Back to Journals » Infection and Drug Resistance » Volume 17
Alginate and Chitosan-Based Hydrogel Enhance Antibacterial Agent Activity on Topical Application
Authors Wathoni N , Suhandi C, Ghassani Purnama MF, Mutmainnah A, Nurbaniyah NS, Syafra DW, Elamin KM
Received 5 January 2024
Accepted for publication 6 February 2024
Published 1 March 2024 Volume 2024:17 Pages 791—805
DOI https://doi.org/10.2147/IDR.S456403
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
Nasrul Wathoni,1 Cecep Suhandi,1 Muhammad Fadhil Ghassani Purnama,1 Annisa Mutmainnah,1 Neng Sani Nurbaniyah,1 Desra Widdy Syafra,1 Khaled M Elamin2
1Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Universitas Padjadjaran, Jatinangor, 45363, Indonesia; 2Graduate School of Pharmaceutical Sciences, Kumamoto University, Kumamoto, 862-0973, Japan
Correspondence: Nasrul Wathoni, Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Universitas Padjadjaran, Jatinangor, 45363, Indonesia, Tel +62-22-842-888-888, Email [email protected]
Abstract: Untreated topical infections can become chronic, posing serious health issues. Optimal skin adherence is crucial in addressing such infections. In this context, chitosan and alginate emerge as promising candidates for use as a foundation in the development of topical hydrogels. The aim of this review is to examine the literature on topical hydrogel formulations that use chitosan and alginate as foundations, specifically in the context of topical antibacterial agents. The research methodology involves a literature review by examining articles published in databases such as PubMed, Scopus, ScienceDirect, and Google Scholar. The keywords employed during the research were “Alginate”, “Chitosan”, “Hydrogel”, and “Antibacterial”. Chitosan and alginate serve as bases in topical hydrogels to deliver various active ingredients, particularly antibacterial agents, as indicated by the search results. Both have demonstrated significant antibacterial effectiveness, as evidenced by a reduction in bacterial colony counts and an increase in inhibition zones. This strongly supports the idea that chitosan and alginate could be used together to make topical hydrogels that kill bacteria that work well. In conclusion, chitosan and alginate-based hydrogels show great potential in treating bacterial infections on the skin surface. The incorporation of chitosan and alginate into hydrogel formulations aids in retaining antibacterial agents, allowing for their gradual release over an optimal period. Therefore, hydrogels specifically formulated with chitosan and alginate have the potential to serve as a solution to address challenges in the treatment of topical bacterial infections.
Keywords: Alginate, chitosan, hydrogel, topical, antibacterial agent
Introduction
Normal and healthy skin naturally provides a robust barrier against pathogen invasion. Compromising this barrier makes individuals vulnerable to infections. Skin injuries such as physical trauma, insect bites, burns, and surgical incisions allow the entry of pathogenic bacteria, leading to skin and soft tissue infections.1 Wound healing is a complex project, and promoting effective skin repair presents a significant clinical challenge.2 Staphylococcus aureus and Streptococcus group A commonly cause primary, secondary, and minor skin wounds3 Healthcare professionals commonly use topical antibiotics to treat and prevent skin infections. This method is well-suited for delivering drugs directly to the site of action, ensuring antibacterial bioavailability in infected tissues. Additionally, the advantages of topical antibiotics include minimal drug usage, low cost, and no disruption of intestinal microbial flora.4 However, topically applied antibiotics may be associated with some local side effects and the development of contact dermatitis.5 The shallow penetration depth of topical antibiotics limits their use to superficial infections, and their widespread, unrestricted use can lead to bacterial resistance.6
The use of topical antimicrobials faces challenges such as an increase in bacterial resistance, local hypersensitivity reactions, and concerns regarding the potential development of antibiotic resistance with antiseptic use.7 Therefore, an optimal drug delivery system for antibacterial medications is crucial to maximize the efficacy of the administered drugs. Currently, the use of hydrogels for topical treatment offers various advantages to address issues associated with topical medications. Through formulation into hydrogels, drug release can be modified to occur gradually over an extended period.8–10 Additionally, the water-based nature of hydrogels is generally more compatible with damaged skin tissues. As evidence, the application of hydrogels has proven to have great potential in the field of wound dressings due to their physical properties closely resembling living tissues and excellent characteristics such as high-water content, oxygen permeability, and softness.11 As known, wounds are areas of damaged tissue vulnerable to pathogen infection. In this regard, several types of polymer-based hydrogels not only function as drug carriers but also possess attractive capabilities to prevent infections.
Among various polymer options available, natural polymers are often preferred due to their biodegradable and relatively non-toxic nature.12,13 Certain types of natural polysaccharide polymers have intrinsic properties that can prevent the growth of pathogenic bacteria.14–17 For example, chitosan possesses antibacterial properties by interacting ionically with negatively charged components on the bacterial cell walls.18 This intrinsic efficacy can be achieved with a minimal chitosan concentration (0.2 mg/dL).19 Although the use of chitosan alone can be utilized to create hydrogels, the formed hydrogel may not have optimal gelling capacity to effectively carry the intended drug.20–22 The solution lies in combining it with another polysaccharide polymer, alginate, which enhances the drug loading capacity in the hydrogel formulation.23 Utilizing a multivalent cross-linker such as Ca2+, the gelation process in alginate forms a cross-linked framework that encapsulates the drug. Hence, the combination of these two polymers holds promise for addressing issues related to topical bacterial infection treatment.24 Consequently, knowledge regarding the prospective use of chitosan and alginate, which may still be further developed with variations in polymer combinations, is crucial as a foundation for the development of hydrogel formulations in topical bacterial infection treatment. To address these issues, this review presents a summary of various types of hydrogels based on alginate, chitosan, and others that exhibit antibacterial activity.
Method
This review was conducted through the systematic search of relevant articles discussing the use of chitosan and alginate-based hydrogels for the delivery of antibacterial agents. Article searches were performed on databases, including PubMed, Scopus, ScienceDirect, and Google Scholar, utilizing the keywords “Alginate”, “Chitosan”, “Hydrogel”, And “Antibacterial”, without restricting the publication year. Inclusion criteria were applied to filter articles to be discussed in this review, including: articles published in English; studies employing hydrogels as a dosage form; the use of chitosan and/or alginate as the hydrogel base; and the inclusion of pharmacologically active substances subsequently tested for their antibacterial efficacy. Exclusion criteria in this article review encompassed: articles with study designs such as case reports, case series, and review articles; studies within articles lacking antibacterial testing of the prepared formulations; and articles with incomplete data, such as those lacking information on the active substances used and bacterial species employed in the antibacterial efficacy testing.
