Back to Journals » International Journal of Women's Health » Volume 16
Alveolar Soft Part Sarcoma in the Female Genital Tract: Case Series with Literature Review and SEER Database Analysis
Authors Long X, Jiang Q, Li R, Wang D, Zou D
Received 13 September 2023
Accepted for publication 13 December 2023
Published 6 January 2024 Volume 2024:16 Pages 17—30
DOI https://doi.org/10.2147/IJWH.S435135
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Xingtao Long,1 Qingming Jiang,2 Rengui Li,1 Dong Wang,1 Dongling Zou1
1Department of Gynaecological Oncology, Chongqing University Cancer Hospital, Chongqing, People’s Republic of China; 2Department of Pathology, Chongqing University Cancer Hospital, Chongqing, People’s Republic of China
Correspondence: Dongling Zou, Department of Gynaecological Oncology, Chongqing University Cancer Hospital, 181 Hanyu Road, Shapingba District, Chongqing, 400000, People’s Republic of China, Tel +8613657690699, Fax +8602365075612, Email [email protected]
Introduction: Alveolar soft part sarcoma (ASPS) is a rare and distinct subtype of soft tissue sarcoma. This study aims to describe the unique presentation of ASPS in the female genital tract.
Methods: Prognostic factors for cancer-specific overall survival (CSS) were evaluated using multivariate analyses.
Results: In our case series, we identified a novel TFE3-PRCC gene fusion in a 24-year-old unmarried patient with cervical ASPS who underwent fertility-sparing surgery and remained recurrence-free for 41 months. The other two patients underwent radical hysterectomy and bilateral salpingo-oophorectomy. At the time of writing, the two patients had been disease-free for 49 and 71 months, fluorescence in situ hybridization showed break-apart signals for the ASPL-TFE3 gene. Among the 55 cases with available information from the PubMed/Medline database, most presented with localized disease, and at the last follow-up, all patients were alive and 45 patients showed no evidence of disease. The 5-year CSS rate in the female genital tract cohort from SEER database was 86.2%. Multivariate analysis revealed that older age was associated with a 1.042-fold increased risk of cancer-specific mortality (HR=1.042, 95% CI 1.022– 1.063, P < 0.001), involvement of soft tissue including the heart was associated with a 4.786-fold higher risk (HR=4.7868, 95% CI 1.681– 13.623, P= 0.003), and regional infiltration and distant metastasis were associated with approximately 8.6-fold and 18-fold higher risk of cancer-specific mortality compared to local disease, respectively (HR=8.652, 95% CI 2.529– 29.63, P = 0.001; HR=18.366, 95% CI 6.153– 54.817, P< 0.001). Patients who underwent radical excision did not show reduced cancer-specific mortality compared to those who underwent local excision (HR=0.492, 95% CI 0.224– 1.081, P = 0.078).
Discussion: Previously unrecognized genetic diversity exists in ASPS. Patients with ASPS in the female genital tract have the lowest likelihood of presenting with a distant disease and are associated with a more favorable survival outcome.
Keywords: ASPSCR1, TFE3, alveolar soft part sarcoma, fusion
Introduction
Alveolar soft-part sarcoma (ASPS) is a rare and distinct subtype of soft tissue sarcoma (STS) that constitutes less than 1% of all STS cases.1 It was first identified in 1952 as a painless mass occurring in the extremities, head, and neck.2 ASPS exhibits a strong female predominance. Primary ASPS in the female genital tract is a rare histological subtype of STS, with only a few reported cases. This subtype is classified separately by the World Health Organization (WHO) in the 5th edition of the classification of female genital tumors and presents significant challenges in terms of diagnosis and treatment.3 Despite this, there is limited knowledge about the patient characteristics and prognosis associated with this condition. ASPS shares clinicopathological and histomorphological features with other Xp11 translocation-related tumors.4 While ASPS patients are prone to distant metastasis, the site of the primary tumor influences the rate of metastasis and prognosis.5 Prognosis for ASPS in the female genital tract has been reported to be better compared to other locations in the body, but the follow-up duration for survival assessment is short.6 ASPS predominantly affects young women, and it remains uncertain whether fertility-sparing local excision is a recommended treatment approach. In order to gain a better understanding of ASPS in the female genital tract, the present study examines the histopathological and molecular pathology profile of ASPS, along with survival outcomes, through the analysis of case series, literature review, and the SEER database. The aim is to provide assistance for clinical treatment decisions.
