Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 17
Advances in Human-Centered Care to Address Contemporary Unmet Needs in Chronic Dialysis
Received 23 December 2023
Accepted for publication 12 March 2024
Published 20 March 2024 Volume 2024:17 Pages 91—104
DOI https://doi.org/10.2147/IJNRD.S387598
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Pravin Singhal
Sumedh Jayanti,1,2 Gopala K Rangan1,2
1Department of Renal Medicine, Westmead Hospital, Sydney, NSW, Australia; 2Michael Stern Laboratory for Polycystic Kidney Disease, Centre for Transplant and Renal Research, Westmead Institute for Medical Research, The University of Sydney, Sydney, NSW, Australia
Correspondence: Gopala K Rangan, Centre for Transplant and Renal Research, Westmead Institute for Medical Research, 176 Hawkesbury Road, Westmead, Sydney, NSW, 2145, Australia, Email [email protected]
Abstract: Advances in the treatment of kidney failure with chronic dialysis have stagnated over the past three decades, with over 50% of patients still managed by conventional in-hospital haemodialysis. In parallel, the demands of chronic dialysis medical care have changed and evolved due to a growing population that has higher frailty and multimorbidity. Thus, the gap between the needs of kidney failure patients and the healthcare capability to provide effective overall management has widened. To address this problem, healthcare policy has increasingly aligned towards a human-centred approach. The paradigm shift of human-centred approach places patients at the forefront of decision-making processes, ensuring that specific needs are understood and prioritised. Integration of human-centred approaches with patient care has been shown to improve satisfaction and quality of life. The aim of this narrative is to evaluate the current clinical challenges for managing kidney failure for dialysis providers; summarise current experiences and unmet needs of chronic dialysis patients; and finally emphasise how human-centred care has advanced chronic dialysis care. Specific incremental advances include implementation of renal supportive care; home-assisted dialysis; hybrid dialysis; refinements to dialysis methods; whereas emerging advances include portable and wearable dialysis devices and the potential for the integration of artificial intelligence in clinical practice.
Keywords: patient-centred care, chronic dialysis, kidney failure, patient preference, renal replacement therapy, innovation
Introduction
Since the advent of haemodialysis and peritoneal dialysis, innovations in chronic dialysis technology and care in patients with kidney failure have been incremental.1 Additionally, such innovations have been unable to address the increasing care needs of a larger chronic dialysis patient population with higher morbidity and mortality.2 Studies have consistently shown that dialysis patients have a significantly reduced quality of life compared to kidney transplant recipients and the general population.3,4 Despite chronic dialysis patients constituting less than 1% of the general population, they require a disproportionate amount of healthcare funding.5,6 To address this major clinical problem, healthcare systems and the nephrology community have pivoted towards human-centered care (involving renal supportive care) to better address needs while simultaneously developing dialysis technologies that improve quality of life.7
The aim of this narrative review is to evaluate the current clinical challenges for managing kidney failure by dialysis providers; summarise current experiences and unmet needs of chronic dialysis patients; and finally emphasise the importance on how human-centred care, leading to advances in chronic dialysis care. As this area is only in the early stages of development with limited evidence, the goal is to demonstrate how these human-centred innovations could potentially establish new care models that facilitate home-based dialysis, promote increased patient independence, and reduce symptom burden. Given major differences in global and regional differences, the content of the review will focus on a high-resource healthcare setting and clinical pathways from an Australian perspective.8–10
Methods
To undertake this narrative review, databases including MEDLINE (OvidSP) and EMBASE (OvidSP) were searched on the 23rd of September 2023 using the following relevant search terms: kidney failure, dialysis, innovations, human-centred care, patient-centred care, technology, patient priorities, patient choice, renal replacement therapy, home-based dialysis, and quality of life. Reference lists of review articles, grey literature, and clinical practice guidelines were also reviewed. A total of 246 articles were screened initially, with 143 articles reviewed in full and 121 included in this review. Studies were excluded if they could not be translated into English, did not focus on human-centred innovations, or if they were not relevant to the dialysis population.