Topical Infection
Propionibacterium Acnes
Propionibacterium acnes is a gram-positive bacterium with a thick peptidoglycan layer in its cell wall. The bacterium is anaerobic and produces lipase enzymes that break down free fatty acids from skin lipids. Fatty acids can trigger tissue inflammation. Therefore, Propionibacterium acnes contributes to the occurrence of acne and is involved in the pathogenesis of acne. Acne is a form of chronic inflammation that affects the pilosebaceous unit, consisting of hair follicles and sebaceous glands.25
Currently, topical preparations of Erythromycin and Clindamycin are used as antibiotics to treat acne with inflammation caused by Propionibacterium acnes. On the market, erythromycin is known as Corsatrocin, Dothrocyn, Duramycin, Erymed, Erysanbe, and Trovilon. Meanwhile, Clindamycin is recognized as Mediklin, Clinbercin, Nufaclind, and Opiclam.26
Corynebacterium
Corynebacterium is a gram-positive bacterium that does not form spores, has a bacillus shape, and is aerobic. Some species of Corynebacterium bacteria can produce toxins, such as endotoxins, which can cause diseases. This bacterium causes erythrasma, a superficial skin infection that affects body folds, such as the armpits and groin. Symptoms of this disease include pink and scaly patches accompanied by itching and a burning sensation. This bacterium causes erythrasma by invading the stratum corneum, followed by proliferating in conditions such as heat and humidity. The bacteria then dissolve keratin fibrils in intercellular spaces and within cells, producing porphyrins. The first-line therapy is topical erythromycin, clindamycin, or miconazole cream. Some common brand names for topical clindamycin include Anerocid, Acne Clin, and Benzasil. Meanwhile, some common brand names for miconazole cream include Monistat-Derm and Micatin.27
Staphylococcus Epidermidis
Staphylococcus epidermidis is a gram-positive bacterium that forms part of the normal human microbiota, including the skin microbiota. Staphylococcus epidermidis survives without oxygen and forms cohesive, large, white colonies with a diameter of about 1–2 mm after overnight growth. It does not break down red blood cells on blood agar. This bacterium is an opportunistic pathogen that can exhibit virulence when attacking the human body. Staphylococcus epidermidis can cause infections resulting from the implantation of medical devices, such as cardiac and orthopedic devices. The formation of biofilm, exopolymers, and other mechanisms can protect this bacterium from antibiotics. The bacteria can enter the skin through scratches, wounds, or other skin damage. Staphylococcus epidermidis can produce enzymes that break down skin oils, triggering the formation of acne, and produce toxins that cause inflammation in the skin.
The treatment of Staphylococcus epidermidis infections involves antibiotics, depending on the severity of the infection and the bacteria’s susceptibility to antibiotics. Medical doctors often use vancomycin to treat methicillin-resistant Staphylococcus epidermidis infections. Vancomycin is known on the market as Klosvan, Vancolon, and Ladervan.28
Staphylococcus Aureus
Staphylococcus aureus belongs to the genus Staphylococcus within the Firmicutes, with a diameter of ~0.8 μm and arranged in “grape-like clusters” under the microscope. It can thrive both aerobically and anaerobically and has an optimal growth temperature of 37°C at a pH of 7.4. Colonies on blood agar plates are thick, shiny, and round with a diameter of 1–2 mm.29 S. aureus does not form spores or flagella, but it possesses a capsule, can produce a golden-yellow pigment, and ferments mannitol.30
Staphylococcus aureus is a gram-positive bacterium that causes various clinical diseases. On healthy skin, S. aureus typically does not cause infections. However, if the bacteria enter the bloodstream, they can lead to various infections, such as skin and soft tissue infections (SSTIs), which are among the most common bacterial infections worldwide. Common topical antibiotics used for the prevention and treatment of skin infections caused by this pathogen include fusidic acid (FA) and mupirocin. These drugs have been employed as the first-line topical treatment for superficial SSTIs (eg, impetigo) in many countries.31
Streptococcus Pyogenes
Streptococcus pyogenes is a bacterium classified in the phylum Firmicutes and is categorized as a Gram-positive bacterium.32 Also known as “group A streptococcus” (GAS), Streptococcus pyogenes is a pathogen that has infected 18.1 million people worldwide, resulting in 500,000 deaths annually.33 S. pyogenes is a facultative anaerobe, thrives best in 5 to 10% carbon dioxide, and forms colonies precisely on blood agar plates. The antigenic characteristics of protein M further divide GAS strains into different serotypes, making it the primary surface protein found in the cell wall of S. pyogenes. More than 80 different serotypes have been identified based on protein.34
Streptococcus pyogenes is one of the bacteria causing skin and soft tissue infections (SSTIs). Furthermore, no other pathogen causes such diverse clinical entities as S. pyogenes. Specifically, this organism induces infections in the superficial keratin layer (impetigo), superficial epidermis (erysipelas), subcutaneous tissue (cellulitis), fascia (necrotizing fasciitis), or muscles (myositis and myonecrosis). Topical therapy for infections caused by Streptococcus pyogenes can be accomplished with mupirocin, where mupirocin is equivalent to oral systemic antimicrobial agents and can be used when the number of lesions is limited.35
Hydrogel as Promising Topical Drug Delivery
Definition of Hydrogel
Hydrogel formulations in medicine are a pharmaceutical dosage form used to deliver drugs into the human or animal body. Hydrogels are water-based materials with the capacity to retain a significant amount of water within their structure. Medicinal hydrogel formulations typically consist of a three-dimensional network that absorbs and retains a substantial amount of water or other fluids, such as drug solutions. The water-absorbing properties of hydrogels make them ideal for various medical applications, including pharmaceutical product manufacturing.36
Hydrogel formulations in medicine can take various forms, including transdermal gels applied to the skin, eyes, or ears, as well as gels used for wound care or drug delivery through medical devices such as catheters or stents. Hydrogels can be designed to release drugs slowly over a specific period of time, allowing for precise and consistent drug dosages. This can enhance treatment efficiency and reduce side effects associated with fluctuations in drug levels in the bloodstream.37
Hydrogel formulations have various applications, such as topical drug delivery (on the skin or eyes), wound healing, or drug delivery through deeper body channels. Hydrogel formulations offer the ability to control drug release, enhance drug penetration into target tissues, and improve patient comfort.38
Commonly Used Excipients
Excipients (also referred to as additives or auxiliary substances) commonly used in pharmaceutical hydrogel formulations are substances that aid in forming, maintaining, and enhancing the quality of the hydrogel preparation. Common excipients utilized in pharmaceutical hydrogel formulations are summarized in Table 1.