Materials and Methods
Data Source and Patients
Clinicopathological characteristics and relevant data from January 2010 to December 2022 were obtained from the electronic medical record system at our institution. These three patients have signed consent forms for information disclosure and a statement confirming consent to publish has been obtained. All undergone tissue pathology testing and fusion gene testing. Case 3 underwent whole exon sequencing. A search was conducted in the PubMed/Medline databases for case reports and series on ASPS of the female genital tract from January 1975 to May 2022. Two independent reviewers screened the identified studies based on titles and abstracts, with any discrepancies resolved through consensus with a third investigator. The search criteria included “Sarcoma, Alveolar Soft Part” [Mesh] OR “Alveolar Soft-Part Sarcoma” OR “Alveolar Soft Part Sarcoma” Alveolar Soft Part Sarcoma, with filters for females and English language. Patients with histopathologically confirmed ASPS in the female genital tract were included, while those with genital tract metastases were excluded. Among the 43 authors considered for inclusion, 4 were excluded (one without histopathology, one without an abstract, one with vaginal metastasis, and one repeat case), resulting in 39 studies meeting the final inclusion criteria. A total of 55 cases were utilized for analysis (Figure 1).7–45 To identify eligible female patients with histologically confirmed primary ASPS (ICD-0 coding 9581/3) between 1975 and 2019, the SEER stat 8.4.1 software with Plus database was used. A multivariate analysis was performed to identify potential risk variables in alveolar soft part sarcoma, male sex as negative prognostic factors for overall survival, so the SEER database only focuses on the female.45 Patients with ASPS that was not the first malignant primary indicator were excluded. Cancer-specific survival (CSS) was defined as the time interval between diagnosis and ASPS-related death. The permission to use information from PubMed/Medline and SEER stat 8.4.1 software with Plus database are not required, as it is publicly available and unrestricted re-use is permitted via an open licence. This study received approval from the ethics committee of Chongqing cancer hospital (CZLS2023318-A).
 |
Figure 1 A flowchart outlining the criteria for patient eligibility. |
Histologic and Immunohistochemical Stains
Tumor samples were fixed in 4% formalin, conventionally dehydrated, embedded in paraffin, and sliced to a thickness of 3 μm. These procedures were carried out using an automated immunohistochemical stainer. A positive reaction was observed under a microscope, and appropriate positive and negative controls were utilized. The following antibodies were available: TFE3 (RMA-0663, 1:300; Maixin Biotech. Co., Ltd, China), HMB45 (MAB-0098, 1:500; Maixin Biotech. Co., Ltd, China), cathepsin K (3F9, 1:300; Abcam, Cambridge, MA, USA), PAX8 (4H7B3, 1:100; Proteintech Group, Rosemont, IL, USA), Melan-A (MAB-0275, 1:100; Maixin Biotech. Co., Ltd, China), CD10 (MAB-0668, 1:100; Maixin Biotech. Co., Ltd, China), desmin (MAB-0766, 1:100; Maixin Biotech. Co., Ltd, China), SMA (MAB-0890, 1:100; Maixin Biotech. Co., Ltd, China), S100 (Kit-0007, 1:2000; Maixin Biotech. Co., Ltd, China). Paraffin-embedded slices with a thickness of 3 μm were stained using the periodic acid-Schiff method.
Fluorescence in situ Hybridization (FISH)
Fluorescence in situ hybridization (FISH) was conducted using the TFE3 fracture-separation probe, where the fluorescent mark of the TFE3 probe and the nucleic acid of the sample were hybridized in situ. The fluorescent signals were distinguished and counted under a microscope following the manufacturer’s instructions. The TFE3 (Xp11.2) gene break probe (F.01106-01, anbiping Co., Ltd, China) was used. Result interpretation: Normal cells displayed two yellow signals (red and green fusion signals). A positive result was determined when the separation distance between the red and green signal widths matched that of two large fused signals. Each sample contained 200 cells, and a sample was considered positive if more than 10% of tumor cells exhibited separation signals.
RNA Sequencing
RNA sequencing was conducted by gene plus Inc (Beijing, China). The Nebnext rRNA Removal Kit (NEB # z1955e) was used to remove targeted ribosomal RNA. All RNA fragments with a percentage > 200 nucleotides (DV200) ≤ 50% underwent fragmentation and library preparation. After rRNA depletion and fragmentation, cDNA synthesis and NGS library preparation were performed using the NEBNext® Ultra™ II Directional RNA Library Prep Kit (NEB, E7760L). The library was quantified using Qubit3.0 (Life Invitrogen, USA), and quality assessment was conducted with LabChip GX Touch (PerkinElmer, USA). Terminal adaptor sequences and low-quality data were removed using fastp (version: 0.19.5), and rRNA reads were eliminated by aligning clean reads to the rRNA database (downloaded from NCBI) using bowtie2 (version: 2.2.8). Clean reads without known rRNA were aligned to the reference human genome (hg19) using STAR (version 020201). Fusions were detected using a customized version of Arriba 1.1.0. and annotated using the in-house software annoFilterArriba (version: 1.0.0) with the NCBI release 104 database. All final candidate fusions were manually verified using an integrative genomics viewer browser. RNA-SeQC assessment was utilized to compute a series of quality control metrics. A threshold of ≥ 80 million mapped reads and ≥ 10 million junction reads per sample was established.
