140. doi:10.4103/tjo.tjo_16_21
4. Walkden A. Amniotic membrane transplantation in ophthalmology: an updated perspective. Clin Ophthalmol. 2020;14:2057–2072. doi:10.2147/OPTH.S208008
5. Železnik RT, Tina S, Marjanca S, Erdani K. Antimicrobial activity of human fetal membranes: from biological function to clinical use. Front Bioeng Biotechnol. 2021;9:1–16.
6. Fénelon M, Catros S, Meyer C, et al. Applications of human amniotic membrane for tissue engineering. Membranes (Basel). 2021;11(6):387. doi:10.3390/membranes11060387
7. Peng WY, He LW, Zeng P, Chen DC, Zhou SY. Tenonplasty combined with amniotic membrane transplantation for patients with severe ocular burns induced anterior segment necrosis. J Burn Care Res. 2020;41(3):668–673. doi:10.1093/jbcr/iraa016
8. Miller DD, Hasan SA, Simmons NL, Stewart MW. Recurrent corneal erosion: a comprehensive review. Clin Ophthalmol. 2019;13:325–335. doi:10.2147/OPTH.S157430
9. Dua HS, Said DG, Messmer EM, et al. Neurotrophic keratopathy. Prog Retin Eye Res. 2018;66:107–113. doi:10.1016/j.preteyeres.2018.04.003
10. Mead OG, Tighe S, Tseng SCG. Amniotic membrane transplantation for managing dry eye and neurotrophic keratitis. Taiwan J Ophthalmol. 2020;10(1):13–21. doi:10.4103/tjo.tjo_5_20
11. Parmar D, Bhole P, Patel P, et al. Amniotic membrane transplant in acute ocular surface burns in Western India. Indian J Ophthalmol. 2021;69:58–64. doi:10.4103/ijo.IJO_2252_19
12. Röck T, Bartz-Schmidt KU, Landenberger J, Bramkamp M, Röck D. Amniotic membrane transplantation in reconstructive and regenerative ophthalmology. Ann Transplant. 2018;23:160–165. doi:10.12659/AOT.906856
13. Yang Y, Fung SM, Chew H, Mireskandari K, Ali A. Amniotic membrane transplantation for Stevens-Johnson syndrome/toxic epidermal necrolysis: the Toronto experience. Br J Ophthalmol. 2021;105(9):1238–1243.
14. Agarwal R, Nagpal R, Todi V, Sharma N. Descemetocele. Surv Ophthalmol. 2021;66(1):2–19. doi:10.1016/j.survophthal.2020.10.004
15. Krysik K, Dobrowolski D, Wylegala E, Lyssek-Boron A. Amniotic membrane as a main component in treatments supporting healing and patch grafts in corneal melting and perforations. J Ophthalmol. 2020;14:4238919.
16. Shahraki T, Arabi A, Feizi S. Pterygium: an update on pathophysiology, clinical features, and management. Ther Adv Ophthalmol. 2021;13:1–21.
17. Marangon FB, Alfonso EC, Miller D, Remonda NM, Muallem MS, Tseng SC. Incidence of microbial infection after amniotic membrane transplantation. Cornea. 2004;23:264–269. doi:10.1097/00003226-200404000-00008
18. Boboridis KG, Mikropoulos DG, Georgiadis NS. Hypopyon after primary cryopreserved amniotic membrane transplantation for sterile corneal ulceration: a case report and review of the literature. Case Rep Ophthalmol Med. 2021;2021:9982354.
19. Alreshidi S, Al-Swailem S. Late-onset granular intra-amniotic infection following amniotic membrane transplantation. Am J Ophthalmol Case Rep. 2021;24:1–4. doi:10.1016/j.ajoc.2021.101221
20. Azuara-Blanco A, Pillai CT, Dua HS. Amniotic membrane transplantation for ocular surface reconstruction. Br J Ophthalmol. 1999;83:399–402. doi:10.1136/bjo.83.4.399
21. Lacorzana J, Campos A, Brocal-Sánchez M, et al. Visual acuity and number of amniotic membrane layers as indicators of efficacy in amniotic membrane transplantation for corneal ulcers: a multicenter study. J Clin Med. 2021;10(15):3234. doi:10.3390/jcm10153234
22. Le Q, Deng SX. The application of human amniotic membrane in the surgical management of limbal stem cell deficiency. Ocul Surf. 2019;17(2):221–229. doi:10.1016/j.jtos.2019.01.003
23. Hamza M, Rizwan Ullah M, Hashmi A, Sahaf I. Amniotic membrane transplantation in ocular surface disorders. Pak J Ophthalmol. 2011;27:138–141.