 |
Table 1 Excipients Commonly Used in Formulating Topical Hydrogel |
Preparation method
The production of hydrogel pharmaceutical formulations involves a series of steps designed to produce a stable hydrogel suitable for specific medical or pharmaceutical applications. The process may vary depending on the materials used and the desired hydrogel formulation. Figure 1 illustrates the common scheme in producing hydrogel formulations.44
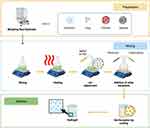 |
Figure 1 Scheme in manufacturing topical chitosan and alginate-based hydrogel dosage form. Created with Biorender.com. |
Preparing and Weighing of Raw Materials
The first step involves measuring and preparing raw materials, including gelling agents, water, preservatives, pH adjusters, consistency enhancers, and other excipients used in the hydrogel formulation.44
Mixing
Raw materials are mixed in specified quantities and order. Mixing is performed carefully to achieve homogeneity and even distribution. This can be done manually or using equipment such as mixers or stirrers.44
Heating
The heating process may be required, depending on the formulation. Some hydrogels require gentle heating to assist in the dissolution of gelling agents and form the gel as the hydrogel cools.44
pH Adjustment
If necessary, the solution’s pH is adjusted according to the drug application requirements. This can be done by adding acid or base.44
Preservatives and Other Excipients Addition
Preservatives and other excipients, such as texture modifiers, drugs, or colorants and fragrances, are added to the mixture according to the desired formulation.44
Gelation
The gelation process begins as the hydrogel starts to cool after mixing and may require gradual cooling or temperature changes to achieve the desired consistency.44
Filtration and Sterilization
The hydrogel can then be filtered to remove coarse particles and subsequently sterilized if intended for medical applications.44
Filling and Packaging
Once the hydrogel is ready, it can be filled into appropriate containers or packaging, such as tubes, bottles, or sachets, depending on the desired end product.44
It is important to note that the hydrogel manufacturing process can vary significantly depending on the type of drug to be applied, such as transdermal hydrogel, eye hydrogel, or wound care hydrogel. Additionally, strict regulations and standards must be followed in the production of hydrogel formulations to ensure drug quality, safety, and efficacy.
Characterization of Hydrogel Dosage Form
Important characteristics for the characterization of hydrogels include pH; scanning electron microscopy (SEM) to provide information about sample composition, surface topography, and other properties; Fourier transform infrared spectroscopy, or FTIR, to identify the chemical structure of a substance; swelling measurements; X-ray diffraction to understand whether the polymer retains its crystalline structure; in-vitro drug release studies to comprehend the release mechanism during the application period; rheology; spreading studies; in vivo skin irritation tests conducted on rabbits to examine for signs of edema and erythema; and tissue pore size measured with tools such as electron microscopes, mercury porosimeters, and others.45 Among the several hydrogel characteristics that were investigated, pH testing and in vitro release are two criteria that might directly influence the antibacterial effectiveness of the administered antibiotic.46–48 The formulation’s pH conditions are crucial to be adjusted to mimic the pH of the infected tissue or mucosa. Formulation pH conditions aligned with the treatment site’s pH can support the drug release process towards bacterial colonies as the target of its action.48 Subsequently, in vitro testing becomes a factor in confirming drug release into the release medium, in this case, the topical infection site, beyond assuming pH values. From these tests, the precise values of the amount of drug that can be released towards infectious bacteria and the duration of drug release can be accurately determined.46,47
Potency of Chitosan and Alginate as Natural Biodegradable Polymers-Based Hydrogel
Alginate
Alginates are anionic polysaccharides, salts of alginic acid predominantly present in brown algae, constituting about 40% of their dry matter.49 The intercellular matrix contains alginate in the form of a gel, which includes ions like sodium, calcium, magnesium, strontium, and barium. Therefore, the presence of multivalent cations makes alginate easily form a gel.50 The ability of alginate to form a gel or gelation varies based on molecular weight, molecular structure, and gelation agent concentration. Alginate gel can be formed through hydrogen bonding at low pH or through ionic interaction with mono- or polyvalent cations.51
Alginate is widely used in the industry because it retains water, forms gel, acts as a thickener, and provides stability. Moreover, alginate is known for its biocompatibility and immunogenicity, as well as its low toxicity.52 Alginate functions as a thickening agent in food, a balance regulator, an emulsifier, and a thin oil-resistant film former.53 Alginate can form films used in cosmetic and pharmaceutical applications, such as drug capsules and wound dressings. Alginate is also developed in tissue engineering technology for the production of hydrogels, beads, and micro/nanoparticles (NPs), for various applications such as wound healing, protein delivery, and cell encapsulation.51,54 Alginate readily forms hydrogels in the presence of cross-linking agents such as divalent cations. Therefore, alginate is essential not only for the textile industry but also for pharmaceuticals, food, and cosmetic ingredients. Although not classified as an antibiotic, alginate has high absorbency, antibacterial properties, and can accelerate wound healing. Therefore, alginate can be used as a primary wound dressing and as a medium for topical drug delivery to infected wounds. Furthermore, alginate’s origin from brown algae and its inclusion of active compounds like iodine with antibacterial properties make it suitable for creating composite hydrogels with antibacterial properties.55
Alginate is one of the natural polymers frequently used in drug and protein delivery systemsAlginate exhibits excellent biocompatibility and non-toxicity, and researchers can produce it using simple methods. Alginate hydrogels can function as delivery vectors for molecules precisely targeting tissues. The use of alginate can alter the physicochemical characteristics of drugs, enhancing their effectiveness and safety in drug delivery systems.55
Alginate is a linear polymer composed of d-mannuronic acid blocks linked by β(1→4) (M) and l-guluronic acid blocks linked by α(1→4) (G). These blocks consist of consecutive G residues (GGGGGG), consecutive M residues (MMMMMM), and alternating G and M residues (GMGMGM). It is known that only G blocks of alginate participate in the formation of cross-links between molecules with divalent cations (such as Ca2+) during the hydrogel formation process.54
Chitosan
Chitosan is a product resulting from the partial deacetylation of chitin, possessing excellent antibacterial properties, adhesive power, oxygen permeability, non-toxicity, and easy biodegradation, holding great potential for medical applications, such as antibacterial medical dressings.56 The crucial aspect of chitosan-based hydrogels for use in medical dressings is their host acceptance. Researchers have employed oxidized polysaccharides, like glucomannan, as environmentally friendly bio-crosslinkers. The researchers utilized the Schiff base reaction (reversible imine bond) to design injectable self-healing hydrogels. The Schiff base reaction principle is the basis for designing the CS/OKGM hydrogel dressing. The reaction oxidizes glucomannan to form oxidized konjac glucomannan (OKGM), which is then cross-linked with CS to create a hydrogel through the Schiff base reaction. Due to the dynamic nature of the Schiff base bond, the hydrogel demonstrates excellent self-healing and injectability capabilities.57