Statistical Analysis
Statistical analysis was performed using SPSS 25 and GraphPad Prism 6.01 software. Categorical variables were compared using the χ2 or Fisher’s exact test. Measurement data were expressed as means and standard deviation (M±SD). Skewed distribution data were expressed as median and interquartile range (M ± QR). Univariate and multivariate Cox regression analyses were conducted to identify independent predictors associated with survival, reporting hazard ratios (HR) and 95% CI. Variables included in the multivariate model were those with P ≤ 0.1 in the univariate analysis. A two-tailed P value of < 0.05 was considered statistically significant.
Results
ASPS in the Female Genital Tract at Our Institution
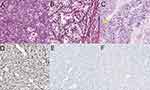
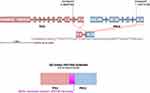
Case 1: A 31-year-old married woman presented with vaginal bleeding. Hysteroscopic examination revealed a 2.5 cm-sized mass protruding from the uterine corpus. The patient underwent total hysterectomy. Fluorescence in situ hybridization demonstrated break-apart signals for the ASPL-TFE3 gene. At the time of writing, the patient had been disease-free for 71 months. Case 2: A 61-year-old woman presented with vaginal bleeding. Gynecologic examination revealed a 4.5 cm-sized mass protruding from the cervix. Fluorescence in situ hybridization showed break-apart signals for the ASPL-TFE3 gene. The patient underwent radical hysterectomy and bilateral salpingo-oophorectomy. At the time of writing, the patient had been disease-free for 49 months. Immunohistochemical staining was positive for TFE3 but negative for HMB45, Melan-A, and Pax-8 in both Case 1 and Case 2. Case 3: A 24-year-old unmarried woman, who had never been pregnant, presented with increased menstrual bleeding for six months. Gynecologic examination revealed a polypoid mass measuring 3×2.5×0.5 cm protruding from the cervical canal. The mass was removed via dilatation and curettage. Gross examination showed a friable, tan-pink surface. Pathological evaluation revealed large, round, or polygonal tumor cells growing in an organoid pattern (Figure 2A) or a nest-like arrangement (Figure 2B), separated by delicate fibrovascular septa. The tumor cells exhibited abundant cytoplasmic and eosinophilic particles. Mitotic activity was infrequent. Periodic acid-Schiff-positive purplish-red crystals were observed in the cytoplasm (Figure 2C). Immunohistochemical staining was positive for TFE3 (Figure 2D) and CD56, but negative for HMB45 (Figure 2E), Melan-A (Figure 2F), Desmin, S-100, Syn, CgA, CD10, and Pax-8. Fluorescence in situ hybridization showed break-apart signals for the TFE3 gene (Figure 3). Whole exon sequencing identified a novel gene fusion between PRCC (NM_005973.4) exon 7 and TFE3 (NM_006521.4) exon 2 (Figure 4). Following curettage, magnetic resonance imaging revealed a residual nodule measuring 1.1×0.8 cm on the uterine isthmus. The patient underwent hysteroscopic resection of the residual nodule, which showed the same pathological features as before with negative margins. Positron emission tomography-computed tomography revealed no tumor after hysteroscopic resection. The patient did not receive additional postoperative treatment. At the time of writing, the patient had been disease-free for 41 months, and the last follow-up date was May 20, 2023. Table 1 summarizes the characteristics of the three patients with ASPS arising in the female genital tract at our institution.