Current Clinical Challenges of Managing Kidney Failure for the Dialysis Provider
Growth of the Chronic Dialysis Population in Australia and Western Sydney
The prevalence of kidney failure is increasing worldwide. In 2022, in Australia, 15,518 patients received chronic dialysis and 3311 new patients commenced dialysis for the first time.11 Within Australia, Western Sydney is a unique geographic cluster. It is characterised by one of the most rapidly growing regions in Australia with a population estimated to be 2.6 million people in 2022 and includes a patient population of significant cultural diversity (as ~60% are born outside Australia, with the top countries of birth being India, China, Lebanon, and South Korea).12 Furthermore, the median weekly personal income is approximately $80 lower than the Australian median weekly income.12 As of 2016, approximately 50,600 adults with chronic kidney disease (CKD) of any stage resided in Western Sydney, which accounts for 12% of the local population, higher than the national incidence of 10%.13 Furthermore, this region currently manages 903 chronic dialysis patients with less than half (43%) receiving a type of home-based dialysis treatment [32% (293 patients) receiving chronic peritoneal dialysis (PD); 11% (100 patients) receiving home HD; 20% (178 patients) receiving hospital HD and 37% (332 patients) receiving satellite HD].11 The annual incidence of kidney failure has been steadily increasing and was 232 new dialysis patients in 2022 compared to 201 in 2021 and between 175 and 201 annually between 2018 and 2021.11
Increasing Multimorbidity and Frailty in Kidney Failure Patients
Similar to the general population, the prevalence of multimorbidity and frailty has increased in chronic dialysis patients.5 Multimorbidity is defined as the presence of at least two significant comorbidities,14 with frailty being a clinical syndrome of increased vulnerability in the elderly population.15 In Australia, the presence of comorbid diabetes in patients commenced on dialysis has increased by 30% over the past 2 decades, with a relatively static presence of coronary artery disease, peripheral artery disease, chronic lung disease, or cerebrovascular disease.5 Recent large-scale cohort studies have shown that greater than 95% of patients with CKD have at least one significant comorbidity.16,17 Multimorbidity is inherently linked to frailty in the CKD population.14 As such, patients with CKD have high rates of frailty (up to 82%) that increase with age and duration of dialysis.14,18 Similar trends are demonstrated in the Australian population.19 Frailty is linked to heightened mortality risk and diminished quality of life. Consequently, managing patients becomes more intricate, demanding a delicate balance between optimising symptom relief and enhancing overall prognosis.18
Hub and Spoke Model of Chronic Dialysis Care Delivery
The model of chronic dialysis care in Australia has advanced gradually over the last 50 years20 and currently operates mainly through government-funded tertiary centres (underpinned by some private-partnerships), which serve as hubs for dialysis and transplantation services.8 These centres extend their reach through smaller facilities that provide satellite dialysis at varying distances, following a “hub and spoke” model.8 Within these centres, dedicated training units support patients in the use of both home HD and PD.8 The key advantages of the Australian system are the provision of universal government-funded healthcare to all dialysis patients8 and a high global ratio of nephrologists to dialysis patients (~10–13 per 1000 patients).21 These reasons likely explain why Australia is one of the nine countries where over 20% of the population opts for home-based dialysis modalities, and this percentage continues to rise.22–24 In contrast, the incidence of home-based dialysis was less than 15% in the United States (US), but this has increased over the last decade.24,25
Are Current Systems for Delivering Chronic Dialysis Services Optimal?