Hydrogel films consist of several polymers used to entrap α-M and enhance the consistency and elasticity of the hydrogel film. Chitosan and alginate are commonly used polymers, along with plasticizers like glycerin and propylene glycol. It has been found that alginate mixed with chitosan can effectively form hydrogel films using α-M, but the physicochemical quality still needs improvement. The safety and efficacy of RAS treatment also need evaluation. The self-healing and injectable properties enable hydrogels to fill various wound shapes or cover skin surfaces that require monitoring. Ions generate electrical conductivity, enabling the hydrogel to function as a sensor and paving the way for research on new multifunctional hydrogel dressings that combine biomedical functions and sensing. The presence of reactive oxygen species (ROS) generated massively around damaged tissues can cause damage to DNA and proteins, inhibiting tissue regeneration.56
Among biopolymers, polysaccharides are the most prominent in health-related applications due to their broad biological activities. They exist in various configurations: fibers, membranes, hydrogels, capsules, nanostructures, micelles, etc. Common biopolymers in recent years include chitosan (CHT) and alginate (ALG), widely used for wound healing and other skin issues.58 Combining biomacromolecules such as chitosan with sodium alginate for film development also demonstrates attractive advantages as the characteristics of each film can be enhanced. Moreover, the combination of CHT and ALG has proven to act as a membrane applied to cell regeneration and wound healing.59 Additionally, several studies suggest that combining various types of essential oils can enhance the properties of hydrogels and membranes, particularly by improving their antimicrobial potential, making them appealing for skin pathology treatment or food packaging.60
Alginate and Chitosan-Based Hydrogel Application for Topical Bacterial Treatment
Table 2 summarizes the use of chitosan and alginate as the foundation for topical hydrogel formulations for antibacterial purposes. The search results indicate that chitosan and alginate have extensive applications, encompassing diverse active substances and various bacterial types. In addition, chitosan and alginate can be combined with other base mixtures, such as gelatin, sodium carboxymethylcellulose (NaCMC), pectin, polyvinyl alcohol (PVA), sodium glycerophosphate, and g-pluronic copolymer. The following is a more detailed discussion of the potential of alginate-chitosan as a hydrogel base for topical antibacterial applications.
 |
Table 2 Applicability of Chitosan and Alginate as a Base for Topical Hydrogel in Topical Bacterial Treatment |
Alginate-Chitosan-Based Hydrogel
Alginate-chitosan hydrogel can apply to various active substances, including inorganic metals, chemical entities, and extracts. Alginate-chitosan-based hydrogel successfully carries zinc oxide (ZnO) for inorganic metal-based active substances. It can be applied to both secondary metabolites and synthetic chemical compounds.88 Researchers have also found this base to be a relevant choice for developing the delivery of marketed active pharmaceutical ingredients, including tetracycline, tobramycin, and vancomycin. One study demonstrates the applicability of alginate-chitosan-based hydrogel in delivering the active ingredient extract, namely Solanum nigrum L. leaf extract. Interestingly, this dosage form is also compatible for the delivery of modified compounds on a nanoscale, such as curcumin loaded in β-cyclodextrin (curcumin-β-cyclodextrin complex).64,89,90 The significant benefit of using alginate-chitosan-based hydrogel is evident from its testing as an antibacterial agent. Researchers have tested this hydrogel’s effectiveness against various types of bacteria, including S. aureus and P. aeruginosa. In addition, they have conducted tests on non-topical bacteria, such as E. coli and B. subtilis, as well as topical fungi, such as G. vaginalis. Search results indicate that studies utilizing alginate-chitosan-based hydrogels can provide a good antibacterial effect. The larger inhibition zone parameters and decreased bacterial colony counts reflect the good antibacterial effect of studies utilizing alginate-chitosan-based hydrogel.
Multiple studies have demonstrated that there are slight differences in the preparation techniques of hydrogels with a combination of chitosan and alginate. In the case of modified chitosan (carboxymethyl cellulose chitosan), polymer preparation is carried out simply using distilled water.61,62 On the other hand, for the base form of chitosan, dissolution is assisted using a dilute solution of acetic acid.63,64 Nevertheless, there is no significant difference between the use of these two polymer types, both in terms of physical characteristics and the performance of antibacterial agent delivery activities. The overall study indicates that loading antibacterial agents into this hydrogel results in a sustained drug release effect.73,78 For instance, the release of hesperidin as the active substance in the hydrogel experiences a gradual release over 14 days, reaching a release of 77.03 ± 8.71%.66 This indicates conformity with various findings suggesting that hydrogels can serve as long-term drug mediators suitable for treating chronic pathological conditions, such as infections, as illustrated in Figure 2.
 |
Figure 2 Mechanism of action of chitosan and alginate-based hydrogel in delivering antibacterial agents for ameliorating topical bacterial infection. Created with Biorender.com. |
Alginate-Chitosan Combined with Other Polymers
Generally, the addition of other polymers to the hydrogel base aims to enhance or improve the characteristics of the formulated hydrogel. Gelatin, a derivative of natural proteins, has been used for medical hydrogel synthesis due to its non-immunogenicity. Moreover, gelatin is widely used both as a single base and in combination because of its capacity to enhance cell adhesion and its excellent biocompatibility.91 The addition of PVA to the base mixture can form a stronger and relatively rigid hydrogel through physical crosslinking processes.92 Hydrogels containing NaCMC have significant potential to meet the requirements of effective wound care dressings, especially when mixed with natural antimicrobials.92 The addition of pectin-cellulose can provide a more stable hydrogel with good biocompatibility and haemostatic ability.93 Additionally, the use of other decorative polymers such as sodium glycerophosphate and Pluronic acid is generally employed to obtain pH or temperature-sensitive release characteristics.94,95 Significant differences in the impact of incorporating additional polymer materials are evident in the drug release profile.96 The use of pluronic copolymer induces a burst effect in the initial release period, reaching 30–40% on the first day.86 Nevertheless, sustained release continues post the burst effect, extending up to day 21. This presents a distinct advantage, especially in conditions of high infection levels, where maintaining a high drug concentration in the initial stages is crucial.
Challenges and Limitations
Although chitosan and alginate exhibit excellent potential as the foundation for hydrogel fabrication, they still present certain limitations. Based on the results discussed in this review, despite chitosan and alginate having relatively low toxicity profiles, there is a lack of direct clinical trials conducted on humans. Consequently, the assurance of safety regarding the use of chitosan and alginate needs further exploration so that advanced-stage testing can prevent undesirable side effects. Additionally, the use of natural polymers like chitosan generally allows dissolution at low pH, while becoming insoluble at high pH values.97 This poses a challenge as the impact of these materials on the pH changes in hydrogel manufacturing processes must be considered. Inappropriate pH conditions can lead to incompatibility with the polymer base, resulting in suboptimal formation of the hydrogel formulation.