 |
Table 1 Summary of Clinicopathological Features of ASPS in the Female Genital Tract at Our Institution |
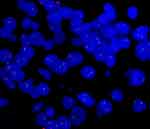 |
Figure 3 Fluorescence in situ hybridization demonstrates a break-apart signal indicating TFE3 rearrangement in case 3. |
Systemic Review of Literature for ASPS of the Female Genital Tract
Table 2 presents the basic information and clinical characteristics of the 55 patients. The median age of the patients was 31 years (range: 8–68 years). The most common symptom was abnormal vaginal bleeding (61.5%). Common sites of involvement included the cervix (45.5%), uterine corpus (34.5%), vagina (12.7%), and vulva (7.3%). The median tumor size was 3 cm (range: 0.16–8.7 cm). Exophytic growth was observed in 55.6% of lesions, such as polypoid, submucosal, and pedicle lesions. The majority of histological growth patterns displayed the classic organoid pattern with well-formed nests. Among the cases, 72.7% exhibited positive periodic acid-Schiff (PAS) and/or Periodic Acid Schiff Diastase (PAS-D) staining. TFE3 immunohistochemical staining was positive in 49.1% of cases, HMB45 in 46.1%, Melan-A in 32.7%, and desmin in 34.6%. TFE3 gene fusion was identified in 34.6% of cases. Only 32.7% of cases underwent both supportive immunohistochemistry and confirmatory genetic analysis. At initial diagnosis, 96.4% of patients presented with localized disease and 3.6% presented with regional disease. Radical resection and local lesion resection were performed as initial treatments in 71.1% and 23.1% of patients, respectively. Node dissection was carried out in 29.4% of patients, and two cases exhibited positive lymph node metastases (pelvic lymph node micrometastasis and para-aortic lymph node metastasis, respectively). Postoperative radiotherapy and/or chemotherapy were administered to 11.76% of patients. Preoperative radiotherapy was given in one case. The median follow-up period was 18 months. No cases of distant metastasis were reported. Local recurrence occurred in four patients, and only two patients experienced relapse, which involved local excision with biopsy and cryotherapy. No deaths were observed at the end of the follow-up, and 45 patients were alive without disease.
 |
Table 2 Summary of Clinicopathological Features of Previously Published Cases of ASPS in the Female Genital Tract |
Analysis of ASPS in SEER Database
We reviewed a total of 174 cases in women and excluded nine cases due to non-first primary tumors, leaving us with 165 patients for analysis. The characteristics of the ASPS are summarized in Table 3. The common sites of occurrence were soft tissue, including the heart (n=134, 81.2%), and the female genital tract (n=17, 10.3%). The median age of the patients was 24±14.054 years (range: 2–78 years). The majority of patients were white (93, 56.4%) and unmarried (101, 61.2%). Regarding disease stage, 83 patients (50.3%) had localized/regional disease. Local excision was performed in 57 patients (34.5%), while radiation and chemotherapy were administered to 76 (46.1%) and 54 (32.7%) patients, respectively. In the female genital tract cohort, the 5-year cancer-specific survival (CSS) rate was 86.2%, whereas in the soft tissue, including the heart, it was 70.5%, and in other parts, it was only 47.1%. Univariate analysis demonstrated significant associations between CSS and age, tumor size, primary site, extent of surgery, stage, radiation, and chemotherapy (P < 0.05), as presented in Table 4. Multivariate analysis revealed that older age was associated with a 1.042-fold increased risk of cancer-specific mortality (HR=1.042, 95% CI 1.022–1.063, P < 0.001). Soft tissue, including the heart, exhibited a 4.786-fold higher risk of cancer-specific mortality (HR=4.786, 95% CI 1.681–13.623, P=0.003). Regional infiltration and distant metastasis carried approximately 8.6-fold and 18-fold higher risks of cancer-specific mortality compared to local disease, respectively (HR=8.652, 95% CI 2.529–29.63, P = 0.001; HR=18.366, 95% CI 6.153–54.817, P < 0.001). Patients who underwent radical excision did not show a reduced risk of cancer-specific mortality compared to those who underwent local excision (HR=0.492, 95% CI 0.224–1.081, P = 0.078), as indicated in Table 4.
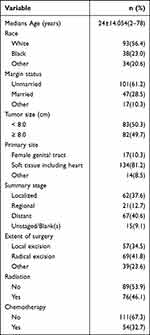 |
Table 3 Summary of Demographic and Treatment Information for ASPS Female Patients in the SEER Database |
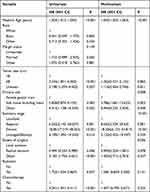 |
Table 4 Univariate and Multivariate Survival Analysis for ASPS Patients in the SEER Database |
Discussion
For the first time, we have identified a novel PRCC-TFE3 gene fusion in ASPS occurring in the female reproductive system. We first report the case of a 24-year-old unmarried patient with cervical ASPS who underwent fertility-sparing surgery without recurrence for 41 months. The median age of the patients was 31 years. The most common symptom was abnormal vaginal bleeding. Common sites of involvement included the cervix and uterine corpus. The median tumor size was 3 cm. Exophytic growth was observed in 55.6% of lesions. ASPS patients with primary involvement of the female genital tract were least likely to present with distant disease and exhibited a favorable survival advantage. The 5-year cancer-specific survival (CSS) rate was 86.2% in the female genital tract cohort. The patients who underwent local resection did not have increased cancer-specific mortality; however, older age, involvement of soft tissue including the heart, and metastasis at presentation were adverse prognostic factors.