The best evidence-based approach for delivering chronic dialysis services is not known, and current methods may not be sustainable and/or fully integrated with other parts of the healthcare system.26 In the Western Sydney Renal Service (a speciality renal network, combining two metropolitan jurisdictions see Figure 1),27 dialysis services are delivered by three major (level 5–6) Hospitals (Westmead, Nepean, and Blacktown), consisting of three in-hospital HD units at each hospital; three public satellite HD units located on the grounds of Auburn, Blacktown, and Mount Druitt hospitals, and a private satellite HD unit at Norwest Hospital (satellite HD consists of patients with the ability to self-care).13 There are also two PD Units for training and service at Blacktown and Nepean hospitals; a single Home HD training unit is located at satellite unit of Blacktown Hospital; one pre-dialysis educator across the network; a newly established renal supportive care team with a renal physician and clinical nurse consultant, and one renal social worker and psychologist.
 |
Figure 1 This figure shows the geographic locations of the Local Health Districts within Sydney. Reproduced from © State of New South Wales NSW Ministry of Health under the Creative Commons Attribution 4.0 licence.27 The Western Renal Service comprising the Western Sydney Local Health District and the Nepean Blue Mountains Local Health District serve a population of 1.3M and a total area of 9959 square kilometres. |
Some of the current pressures on the effectiveness of dialysis service delivery in Australia include workforce capacity to provide pre-dialysis education and support chronic dialysis patients due to population growth in kidney failure;28 declining number of patients capable of undertaking home-based dialysis22 and infection control problems due to COVID-19.29 Policy changes to address these problems include employment of highly skilled nurse consultants;21,28 use of remote patient monitoring;30 encouraging family-assisted automated PD;31 and forward government planning.32
What are the Emerging Problems with Current Clinical Pathways?
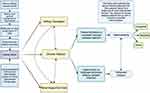
Figure 2 summarises the standard clinical pathways and transitions for patients with kidney failure, which includes three options, which are chronic dialysis, kidney transplantation, or conservative management without dialysis (renal supportive care). In Australia, the conventional clinical pathway for kidney failure involves referral from a general practitioner to a nephrologist with CKD to provide treatment advice to slow disease progression. This includes treatment of the underlying cause of CKD; control of blood pressure, diabetes, and proteinuria, with lifestyle interventions, treatment with renin angiotensin aldosterone inhibitors and consideration of SGLT2 inhibitor therapy.33 Typically, when the eGFR falls below 30 mL/min/1.73 m2, the nephrologist refers the patient for pre-dialysis education to enable patients to make an informed choice regarding dialysis modality or pre-emptive kidney transplantation (if a donor is available). This is followed by the surgical review for planning long-term chronic dialysis access (either arteriovenous fistula creation or PD catheter insertion).34
The standard clinical pathway to renal replacement therapy attempts to ensure that there is a smooth transition from pre-dialysis care to chronic dialysis, minimising the risk of acute hospitalisation and maintaining outpatient care and uptake of home-based chronic dialysis. However, over the last five years in Australia, there have been more patients deviating from this path, presenting to hospital late with kidney failure and thus requiring emergency and acute HD or PD. For example, in 2023, 23.6% of patients (39/165) patients in Western Sydney presented late, a notable increase from 2022, where only 8.2% (19/232) required acute dialysis.11 In addition, among those requiring acute dialysis in 2023, 25 out of 39 patients were from culturally diverse backgrounds, posing challenges in meeting individual care needs. The reasons for the increase are complex and poorly understood, but hypotheses include insufficient pre-dialysis educators together with reduced patient engagement with healthcare due to the pandemic and cultural barriers as well as misinformation.35–37 Additionally (similar to other countries), managing patients who are non-permanent residents (who do not have national insurance coverage) is an emerging challenge, adding to the financial burden of the dialysis process.38
What is the Financial Burden of Kidney Failure
Kidney disease disproportionately affects populations of lower socioeconomic status, and healthcare funding and infrastructure fall behind other medical specialties.6 For reasons that are not clear, this is despite kidney failure patients representing approximately 1% of the population but needing greater than 6% of national healthcare funding.6 The financial costs for treating kidney failure are significant and rising. Approximately 80% of the allocated expenditure attributed to patients with CKD in Australia was allocated to public hospital services.5 Furthermore, the rate of increase in expenditure for CKD patients is rising faster than the rate of general healthcare expenses.5 This high burden of public hospital expenditure is likely to be true for CKD patients in Western Sydney, with over 60% of this attributed to those with kidney failure on dialysis.5