Future Perspective
Hydrogels have become a recent favorite in the field of topical drug delivery, particularly for the delivery of antibacterial drugs that are generally chronic in nature. Hydrogels offer relevant benefits, primarily due to their excellent adhesive properties, allowing them to adhere for a considerable duration. Chitosan and alginate, as hydrogel bases, exhibit characteristics suitable for topical applications. As natural polymers, they also have the advantage of relatively low toxicity. Although their potential for delivering antibacterial drugs is evident, researchers have only tested their topical use against bacteria such as S. aureus and P. aeruginosa. Considering their positive impact on specific bacteria, it is plausible that this drug delivery system may show similar efficacy against other bacterial strains. Therefore, researchers need to conduct further studies to determine the potential of the alginate-chitosan-based hydrogel in combating other bacteria that cause skin infections, such as P. acnes, Corynebacterium, S. epidermidis, and S. pyogenes. Furthermore, the positive impact extends beyond the combination of alginate and chitosan alone; exploring the potential of combining other polymers is also crucial. In addition to NaCMC, gelatin, pectin, and PVA, more polymers that support the properties of alginate-chitosan-based hydrogels need to be studied in order to find the best hydrogel formulation. As a recommendation, it is strongly advised to carry out clinical validation to assess the antibacterial effectiveness and safety of hydrogels constructed from chitosan and alginate. This ensures that the products derived from these hydrogels not only possess evidential support but can also be widely utilized by the general public in the market.
Conclusion
As natural polymers, chitosan and alginate exhibit considerable potential as bases for hydrogel formulations. Hydrogel formulations based on chitosan and alginate can be obtained through a simple technique, namely the gelation process. As bases for topical hydrogels, chitosan and alginate are compatible with a wide range of active substances, including isolated compounds, synthetic compounds, and natural extracts. Furthermore, the use of chitosan and alginate can be further combined with other polymers to form hydrogels with specific criteria, such as PVA, NaCMC, gelatin, pectin, pluronic copolymer, and sodium glycerophosphate. The utilization of chitosan-alginate hydrogels can extend the release duration of antibacterial agents and has demonstrated effectiveness in reducing bacterial colony counts and inhibiting growth zones in plate disc tests.
Acknowledgments
We would like to thank The Rector of Universitas Padjadjaran for the APC.
Funding
Kedaireka Matching Fund, Ministry of Education and Culture, Republic of Indonesia (20/E1/PPK/KS.03.00/2023).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Okamoto S, Ogai K, Mukai K, Sugama J. Association of skin microbiome with the onset and recurrence of pressure injury in bedridden elderly people. Microorganisms. 2021;9(8). doi:10.3390/microorganisms9081603
2. Gould L, Li WW. Defining complete wound closure: closing the gap in clinical trials and practice. Wound Repair Regener. 2019;27(3). doi:10.1111/wrr.12707
3. Buzzá HH, Alves F, Tomé AJB, et al. Porphyrin nanoemulsion for antimicrobial photodynamic therapy: effective delivery to inactivate biofilm-related infections. Proc Natl Acad Sci U S A. 2022;119(46). doi:10.1073/pnas.2216239119
4. Punjataewakupt A, Napavichayanun S, Aramwit P. The downside of antimicrobial agents for wound healing. Eur J Clin Microbiol Infect Dis. 2019;38(1). doi:10.1007/s10096-018-3393-5
5. Landeck L, John SM, Geier J. Topical ophthalmic agents as allergens in periorbital dermatitis. Br J Ophthalmol. 2014;98(2). doi:10.1136/bjophthalmol-2013-304197
6. Bandyopadhyay D. Topical antibacterials in dermatology. Indian J Dermatol. 2021;66(2). doi:10.4103/ijd.IJD_99_18
7. Neri I, Miraglia Del Giudice M, Novelli A, Ruggiero G, Pappagallo G, Galli L. Ideal features of topical antibiotic therapy for the treatment of impetigo: an Italian expert consensus report. Curr Ther Res Clin Exp. 2023;98. doi:10.1016/j.curtheres.2022.100690
8. Moeini A, Pedram P, Makvandi P, Malinconico M, Ayala G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: a review. Carbohydr Polym. 2020;233. doi:10.1016/j.carbpol.2020.115839
9. Siavash M, Noursina A. The ideal wound dressing. Burns. 2023. doi:10.1016/j.burns.2023.04.007
10. Rezvani Ghomi E, Khalili S, Nouri Khorasani S, Esmaeely Neisiany R, Ramakrishna S. Wound dressings: current advances and future directions. J Appl Polym Sci. 2019;136(27). doi:10.1002/app.47738
11. Williamson DA, Carter GP, Howden BP. Current and emerging topical antibacterials and antiseptics: agents, action, and resistance patterns. Clin Microbiol Rev. 2017;30(3). doi:10.1128/CMR.00112-16
12. Alsaab HO, Alharbi FD, Alhibs AS, et al. PLGA-based nanomedicine: history of advancement and development in clinical applications of multiple diseases. Pharmaceutics. 2022;14(12). doi:10.3390/pharmaceutics14122728
13. Yanat M, Schroën K. Preparation methods and applications of chitosan nanoparticles; with an outlook toward reinforcement of biodegradable packaging. React Funct Polym. 2021;161. doi:10.1016/j.reactfunctpolym.2021.104849
14. Schmieg B, Döbber J, Kirschhöfer F, Pohl M, Franzreb M. Advantages of hydrogel-based 3D-printed enzyme reactors and their limitations for biocatalysis. Front Bioeng Biotechnol. 2019;6. doi:10.3389/fbioe.2018.00211
15. Aswathy SH, Narendrakumar U, Manjubala I. Commercial hydrogels for biomedical applications. Heliyon. 2020;6(4). doi:10.1016/j.heliyon.2020.e03719