24. Sangwan V, Burman S, Tejwani S, Mahesh S, Murthy R. Amniotic membrane transplantation: a review of current indications in the management of ophthalmic disorders. Indian J Ophthalmol. 2007;55:251–260. doi:10.4103/0301-4738.33036
25. Jirsova K, Jones GLA. Amniotic membrane in ophthalmology: properties, preparation, storage and indications for grafting-a review. Cell Tissue Bank. 2017;18(2):193–204. doi:10.1007/s10561-017-9618-5
26. Kiuchi Y, Yanagi M, Nakamura T. Efficacy of amniotic membrane-assisted bleb revision for elevated intraocular pressure after filtering surgery. Clin Ophthalmol. 2010;4:839–843. doi:10.2147/OPTH.S12311
27. Arora R, Mehta D, Jain V. Amniotic membrane transplantation in acute chemical burns. Eye. 2005;19:273–278. doi:10.1038/sj.eye.6701490
28. Alsharhani W, Alshahrani S, Showail M, et al. Characteristics and recurrence of pterygium in Saudi Arabia: a single center study with a long follow-up. BMC Ophthalmol. 2021;21:207. doi:10.1186/s12886-021-01960-0
29. Echevarría-Lucas L, Senciales-González JM, Medialdea-Hurtado ME, Rodrigo-Comino J. Impact of climate change on eye diseases and associated economical costs. Int J Environ Res Public Health. 2021;18(13):7197. doi:10.3390/ijerph18137197
30. Röck T, Bramkamp M, Bartz-Schmidt KU, Röck D, Retrospective A. Study to compare the recurrence rate after treatment of pterygium by conjunctival autograft, primary closure, and amniotic membrane transplantation. Med Sci Monit. 2019;25:7976–7981. doi:10.12659/MSM.915629
31. Friesacher A, Alder M, Ruesch R, Valmaggia C, Todorova M. Necrotizing scleritis after pterygium excision with mitomycin C. Acta Ophthalmol. 2021;99:343.
32. Schuerch K, Baeriswyl A, Frueh BE, Tappeiner C. Efficacy of amniotic membrane transplantation for the treatment of corneal ulcers. Cornea. 2019;39:479. doi:10.1097/ICO.0000000000002179
33. Liu J, Li L, Li X. Effectiveness of cryopreserved amniotic membrane transplantation in corneal ulceration: a meta-analysis. Cornea. 2019;38(4):454–462. doi:10.1097/ICO.0000000000001866
34. Soleimani M, Naderan M. Management strategies of ocular chemical burns: current perspectives. Clin Ophthalmol. 2020;14:2687–2699. doi:10.2147/OPTH.S235873
35. Al-Yousuf N, Al Alawi E, Mahmood A, et al. Changing indications for penetrating keratoplasty in Bahrain in a tertiary referral centre. Clin Ophthalmol. 2021;15:1503–1510. doi:10.2147/OPTH.S304812
36. Siu GD, Young AL, Cheng LL. Long-term symptomatic relief of bullous keratopathy with amniotic membrane transplant. Int Ophthalmol. 2015;35(6):777–783. doi:10.1007/s10792-015-0038-x
37. Altan-Yaycioglu R, Akova YA, Oto S. Amniotic membrane transplantation for treatment of symblepharon in a patient with recessive dystrophic epidermolysis bullosa. Cornea. 2006;25(8):971–973. doi:10.1097/01.ico.0000225708.70135.d0
38. Koulisis N, Moysidis SN, Siegel LM, Song JC. Long-term follow-up of amniotic membrane graft for the treatment of symblepharon in a patient with recessive dystrophic epidermolysis Bullosa. Cornea. 2016;35(9):1242–1244. doi:10.1097/ICO.0000000000000861
39. Brocks D, Mead OG, Tighe S, Tseng SCG. Self-retained cryopreserved amniotic membrane for the management of corneal ulcers. Clin Ophthalmol. 2020;14:1437–1443. doi:10.2147/OPTH.S253750
40. Dadkhah F, Firouzeh A, Shabani I, Shabani A. A review on modifications of amniotic membrane for biomedical applications. Front Bioeng Biotechnol. 2021;8:606982. doi:10.3389/fbioe.2020.606982
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