The percentage of home-based dialysis carries significant implications for overall dialysis costs. Notably, PD and home HD in Australia cost approximately $15,000 to $20,000 less per patient annually compared to satellite or in-hospital HD (2009 prices: home HD $49,137, PD $53,112, satellite HD $65,315, in-hospital HD $79,072). Whilst these costs do not account for the underlying patient comorbidities that may necessitate someone to require in-hospital dialysis compared to a home-based modality, they demonstrate the significant economic benefits associated with widespread adoption of home-based dialysis modalities in those who are suitable.13
Current Experiences and Unmet Needs for the Dialysis User
From limited data available, a view of chronic dialysis patient needs and preferences to inform future innovation is emerging. In a mixed-methods study assessing the important characteristics of dialysis for pre-dialysis and dialysis patients across two dialysis units in Sydney, found that patient groups not only valued survival but also placed strong favour on the convenience of home-based dialysis, increased independence, and the opportunity for dialysis-free days.39 A system that better reflects these values therefore has the potential to improve patient morbidity and mortality. Additionally, such a system should also promote kidney transplantation to improve patient quality of life, patient survival, and cost-effectiveness.1 Based on this knowledge, several themes emerge:
Need for Focus on Symptom Management
Many dialysis patients place a greater emphasis on symptom management rather than survival or biochemical markers.40 Physical symptoms of fatigue and cramping and psychosocial symptoms of anxiety and depression were key areas that patients prioritised for future research.41 Furthermore, the 2023 recommendations from Kidney Disease: Improving Global Outcomes (KDIGO) emphasise the importance of integrating patient preferences into the decision-making process surrounding renal replacement therapy and underscore the significance of considering the impact of symptoms, not just the frequency.42 This highlights the increasing traction in the dialysis community on the importance of renal supportive care. Despite this, there has been a dearth of new interventions specifically addressing symptom management. The limited number of interventions is likely due to a lack of data on the importance of monitoring patient-reported outcomes across clinical trials.41
Increasing Barriers to Undertake Home-Based Dialysis
Chronic dialysis patients value their independence and the ability to do dialysis at home.41,43 Currently, only less than half of Western Sydney dialysis patients decide to undertake home-based chronic dialysis, which is below proposed national targets.44 The disinterest in home-based dialysis therapies may, in part, be due to insufficient resources for pre-dialysis education to overcome perceived fears, and the absence of culturally sensitive patient educational approaches and materials.44,45
With regard to PD, the reasons for the reduced uptake and technique survival of PD are complex and include inadequate patient education and/or social factors that impair the ability to self-care.46 An online survey in Australia suggested large variations in standardised curricula and competency assessments between different PD units.47 This could account for the marked difference in PD peritonitis rates between centres.48,49 Furthermore, the uptake of PD in patients residing in nursing homes is reduced46 despite evidence showing that training of nursing home staff is achievable with good patient outcomes.50 Internationally, varying administrative factors influence the uptake of PD, as in the US reimbursement per patient for HD is higher than PD,51,52 whereas in Australia and New Zealand, the cost of providing in-centre HD is ~1.5-fold higher than home-based therapies, and thus there is a preference for PD.53 Addressing these deficiencies will likely boost PD adoption and reduce complications, enabling patients to continue to perform dialysis at home. Similarly, despite home HD allowing for more intensive treatment protocols that improves patient uraemic symptoms,54 its reduced uptake may be related to patient discomfort at self-cannulation and perceived lack of support in troubleshooting problems.55 A key concern for HD patients was the lack of assistance at home.56 Furthermore, prior to the COVID-19 pandemic, there was a lag in the uptake of telehealth and remote monitoring to support home HD patients,57 potentially limiting the number of patients benefiting from it.