ASPS originating from the female genital tract is a rare condition that poses significant diagnostic challenges.3 ASPS and Xp11 translocation PEComa have very similar microscopic features and immunoprofiles, being characteristically diffusely positive for TFE3 and negative for PAX-8. Clues to the diagnosis of ASPS include negative labeling for melanotic markers (HMB45 or Melan-A) and the fusion gene of ASPSCR1-TFE3.46 TFE3 translocation RCCs are positive for PAX-8 and CD10 and usually do not express pigment markers.47 The ASPSCR1-TFE3 fusion gene has long been known as a disease-defining molecular event that underpins the diagnosis of ASPS;41 however, a novel CSCP-TFE3 gene fusion was identified using whole exon sequencing at our institution. Dickson et al48 have identified three novel TFE3 fusion partners, namely HNRNPH3, DVL2, and PRCC, associated with ASPS in their soft tissue counterparts. These ‘MiT family translocation-associated neoplasms’ contain diverse TFE3 fusion products. The fusion of DVL2 and PRCC with TFE3 has been discovered in RCC,49 and a ASPSCR1 fusion partner has been identified in TFE3-rearranged PEComa.50 The potential under-recognized genetic diversity of ASPS. The next-generation sequencing strategy for fusion gene evaluation in the diagnosis of ASPS is more comprehensive and accurate.
A systematic analysis of the reported 55 cases in the female genital tract revealed that consistent with the report by Schoolmeester,41 ASPS arising in the female genital tract predominantly affected young individuals (median age 31 years). These tumors showed a strong preference for the uterine cervix, uterine corpus, and vagina, and typically exhibited small sizes (mean 3 cm). In half of the patients, exophytic growth of lesions, such as polypoid, submucosal, and pedicle lesions, was observed. 70.9% of the cases described typical morphology and PAS and/or PAS-D staining, however, Schoolmeester et al have noted these crystals are not always present.41 Only 32.7% of the cases had either supportive immunohistochemistry and confirmatory genetic analysis. All patients were alive at the last follow-up. Compared to previous reports,1 patients with ASPS of the female genital tract presented at earlier stages and had higher rates of resection. Unlike most patients with ASPS in soft tissue sites who remain asymptomatic,46 it is interesting to note that half of the female genital tract tumors appeared as exogenous masses and were associated with abnormal vaginal bleeding, which can aid in early diagnosis. The standard treatment for uterine sarcoma is total hysterectomy.51 In our review of the PubMed/MEDLINE databases, we found 12 cases of ASPS of the female genital tract in which lesion removal was performed. Local tumor recurrence data were available for four patients, and only two patients experienced relapse, both of whom underwent local excision with biopsy and cryotherapy. Lieberman et al reported that local recurrence of ASPS was only observed in cases with residual disease left behind.52 These results suggest that achieving R0 excision is crucial (and perhaps sufficient) for satisfactory local control of ASPS.
The 5-year cancer-specific survival (CSS) rate was 86.2% in the female genital tract cohort, indicating a favorable prognosis consistent with previous literature.33 Ogura reported one of the largest series based on data from a single center and identified smaller tumor size and absence of metastasis as favorable prognostic factors.53 Other studies have also suggested differences in prognosis based on primary site, sex, and age.54 Multivariate analysis using the SEER database confirmed that primary site was an independent risk factor for prognosis. The favorable prognosis of ASPS in the female genital tract may be attributed to early detection facilitated by bleeding and exogenous masses, leading to improved resectability. However, older age was identified as an adverse prognostic factor based on the SEER database. Differences in the biological characteristics between pediatric and adult ASPS may explain this observation.55 Our analysis also indicated that the prognosis of local resection was not statistically different from radical resection. At our institution, we treated a 24-year-old unmarried patient with ASPS of the cervix who underwent fertility-sparing surgery and remained recurrence-free for 41 months. Fertility-sparing local excision may be considered as a clinical decision for patients with localized disease, although long-term follow-up for prognosis is necessary. Consistent with previous reports, there was no survival benefit from traditional radiotherapy or chemotherapy.56
Our study has several limitations. Firstly, some cases may have been misdiagnosed due to the absence of immunohistochemistry and fusion gene detection in the PubMed/MEDLINE databases, which could have affected the accuracy of the differential diagnosis. Secondly, the SEER database lacks information on pediatric patients, limiting our understanding of ASPS in this population. Thirdly, important details such as the surgical margin and information on disease progression or recurrence were not available in the SEER database. Lastly, it is unclear whether patients in the study underwent metastasectomy.
In conclusion, the potential under-recognized genetic diversity of ASPS. The favorable prognosis observed in ASPS of the female genital tract can be attributed to early detection and improved resectability. Furthermore, our findings indicate that patients who underwent local resection did not experience increased cancer-specific mortality.