16. Kibungu C, Kondiah PPD, Kumar P, Choonara YE. This review recent advances in chitosan and alginate‐based hydrogels for wound healing application. Front Mater. 2021;8. doi:10.3389/fmats.2021.681960
17. Ghobashy MM. The application of natural polymer-based hydrogels for agriculture. In: Hydrogels Based on Natural Polymers. Elsevier; 2019. doi10.1016/B978-0-12-816421-1.00013-6
18. Sanchez-Salvador JL, Balea A, Monte MC, Negro C, Blanco A. Chitosan grafted/cross-linked with biodegradable polymers: a review. Int J Biol Macromol. 2021;178. doi:10.1016/j.ijbiomac.2021.02.200
19. Yilmaz Atay H. Antibacterial activity of chitosan-based systems. Function Chitosan. 2020;457–489. doi:10.1007/978-981-15-0263-7_15
20. Long S, Xie C, Lu X. Natural polymer-based adhesive hydrogel for biomedical applications. Biosurf Biotribol. 2022;8(2). doi:10.1049/bsb2.12036
21. Bao Z, Xian C, Yuan Q, Liu G, Wu J. Natural polymer-based hydrogels with enhanced mechanical performances: preparation, structure, and property. Adv Healthc Mater. 2019;8(17). doi:10.1002/adhm.201900670
22. Abdel Maksoud MIA, Ghobashy MM, Kodous AS, et al. Insights on magnetic spinel ferrites for targeted drug delivery and hyperthermia applications. Nanotechnol Rev. 2022;11(1). doi:10.1515/ntrev-2022-0027
23. Motelica L, Ficai D, Oprea O, et al. Biodegradable alginate films with ZnO nanoparticles and citronella essential oil-A novel antimicrobial structure. Pharmaceutics. 2021;13(7). doi:10.3390/pharmaceutics13071020
24. Tomić SL, Babić Radić MM, Vuković JS, Filipović V, Nikodinovic-Runic J, Vukomanović M. Alginate-based hydrogels and scaffolds for biomedical applications. Mar Drugs. 2023;21(3). doi:10.3390/md21030177
25. Suhandi C, Mohammed AFA, Wilar G, El-Rayyes A, Wathoni N. Effectiveness of mesenchymal stem cell secretome on wound healing: a systematic review and meta-analysis. Tissue Eng Regen Med. 2023. doi:10.1007/s13770-023-00570-9
26. Ma Y, Zhang N, Wu S, Huang H, Cao Y. Antimicrobial activity of topical agents against Propionibacterium acnes: an in vitro study of clinical isolates from a hospital in Shanghai, China. Front Med. 2016;10(4). doi:10.1007/s11684-016-0480-9
27. Salemi SZ, Memar MY, Kafil HS, et al. The prevalence and antibiotics susceptibility patterns of Corynebacterium minutissimum isolates from skin lesions of patients with suspected erythrasma from Tabriz, Iran. Can J Infect Dis Med Microbiol. 2022;2022. doi:10.1155/2022/4016173
28. Pinheiro L, Brito CI, Pereira VC, de Oliveira A, Camargo CH. Reduced susceptibility to vancomycin and biofilm formation in methicillin-resistant Staphylococcus epidermidis isolated from blood cultures. Mem Inst Oswaldo Cruz. 2014;109(7). doi:10.1590/0074-0276140120
29. Guo Y, Song G, Sun M, Wang J, Wang Y. Prevalence and therapies of antibiotic-resistance in Staphylococcus aureus. Front Cell Infect Microbiol. 2020;10. doi:10.3389/fcimb.2020.00107
30. Tayeb-Fligelman E, Tabachnikov O, Moshe A, et al. The cytotoxic Staphylococcus aureus PSMα3 reveals a cross-α amyloid-like fibril. Science. 2017;355(6327). doi:10.1126/science.aaf4901
31. Nong Y, Taiaroa G, Pasricha S, et al. Clinical relevance of topical antibiotic use in coselecting for multidrug-resistant staphylococcus aureus: insights from in vitro and ex vivo models. Antimicrob Agents Chemother. 2021;65(5). doi:10.1128/AAC.02048-20
32. Jespersen MG, Lacey JA, Tong SYC, Davies MR. Global genomic epidemiology of Streptococcus pyogenes. Infect Genet Evol. 2020;86. doi:10.1016/j.meegid.2020.104609
33. Avire NJ, Whiley H, Ross K. A review of streptococcus pyogenes: public health risk factors, prevention and control. Pathogens. 2021;10(2). doi:10.3390/pathogens10020248
34. Chowdhury S, Khakzad H, Bergdahl GE, et al. Streptococcus pyogenes forms serotype- and local environment-dependent interspecies protein complexes. mSystems. 2021;6(5). doi:10.1128/msystems.00271-21
35. Stevens DL, Bryant AE. Impetigo, Erysipelas and Cellulitis. Search life-sciences literature; 2016.
36. Thakur S, Thakur VK, Arotiba OA. History, Classification, Properties and Application of Hydrogels: An Overview. Springer; 2018. doi:10.1007/978-981-10-6077-9_2
37. Elsayed MM. Hydrogel preparation technologies: relevance kinetics, thermodynamics and scaling up aspects. J Polym Environ. 2019;27(4). doi:10.1007/s10924-019-01376-4
38. Jiang P, Yan C, Ji Z, et al. Drawing high-definition and reversible hydrogel paintings with grayscale exposure. ACS Appl Mater Interfaces. 2019;11(45). doi:10.1021/acsami.9b14342
39. Ickenstein LM, Garidel P. Hydrogel formulations for biologicals: current spotlight from a commercial perspective. Ther Deliv. 2018;9(3). doi:10.4155/tde-2017-0085
40. Li J, Mooney DJ. Designing hydrogels for controlled drug delivery. Nat Rev Mater. 2016;1(12). doi:10.1038/natrevmats.2016.71
41. Almoshari YH. Novel hydrogels for topical applications: an updated comprehensive review based on source. Gels. 2022;8(3). doi:10.3390/gels8030174
42. Thang NH, Chien TB, Cuong DX. Polymer-based hydrogels applied in drug delivery: an overview. Gels. 2023;9(7). doi:10.3390/gels9070523
43. Ciolacu DE, Nicu R, Ciolacu F. Cellulose-based hydrogels as sustained drug-delivery systems. Materials. 2020;13(22). doi:10.3390/ma13225270
44. Yang J, Chen Y, Zhao L, et al. Preparation of a chitosan/carboxymethyl chitosan/AgNPs polyelectrolyte composite physical hydrogel with self-healing ability, antibacterial properties, and good biosafety simultaneously, and its application as a wound dressing. Compos B Eng. 2020:197. doi:10.1016/j.compositesb.2020.108139
45. Ahmed EM. Hydrogel: preparation, characterization, and applications: a review. J Adv Res. 2015;6(2). doi:10.1016/j.jare.2013.07.006
46. Gopala Kumari SV, Manikandan NA, Pakshirajan K, Pugazhenthi G. Sustained drug release and bactericidal activity of a novel, highly biocompatible and biodegradable polymer nanocomposite loaded with norfloxacin for potential use in antibacterial therapy. J Drug Deliv Sci Technol. 2020;59. doi:10.1016/j.jddst.2020.101900