Patient Interest for Portable Dialysis Technology
Over the last decade, there has been increasing interest in portable dialysis devices to enhance key patient-centred outcomes like satisfaction and independence.1,58 Progress has been hampered by limited funding for research that has impeded translation to clinical practice.6 Qualitative studies have identified patient preferences for such a device, which include heightened flexibility and independence. For example, one of the goals for haemodialysis patients was to travel internationally without issue.59 Also, patients wanted such devices to be simple to operate and compact, ideally worn like a vest.60 Currently, a multi-centre Kidney Health Initiative mixed methods study is underway to establish patient preferences for a device.61 This study will assist in the widespread development of portable dialysis technology.
As summarised in Figure 3 these factors (rapid population growth, cultural diversity, financial disadvantage, sub-optimal healthcare literacy, frailty, multimorbidity, late presentation) have all led to a significant imbalance between optimal healthcare service delivery and patient satisfaction.
Human-Centred Care as a Model to Address Unmet Needs in Chronic Dialysis Care
The negative impacts of dialysis on quality of life and the recognition of patient needs, as well as cost of managing complex care, have led to a push for innovations in dialysis delivery.62 As noted earlier, key patient priorities include independence, the ability to do dialysis at home and symptom control.41,43,63 Recent studies have shown that chronic dialysis patients prioritise the management of fatigue, pain, and optimisation of mental health.64 Therefore, the main ways in which dialysis delivery could be improved involve prioritising home-based dialysis, either through changes in dialysis pathways or using emerging technologies that allow for portable dialysis. Furthermore, there is an increasing trend towards incorporating symptom recognition and management in healthcare protocols.
Over the last decade, the focus on human-centred care (or patient-centred care) has been a paradigm shift in healthcare.65 It is defined as an approach to the planning, delivery, and evaluation of healthcare (systems, devices, and services) that places the needs of patients and families as the prime’ end-user.66 It was conceptualised with the aim of improving patient-relevant outcomes within healthcare systems.66 This approach entails placing patients and families at the core of organisational processes and transforming existing facilities and services to integrate patient viewpoints, which includes increasing the “usability” with innovative technology.7 Studies have suggested that this approach improves patient satisfaction,67 may improve adherence to treatment,68 reduces inpatient mortality,69 with shorter hospital stays70,71 and lower healthcare costs.72
In response to the mismatches between patient needs and the current dialysis system, efforts are underway to develop human-led innovations to disrupt the standard clinical dialysis pathways. These include both new technologies and enhancing the identification and management of distressing chronic and disabling symptoms, which are prioritised by patients (and have been overlooked historically by healthcare providers). For descriptive purposes, we have categorised these either as (i) incremental innovations (that facilitate current clinical pathways) or (ii) disruptive innovations (that radically change current clinical pathways). While these advances do not address all the unmet needs of the dialysis provider or the end-user, they emphasise a human-centred approach to solving problems by putting the needs of the patients as the priority.
Incremental Advances in Human-Centred Dialysis Care
Over the last decade, several incremental changes to the standard dialysis pathways have been implemented. These include renal supportive care pathways; improved home-based support structures; and hybrid/incremental dialysis or PD/HD to improve the uptake of home HD or PD.