Acknowledgments
We thank Ms Lingling Yang, for contributing to the statistics analysis.
Author Contributions
The contribution of the individual authors: All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The present study was supported by the Beijing Kanghua Foundation for the Development of Traditional Chinese and Western Medicine “Le foundation” (KH-2020-LJJ-044, Xingtao Long).
Disclosure
The authors have no conflict of interest that could have appeared to influence the work reported in this paper.
References
1. Paoluzzi L, Maki RG. Diagnosis, prognosis, and treatment of alveolar soft-part sarcoma: a review. JAMA Oncol. 2019;5(2):254–260. doi:10.1001/jamaoncol.2018.4490
2. Christopherson WM, Foote FW
3. Höhn AK, Brambs CE, Hiller GGR, et al. 2020 WHO classification of female genital tumors. Geburtshilfe Frauenheilkd. 2021;81(10):1145–1153. doi:10.1055/a-1545-4279
4. Wang XT, Fang R, Zhang RS, et al. Malignant melanotic Xp11 neoplasms exhibit a clinicopathologic spectrum and gene expression profiling akin to alveolar soft part sarcoma: a proposal for reclassification. J Pathol. 2020;251(4):365–377. doi:10.1002/path.5470
5. Hagerty BL, Aversa J, Diggs LP, et al. Characterization of alveolar soft part sarcoma using a large national database. Surgery. 2020;168(5):825–830. doi:10.1016/j.surg.2020.06.007
6. Huang SW, Huang HY, Lin H. Alveolar soft part sarcoma of the uterine corpus: a 13-year follow-up case report and review of the literature. Taiwan J Obstet Gynecol. 2023;62(5):769–773. doi:10.1016/j.tjog.2023.07.025
7. Kasai K, Yoshida Y, Okumura M. Alveolar soft part sarcoma in the vagina: clinical features and morphology. Gynecol Oncol. 1980;9(2):227–236. doi:10.1016/0090-8258(80)90031-1
8. Shen JT, D’ablaing G, Morrow CP. Alveolar soft part sarcoma of the vulva: report of first case and review of literature. Gynecol Oncol. 1982;13(1):120–128. doi:10.1016/0090-8258(82)90017-8
9. Chapman GW, Benda J, Williams T. Alveolar soft-part sarcoma of the vagina. Gynecol Oncol. 1984;18(1):125–129. doi:10.1016/0090-8258(84)90016-7
10. O’Toole RV, Tuttle SE, Lucas JG, et al. Alveolar soft part sarcoma of the vagina: an immunohistochemical and electron microscopic study. Int J Gynecol Pathol. 1985;4(3):258–265. doi:10.1097/00004347-198509000-00011
11. Flint A, Gikas PW, Roberts JA. Alveolar soft part sarcoma of the uterine cervix. Gynecol Oncol. 1985;22(2):263–267. doi:10.1016/0090-8258(85)90037-x
12. Gray GF
13. Kopolovic J, Weiss DB, Dolberg L, et al. Alveolar soft-part sarcoma of the female genital tract. Case report with ultrastructural findings. Arch Gynecol. 1987;240(2):125–129. doi:10.1007/BF02134046
14. Foschini MP, Eusebi V, Tison V. Alveolar soft part sarcoma of the cervix uteri. A case report. Pathol Res Pract. 1989;184(3):354–8; discussion 359–60. doi:10.1016/S0344-0338(89)80099-8
15. Abeler V, Nesland JM. Alveolar soft-part sarcoma in the uterine cervix. Arch Pathol Lab Med. 1989;113(10):1179–1183.
16. Sahin AA, Silva EG, Ordonez NG. Alveolar soft part sarcoma of the uterine cervix. Mod Pathol. 1989;2(6):676–680.
17. Nolan NP, Gaffney EF. Alveolar soft part sarcoma of the uterus. Histopathology. 1990;16(1):97–99. doi:10.1111/j.1365-2559.1990.tb01071.x
18. Carinelli SG, Giudici MN, Brioschi D, et al. Alveolar soft part sarcoma of the vagina. Tumori. 1990;76(1):77–80. doi:10.1177/030089169007600121
19. Guillou L, Lamoureux E, Masse S, et al. Alveolar soft-part sarcoma of the uterine corpus: histological, immunocytochemical and ultrastructural study of a case. Virchows Arch a Pathol Anat Histopathol. 1991;418(5):467–471. doi:10.1007/BF01605935
20. Morimitsu Y, Tanaka H, Iwanaga S, et al. Alveolar soft part sarcoma of the uterine cervix. Acta Pathol Jpn. 1993;43(4):204–208. doi:10.1111/j.1440-1827.1993.tb01133.x
21. Chang HC, Hsueh S, Ho YS, et al. Alveolar soft part sarcoma of the vagina. A case report. J Reprod Med. 1994;39(2):121–125.