47. Li S, Shi X, Xu B, et al. In vitro drug release and antibacterial activity evaluation of silk fibroin coated vancomycin hydrochloride loaded poly (lactic-co-glycolic acid) (PLGA) sustained release microspheres. J Biomater Appl. 2022;36(9). doi:10.1177/08853282211064098
48. Saliani M, Jalal R, Goharshadi EK. Effects of pH and temperature on antibacterial activity of zinc oxide nanofluid against Escherichia coli O157: H7 and staphylococcus aureus. Jundishapur J Microbiol. 2015;8(2). doi:10.5812/jjm.17115
49. Zakharova L, Pashirova T, Kashapov R, Gabdrakhmanov D, Sinyashin O. Drug delivery mediated by confined nanosystems: structure-activity relations and factors responsible for the efficacy of formulations. In: Nanostructures for Drug Delivery. Elsevier; 2017. doi10.1016/B978-0-323-46143-6.00024-5
50. Sosnik A. Alginate particles as platform for drug delivery by the oral route: state-of-the-art. ISRN Pharm. 2014;2014. doi:10.1155/2014/926157
51. Yang J, Han S, Zheng H, Dong H, Liu J. Preparation and application of micro/nanoparticles based on natural polysaccharides. Carbohydr Polym. 2015;123. doi:10.1016/j.carbpol.2015.01.029
52. Silva SS, Fernandes EM, Pina S, et al. 2.11 Polymers of biological origin. In: Comprehensive Biomaterials II. Elsevier; 2017. doi:10.1016/B978-0-12-803581-8.10134-1
53. Hassabo AG, Mohamed AL. Extraction, structural properties, and applications of alginic acid. In: Natural Gums. Elsevier; 2023. doi10.1016/b978-0-323-99468-2.00023-1
54. Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37(1). doi:10.1016/j.progpolymsci.2011.06.003
55. Jarrah R, Sammak S, Onyedimma C, et al. The role of alginate hydrogels as a potential treatment modality for spinal cord injury: a comprehensive review of the literature. Neurospine. 2022;19(2). doi:10.14245/ns.2244186.093
56. Milanda T, Cindana Mo FR, Mohammed AFA, et al. Alginate/chitosan-based hydrogel film containing α-mangostin for recurrent aphthous stomatitis therapy in rats. Pharmaceutics. 2022;14(8). doi:10.3390/pharmaceutics14081709
57. Chen H, Cheng J, Ran L, et al. An injectable self-healing hydrogel with adhesive and antibacterial properties effectively promotes wound healing. Carbohydr Polym. 2018:201. doi:10.1016/j.carbpol.2018.08.090
58. Duceac IA, Coseri S. Biopolymers and their derivatives: key components of advanced biomedical technologies. Biotechnol Adv. 2022;61. doi:10.1016/j.biotechadv.2022.108056
59. Baysal K, Aroguz AZ, Adiguzel Z, Baysal BM. Chitosan/alginate crosslinked hydrogels: preparation, characterization and application for cell growth purposes. Int J Biol Macromol. 2013;59. doi:10.1016/j.ijbiomac.2013.04.073
60. Istiqomah A, Utami MR, Firdaus M, Suryanti V, Kusumaningsih T. Antibacterial chitosan-Dioscorea alata starch film enriched with essential oils optimally prepared by following response surface methodology. Food Biosci. 2022;46. doi:10.1016/j.fbio.2022.101603
61. Hu H, Zhong D, Li W, et al. Microalgae-based bioactive hydrogel loaded with quorum sensing inhibitor promotes infected wound healing. Nano Today. 2022:42. doi:10.1016/j.nantod.2021.101368
62. Cheng M, Cui Y, Guo Y, et al. Design of carboxymethyl chitosan-reinforced pH-responsive hydrogels for on-demand release of carvacrol and simulation of release kinetics. Food Chem. 2023:405. doi:10.1016/j.foodchem.2022.134856
63. Khan YA, Ozaltin K, Bernal-Ballen A, Di Martino A. Chitosan-alginate hydrogels for simultaneous and sustained releases of ciprofloxacin, amoxicillin and vancomycin for combination therapy. J Drug Deliv Sci Technol. 2021;61. doi:10.1016/j.jddst.2020.102126
64. Kiti K, Suwantong O. Bilayer wound dressing based on sodium alginate incorporated with curcumin-β-cyclodextrin inclusion complex/chitosan hydrogel. Int J Biol Macromol. 2020;164. doi:10.1016/j.ijbiomac.2020.09.013
65. Bagher Z, Ehterami A, Nasrolahi M, Azimi M, Salehi M. Hesperidin promotes peripheral nerve regeneration based on tissue engineering strategy using alginate/chitosan hydrogel: in vitro and in vivo study. Int J Polym Mater Polym Biomater. 2021;70(5). doi:10.1080/00914037.2020.1713781
66. Bagher Z, Ehterami A, Safdel MH, et al. Wound healing with alginate/chitosan hydrogel containing hesperidin in rat model. J Drug Deliv Sci Technol. 2020:55. doi:10.1016/j.jddst.2019.101379
67. Permana AD, Asri RM, Amir MN, et al. Development of thermoresponsive hydrogels with mucoadhesion properties loaded with metronidazole gel-flakes for improved bacterial vaginosis treatment. Pharmaceutics. 2023;15(5). doi:10.3390/pharmaceutics15051529
68. Feyissa Z, Edossa GD, Gupta NK, Negera D. Development of double crosslinked sodium alginate/chitosan based hydrogels for controlled release of metronidazole and its antibacterial activity. Heliyon. 2023;9(9). doi:10.1016/j.heliyon.2023.e20144
69. Tentor F, Siccardi G, Sacco P, et al. Long lasting mucoadhesive membrane based on alginate and chitosan for intravaginal drug delivery. J Mater Sci Mater Med. 2020;31(3). doi:10.1007/s10856-020-6359-y
70. Zhang M, Fan Z, Zhang J, et al. Multifunctional chitosan/alginate hydrogel incorporated with bioactive glass nanocomposites enabling photothermal and nitric oxide release activities for bacteria-infected wound healing. Int J Biol Macromol. 2023:232. doi:10.1016/j.ijbiomac.2023.123445
71. Najafpour F, Arabzadeh S, Kalalinia F, et al. Evaluation of wound healing effect of Solanum nigrum L. leaf extract-loaded sodium alginate nanoparticles embedded in chitosan hydrogel, In vivo study. Nanomed J. 2022;9(1). doi:10.22038/NMJ.2022.62218.1644