Renal Supportive Care and Optimisation of Symptom Management
Without doubt, the most important human-centred advance in chronic dialysis patients has been the introduction of renal supportive care. Since 2013, Australian renal units have embedded these pathways into routine nephrology clinical practice (described in detail in previous reviews).73,74 It is defined as holistic, multi-disciplinary patient-centred approaches that combine the principles of palliative care with general symptoms experienced by patients with kidney failure.73,74 In a prospective observational cohort study of 127 chronic dialysis patients referred to renal supportive care clinics, there were significant improvements over time in depression, some symptoms (lack of energy, insomnia, itching, pain, and skin changes) but without change in dialysis delivery.75 In addition, renal supportive care programs are highly favoured and provide empowerment for patients and their families with healthcare decisions,76 and significantly reduce the number and length of hospitalisations.77 Four multicentre clinical trials that aim to provide further high-quality evidence on the role of renal supportive care in chronic HD patients, are currently in progress. These are summarised in Table 1.
 |
Table 1 Current Clinical Trials Addressing Symptom Management in Haemodialysis Patients |
Home-Assisted Dialysis
Home-assisted dialysis, where nursing and clinical support is provided to home HD patients, is gaining traction. In Western Sydney, home HD patients historically have received support from both senior nurse practitioners and nurse consultants, who troubleshoot problems ranging from cannulation to issues with the dialysis prescription. In addition, over the last 10 years, services affiliated with hospitals or independent accredited national healthcare providers (such as Dialysis Australia) provide home nursing support to enable patients them to undertake HD or automated PD at home.82,83 Internationally, home haemodialysis has been made more accessible through increased telemonitoring with regular input from nursing staff, and upgrades in technology allowing for more efficient storage.84 This type of active nurse-assisted home dialysis program addresses patient priorities whilst maintaining the mortality benefits of home dialysis therapies with significant health-economic advantages.83,85,86
Other models of care include the empowered in-centre units where patients have an active role in their haemodialysis treatment while benefiting from the safety net provided by the centre.87,88 In addition, in the US, home-assisted dialysis is being formalised through the creation of transitional care units (TCUs), which offer a comprehensive 4-week educational and decision support program to patients newly initiated on dialysis.89 A retrospective cohort study of 724 patients initiating dialysis across 48 TCUs compared to 2892 matched controls with no TCU history showed that patients initiating dialysis in a TCU were significantly more likely to utilise home dialysis and more likely to be referred for a kidney transplant at 14 months.90
Incremental and Hybrid Dialysis
There is renewed interest in incremental dialysis, which is defined as HD administered less than thrice weekly or limiting dialysis prescription by duration to allow for a more gradual transition.91 This approach addresses patient concerns about the potential loss of independence and ability to work when adhering to a conventional haemodialysis regimen. A recent Australian study among individuals who were either presently undergoing HD or on the verge of starting demonstrated that all 26 participants preferred an incremental approach to HD. Their foremost priorities were about quality of life and preserving residual kidney function.92 Similarly, patients with failing PD do not necessarily need to be transitioned immediately to HD but rather had a hybrid model of PD and HD can be utilised.93 This strategy was employed more frequently during the COVID-19 pandemic where patients with hybrid PD/HD needed in-hospital HD only 1–2 times per week rather than the usual three when completely transitioned from PD to HD.94 Several studies have shown that combined therapy provides the improved dialysis adequacy that HD offers whilst still optimising patient quality of life with the home-based advantage of PD.95–97 The recognition of incremental and hybrid dialysis as being important modalities to address patient needs has led to their integration in nephrology education programs.98
Refinements to Dialysis Technology
Several refinements to dialysis membranes and HD methods may improve symptom control and survival. Until recently, progress to advance dialysis membranes has been slow.99 The key limitations are suboptimal removal of uraemic toxins and a non-continuous function when compared to natural kidneys.99 The use of mixed matrix membranes, which contain polyethersulfone, polyvinylpyrrolidone, and activated carbon, may allow for increased clearance of uraemic toxins. These membranes may also have improved haemocompatibility, potentially allowing for continuous kidney replacement therapy without an increased risk of clotting or infection.100 Enhanced toxin removal could improve patient symptoms and significantly enhance the quality of life for dialysis patients.101