22. Burch DJ, Hitchcock A, Masson GM. Alveolar soft part sarcoma of the uterus: case report and review of the literature. Gynecol Oncol. 1994;54(1):91–94. doi:10.1006/gyno.1994.1172
23. Nielsen GP, Oliva E, Young RH, et al. Alveolar soft-part sarcoma of the female genital tract: a report of nine cases and review of the literature. Int J Gynecol Pathol. 1995;14(4):283–292. doi:10.1097/00004347-199510000-00001
24. Heimann P, Devalck C, Debusscher C, et al. Alveolar soft-part sarcoma: further evidence by FISH for the involvement of chromosome band 17q25. Genes Chromosomes Cancer. 1998;23(2):194–197. doi:10.1002/(SICI)1098-2264(199810)23:2<194::AID-GCC14>3.0.CO;2-O
25. Radig K, Buhtz P, Roessner A. Alveolar soft part sarcoma of the uterine corpus. Report of two cases and review of the literature. Pathol Res Pract. 1998;194(1):59–63. doi:10.1016/S0344-0338(98)80013-7
26. Roma AA, Yang B, Senior ME, et al. TFE3 immunoreactivity in alveolar soft part sarcoma of the uterine cervix: case report. Int J Gynecol Pathol. 2005;24(2):131–135. doi:10.1097/01.pgp.0000148343.07759.e1
27. Kasashima S, Minato H, Kobayashi M, et al. Alveolar soft part sarcoma of the endometrium with expression of CD10 and hormone receptors. APMIS. 2007;115(7):861–865. doi:10.1111/j.1600-0463.2007.apm_635.x
28. Gitau GM, Shepherd JH, Hughes G, et al. Alveolar soft part sarcoma of the uterine cervix. Int J Gynecol Cancer. 2008;18(4):853–856. doi:10.1111/j.1525-1438.2007.01073.x
29. Petersson F, Michal M. Minute alveolar soft part sarcoma of the endocervix: the smallest ever published case. Appl Immunohistochem Mol Morphol. 2009;17(6):553–556. doi:10.1097/PAI.0b013e3181a7bc86
30. Guntupalli S, Anderson ML, Bodurka DC. Alveolar soft part sarcoma of the cervix: case report and literature review. Arch Gynecol Obstet. 2009;279(2):263–265. doi:10.1007/s00404-008-0700-x
31. Mu YL, Liu M, Shi M, et al. Clinical analysis of alveolar soft-tissue sarcoma of the uterine cervix: a case report. Chin Med J. 2010;123(12):1612–1614.
32. Williams A, Bartle G, Sumathi VP, et al. Detection of ASPL/TFE3 fusion transcripts and the TFE3 antigen in formalin-fixed, paraffin-embedded tissue in a series of 18 cases of alveolar soft part sarcoma: useful diagnostic tools in cases with unusual histological features. Virchows Arch. 2011;458(3):291–300. doi:10.1007/s00428-010-1039-9
33. Hasegawa K, Ichikawa R, Ishii R, et al. A case of primary alveolar soft part sarcoma of the uterine cervix and a review of the literature. Int J Clin Oncol. 2011;16(6):751–758. doi:10.1007/s10147-011-0233-3
34. Kang WD, Heo SH, Choi YD, et al. Alveolar soft part sarcoma of the uterine cervix in a woman presenting with postmenopausal bleeding: a case report and literature review. Eur J Gynaecol Oncol. 2011;32(3):359–361.
35. Zhang LL, Tang Q, Wang Z, et al. Alveolar soft part sarcoma of the uterine corpus with pelvic lymph node metastasis: case report and literature review. Int J Clin Exp Pathol. 2012;5(7):715–719.
36. Feng M, Jiang W, He Y, et al. Primary alveolar soft part sarcoma of the uterine cervix: a case report and literature review. Int J Clin Exp Pathol. 2014;7(11):8223–8226.
37. Lee HJ. Alveolar soft part sarcoma of the uterine cervix: a case report and review of the literature. Korean J Pathol. 2014;48(5):361–365. doi:10.4132/KoreanJPathol.2014.48.5.361
38. Jabbour MN, Seoud M, Al-Ahmadie H, et al. ASPL-TFE3 translocation in vulvovaginal alveolar soft part sarcoma. Int J Gynecol Pathol. 2014;33(3):263–267. doi:10.1097/PGP.0b013e318290407c
39. Giordano G, D’Adda T, Varotti E, et al. Primary alveolar soft part sarcoma of uterine corpus: a case report with immunohistochemical, ultrastructural study and review of literature. World J Surg Oncol. 2016;14(1):24. doi:10.1186/s12957-016-0780-1
40. Zhang H, Wang Y, Liu Y, et al. Alveolar soft part sarcoma of uterine cervix in a postmenopausal woman: a case report and review of literature. Int J Clin Exp Pathol. 2017;10(9):9812–9815.