72. Jafari H, Ghaffari-bohlouli P, Podstawczyk D, Nie L, Shavandi A. Tannic acid post-treatment of enzymatically crosslinked chitosan-alginate hydrogels for biomedical applications. Carbohydr Polym. 2022;295. doi:10.1016/j.carbpol.2022.119844
73. Chen H, Xing X, Tan H, et al. Covalently antibacterial alginate-chitosan hydrogel dressing integrated gelatin microspheres containing tetracycline hydrochloride for wound healing. Mater Sci Eng C. 2017;70(Part 2). doi:10.1016/j.msec.2016.08.086
74. Zhou K, Wang X, Chen D, et al. Enhanced treatment effects of tilmicosin against staphylococcus aureus cow mastitis by self-assembly sodium alginate-chitosan nanogel. Pharmaceutics. 2019;11(10). doi:10.3390/pharmaceutics11100524
75. Xiao W, Gu S, Yuan W. Injectable nanocomposite hydrogels with the NIR-triggered low-temperature photothermal effect and low-dose antibiotic release. ACS Appl Polym Mater. 2023;5(7). doi:10.1021/acsapm.3c00676
76. Shi M, Xu Y, Li S, Wang L, Gu J, Zhang YX. The development of a polysaccharide-based hydrogel encapsulating tobramycin-loaded gelatine microspheres as an antibacterial system. Gels. 2023;9(3). doi:10.3390/gels9030219
77. Pawar V, Topkar H, Srivastava R. Chitosan nanoparticles and povidone iodine containing alginate gel for prevention and treatment of orthopedic implant associated infections. Int J Biol Macromol. 2018;115. doi:10.1016/j.ijbiomac.2018.04.166
78. Zhang M, Qiao X, Han W, Jiang T, Liu F, Zhao X. Alginate-chitosan oligosaccharide-ZnO composite hydrogel for accelerating wound healing. Carbohydr Polym. 2021;266. doi:10.1016/j.carbpol.2021.118100
79. Lu Y, Xu J, Su Y, et al. A biocompatible double-crosslinked gelatin/ sodium alginate/dopamine/quaterniazed chitosan hydrogel for wound dressings based on 3D bioprinting technology. Int J Bioprint. 2022;9(2). doi:10.18063/IJB.689
80. Cao J, Xiao L, Shi X. Injectable drug-loaded polysaccharide hybrid hydrogels for hemostasis. RSC Adv. 2019;9(63). doi:10.1039/c9ra07116d
81. Seifi S, Shamloo A, Tavoosi SN, Almasi-Jaf A, Shaygani H, Sayah MR. A novel multifunctional chitosan-gelatin/carboxymethyl cellulose-alginate bilayer hydrogel containing human placenta extract for accelerating full-thickness wound healing. Int J Biol Macromol. 2023. doi:10.1016/j.ijbiomac.2023.126929
82. Amante C, Esposito T, Del Gaudio P, et al. A novel three-polysaccharide blend in situ gelling powder for wound healing applications. Pharmaceutics. 2021;13(10). doi:10.3390/pharmaceutics13101680
83. Chen M, Zhai X, Pan Y, Tan H. Covalent and environment-responsive biopolymer hydrogel for drug delivery and wound healing. J Macromol Sci Part A. 2021;58(11). doi:10.1080/10601325.2021.1929316
84. Ghasemi AH, Farazin A, Mohammadimehr M, Naeimi H. Fabrication and characterization of biopolymers with antibacterial nanoparticles and Calendula officinalis flower extract as an active ingredient for modern hydrogel wound dressings. Mater Today Commun. 2022;31. doi:10.1016/j.mtcomm.2022.103513
85. Sun M, Cheng L, Xu Z, et al. Preparation and characterization of vancomycin hydrochloride-loaded mesoporous silica composite hydrogels. Front Bioeng Biotechnol. 2022:10. doi:10.3389/fbioe.2022.826971
86. Dang LH, Nguyen TH, Tran HLB, Doan VN, Tran NQ. Injectable nanocurcumin-formulated chitosan-g-pluronic hydrogel exhibiting a great potential for burn treatment. J Healthc Eng. 2018;2018. doi:10.1155/2018/5754890
87. Haki M, Shamloo A, Eslami SS, Mir-Mohammad-Sadeghi F, Maleki S, Hajizadeh A. Fabrication and characterization of an antibacterial chitosan-coated allantoin-loaded NaCMC/SA skin scaffold for wound healing applications. Int J Biol Macromol. 2023;253. doi:10.1016/j.ijbiomac.2023.127051
88. Suhandi C, Alfathonah SS, Hasanah AN. Potency of xanthone derivatives from garcinia mangostana L. for COVID-19 treatment through angiotensin-converting enzyme 2 and main protease blockade: a computational study. Molecules. 2023;28(13). doi:10.3390/molecules28135187
89. Suhandi C, Wilar G, Lesmana R, et al. Propolis-based nanostructured lipid carriers for α-mangostin delivery: formulation, characterization, and in vitro antioxidant activity evaluation. Molecules. 2023;28(16). doi:10.3390/molecules28166057
90. Suharyani I, Suhandi C, Rizkiyan Y, et al. Molecular docking in prediction of α-mangostin/cyclodextrin inclusion complex formation. In:
91. Andreazza R, Morales A, Pieniz S, Labidi J. Gelatin-based hydrogels: potential biomaterials for remediation. Polymers. 2023;15(4). doi:10.3390/polym15041026
92. Oliveira RN, McGuinness GB. Blended Gels of Sodium Carboxymethyl Cellulose Incorporating Antimicrobials for Absorbance and Wound Healing Applications. Springer; 2018. doi:10.1007/978-3-319-76573-0_39-1
93. Chen W, Yuan S, Shen J, Chen Y, Xiao Y. A composite hydrogel based on pectin/cellulose via chemical cross-linking for hemorrhage. Front Bioeng Biotechnol. 2021;8. doi:10.3389/fbioe.2020.627351
94. Deng A, Kang X, Zhang J, Yang Y, Yang S. Enhanced gelation of chitosan/β-sodium glycerophosphate thermosensitive hydrogel with sodium bicarbonate and biocompatibility evaluated. Mater Sci Eng C. 2017;78. doi:10.1016/j.msec.2017.04.109
95. Aslam M, Barkat K, Malik NS, et al. pH sensitive pluronic acid/agarose-hydrogels as controlled drug delivery carriers: design, characterization and toxicity evaluation. Pharmaceutics. 2022;14(6). doi:10.3390/pharmaceutics14061218
96. Ghobashy MM, Elbarbary AM, Hegazy DE, Maziad NA. Radiation synthesis of pH-sensitive 2-(dimethylamino)ethyl methacrylate/ polyethylene oxide/ZnS nanocomposite hydrogel membrane for wound dressing application. J Drug Deliv Sci Technol. 2022;73. doi:10.1016/j.jddst.2022.103399
97. El-banna FS, Mahfouz ME, Leporatti S, El-Kemary M, Hanafy NAN. Chitosan as a natural copolymer with unique properties for the development of hydrogels. Appl Sci. 2019;9(11). doi:10.3390/app9112193
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