Similarly, haemodiafiltration (HDF), as a concept, has been around for decades and combines the principle of diffusion for solute removal in traditional HD with convective solute removal in haemofiltration.102 Registry studies and clinical trials have demonstrated the potential for HDF in reducing all-cause mortality103,104 and improving intra-dialytic patient symptoms such as cramping and hypotension.105 Despite these promising findings, concerns about participant attrition during follow-up in these trails may have hindered widespread HDF adoption outside of Europe and Japan.106 This is despite an individual-participant meta-analysis of four randomised controlled trials showing a reduction in all-cause mortality and cardiovascular mortality with high-dose HDF compared to HD.107 Recently, the landmark CONVINCE study, a randomised-controlled trial of 1360 patients randomised to high-dose HDF and HD, demonstrated an absolute risk reduction in mortality of 4.6% (HR 0.77, 95% CI: 0.65–0.93).108 The anticipation is that the outcomes of the CONVINCE study will serve as a catalyst, paving the way for the widespread adoption of HDF. A pivotal step in this trajectory involves seeking Food and Drug Administration (FDA) approval for online generation HDF, ultimately facilitating the transition of HD centres to incorporate HDF equipment.109
Disruptive Advances in Human-Centred Dialysis Care
Radical changes to dialysis technology may in the future lead to major disruption to the traditional models of care for kidney failure. Current medical advancements in this area centre around developing portable and/or wearable dialysis devices; establishing implantable “bioartificial kidneys” without immunosuppression; and artificial intelligence. The need for social distancing during the COVID-19 pandemic created an aggressive push for such technological innovations.110
Portable/Wearable Dialysis Devices
Several groups are evaluating the feasibility of wearable or portable dialysis devices (both HD and PD) for the treatment of kidney failure. Gura et al piloted a portable HD device that could be worn as a belt over 24 hours and found that the five patients who completed the study had effective solute clearance and maintenance of fluid haemostasis without any adverse effects.111 A similar belt-like device that instead regenerated PD fluid (using a modified sorbent system with a single lumen PD catheter) was tested in 15 PD patients for 72 hours. Whilst 60% of patients complained of abdominal pain, there was optimal fluid removal and reductions in urea, creatinine, and beta2-microglobulin.112 Despite these pilot studies showing the proof-of-concept for portable or wearable dialysis devices, large-scale clinical trials have yet to be conducted.113 The challenges limiting wide-spread development have included the need to enable continuous regeneration of a small volume dialysate, miniaturisation with current devices being around 10–30 kg and the optimisation of toxin removal.114
Development of Bioartificial Implantable Kidneys
“kidneys” that replicate the metabolic and endocrine activity of kidneys could in the future replace the need for standard thrice weekly haemodialysis. The bioartificial kidney consists of renal tubular cells cultured on a membrane, which is then surgically implanted and connected to the urinary bladder. Initial devices had problems with membrane storage due to cellular degradation, and device rejection by the animal’s immune system.115 In 2023, a bioartificial kidney system created with tubular cells cultured on silicon nanopore membranes was implanted into pigs, with minimal cellular damage despite no systemic anticoagulation or immunosuppression.116 Consequently, patient studies are likely to follow.
Artificial Intelligence in Dialysis Care
Artificial intelligence (AI) holds the promise of empowering clinicians to deliver highly personalised care to their patients. Current studies have shown that AI could help to ensure optimum haemoglobin by recommending suitable erythropoietin-stimulating agents based on the patient profile117 or predict the risk of intradialytic hypotension by considering patient clinical factors.118 Furthermore, a recent cross-sectional study of 195 patients showed that patients rated AI responses to questions higher in terms of quality and empathy than physicians,119 which could potentially pave the way for the use of AI in pre-dialysis education. In the future, there could be a system that integrates multiple facets of a patient’s clinical and social situation into a thorough management plan. Key issues that slow the adoption of AI in medicine include concerns with data privacy and security; issues regarding maintaining transparency and accountability of decision-making; and the formidable task of attaining the requisite computing power for timely processing of healthcare data.120
Human-Centred Care to Address Problems Faced by Dialysis Providers
In Australia, government funding of the latest evidence-based innovations is required to change the healthcare practice in dialysis patients. The substantial economic impact of dialysis and CKD underscores the necessity for government priorities to be strategically aligned with the efficiency and cost-effectiveness of dialysis systems. In the US, this is often through a singular focus on in-centre HD due to corporate ownership of dialysis units.121 However, it is expected that human-centred care will improve patient outcomes and may also alleviate the economic burden of kidney failure. For example, a nurse-assisted home dialysis program in the Middle East offered economic benefits with a 27% reduction in costs.86 This will render the adoption of innovative changes in dialysis technology and delivery not only feasible but highly advantageous to healthcare providers.