41. Schoolmeester JK, Carlson J, Keeney GL, et al. Alveolar soft part sarcoma of the female genital tract: a morphologic, immunohistochemical, and molecular cytogenetic study of 10 cases with emphasis on its distinction from morphologic mimics. Am J Surg Pathol. 2017;41(5):622–632. doi:10.1097/PAS.0000000000000796
42. Aiba T, Uehara K, Tsukushi S, et al. Perineal alveolar soft part sarcoma treated by laparoscopy-assisted total pelvic exenteration combined with pubic resection. Asian J Endosc Surg. 2017;10(2):198–201. doi:10.1111/ases.12342
43. Gomez M, Whiting K, Naous R. Alveolar soft part sarcoma presenting as a uterine polyp: a case report. SAGE Open Med Case Rep. 2020;8:2050313X20910598. doi:10.1177/2050313X20910598
44. Vishwajeet V, Elhence P, Singh P, et al. Alveolar soft part sarcoma of uterine corpus in a young female: a case report with review of literature. Int J Gynecol Pathol. 2021;40(3):272–277. doi:10.1097/PGP.0000000000000700
45. Lee Y, Na K, Woo HY, et al. Alveolar soft part sarcoma of the uterus: clinicopathological and molecular characteristics. Diagnostics. 2022;12(5):1102. doi:10.3390/diagnostics12051102
46. Wang H, Jacobson A, Harmon DC, et al. Prognostic factors in alveolar soft part sarcoma: a SEER analysis. J Surg Oncol. 2016;113(5):581–586. doi:10.1002/jso.24183
47. Wang XT, Xia QY, Ni H, et al. SFPQ/PSF-TFE3 renal cell carcinoma: a clinicopathologic study emphasizing extended morphology and reviewing the differences between SFPQ-TFE3 RCC and the corresponding mesenchymal neoplasm despite an identical gene fusion. Hum Pathol. 2017;63:190–200. doi:10.1016/j.humpath.2017.02.022
48. Dickson BC, Chung CT, Hurlbut DJ, et al. Genetic diversity in alveolar soft part sarcoma: a subset contain variant fusion genes, highlighting broader molecular kinship with other MiT family tumors. Genes Chromosomes Cancer. 2020;59(1):23–29. doi:10.1002/gcc.22803
49. Ellis CL, Eble JN, Subhawong AP, et al. Clinical heterogeneity of Xp11 translocation renal cell carcinoma: impact of fusion subtype, age, and stage. Mod Pathol. 2014;27(6):875–886. doi:10.1038/modpathol.2013.208
50. Zhao M, Huang Y, Yin X, et al. PEComa with ASPSCR1: TFE3 fusion: expanding the molecular genetic spectrum of TFE3 -rearranged PEComa with an emphasis on overlap with alveolar soft part sarcoma. Histopathology. 2023. doi:10.1111/his.15087
51. Maccaroni E, Lunerti V, Agostinelli V, et al. New insights into hormonal therapies in uterine sarcomas. Cancers. 2022;14(4):921. doi:10.3390/cancers14040921
52. Lieberman PH, Brennan MF, Kimmel M, et al. Alveolar soft-part sarcoma. A clinico-pathologic study of half a century. Cancer. 1989;63(1):1–13. doi:10.1002/1097-0142(19890101)63:1<1::AID-CNCR2820630102>3.0.CO;2-E
53. Ogura K, Beppu Y, Chuman H, et al. Alveolar soft part sarcoma: a single-center 26-patient case series and review of the literature. Sarcoma. 2012;2012:907179. doi:10.1155/2012/907179
54. O’Sullivan Coyne G, Naqash AR, Sankaran H, et al. Advances in the management of alveolar soft part sarcoma. Curr Probl Cancer. 2021;45(4):100775. doi:10.1016/j.currproblcancer.2021.100775
55. Kunisada T, Nakata E, Fujiwara T, et al. Soft-tissue sarcoma in adolescents and young adults. Int J Clin Oncol. 2023;28(1):1–11. doi:10.1007/s10147-022-02119-7
56. Fujiwara T, Kunisada T, Nakata E, et al. Advances in treatment of alveolar soft part sarcoma: an updated review. Jpn J Clin Oncol. 2023;53(11):1009–1018. doi:10.1093/jjco/hyad102
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.