Conclusion
Human-centred innovations in dialysis care offer a transformative solution to enhance outcomes for all stakeholders in all regions. By understanding and addressing patient needs, integration of new technology and the implementation of system changes are expected to improve outcomes, and this will need to be evaluated in future studies. Whilst these innovations have thus far been reviewed in high resource healthcare settings, the general principles of human-centred care (based on the needs of a population in a specific region) are applicable to all countries. As would be expected, the means of delivering innovations inherently vary between settings dependent on the healthcare system and available resources. After years of slow progress, these human-led developments could lead to new systems that could radically change how kidney failure is treated. Furthermore, these patient-led initiatives should serve to alleviate the economic burden of kidney failure. This may pave the way for a future where the quality of life for dialysis patients matches that of transplant recipients, or even a future without the need for dialysis altogether. Clearly, these changes will need to be based on rigorous and robust practice-changing clinical trials.
Acknowledgments
The authors thank Frederika Sciberras (Data manager, Department of Renal Medicine, Westmead Hospital) for assistance with obtaining data regarding dialysis modalities in the Western Renal Service.
Disclosure
Dr Gopala Rangan reports Danone Research, recipient of investigator initiated grant (2015–2024) Otsuka Australia, recipient of investigator initiated grant (2019). The authors report no other conflicts of interest in this work.
References
1. Bonventre JV, Hurst FP, West M, Wu I, Roy-Chaudhury P, Sheldon M. A technology roadmap for innovative approaches to kidney replacement therapies: a catalyst for change. Clin J Am Soc Nephrol. 2019;14(10):1539–1547. doi:10.2215/CJN.02570319
2. Wyld M, Morton RL, Hayen A, Howard K, Webster AC, Turner N. A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLoS Med. 2012;9(9):e1001307. doi:10.1371/journal.pmed.1001307
3. Johnson JP, McCauley CR, Copley JB. The quality of life of hemodialysis and transplant patients. Kidney Int. 1982;22(3):286–291. doi:10.1038/ki.1982.167
4. Tonelli M, Wiebe N, Knoll G, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11(10):2093–2109. doi:10.1111/j.1600-6143.2011.03686.x
5. Australian Institute of Health and Welfare (AIHW). Chronic kidney disease: Australian facts; 2023. Available from: https://www.aihw.gov.au/reports/chronic-kidney-disease/chronic-kidney-disease.
6. Linde PG, Archdeacon P, Breyer MD, et al. Overcoming barriers in kidney health—forging a platform for innovation. J Am Soc Nephrol. 2016;27(7):1902–1910. doi:10.1681/ASN.2015090976
7. Australian Commission on Safety and Quality in Health Care (ACSQHC). Partnering with Consumers Case Study: Royal Prince Alfred Hospital and Sydney Local Health District; 2021. Available from: https://www.safetyandquality.gov.au/sites/default/files/2021-10/partnering_with_consumers_case_study_royal_prince_alfred_hospital_and_sydney_local_health_district.pdf.
8. Damasiewicz MJ, Polkinghorne KR. Global dialysis perspective: Australia. Kidney360. 2020;1(1):48–51. doi:10.34067/KID.0000112019
9. Corcoran RM. Maintenance dialysis: the Western Sydney experience. Commun Health Stud. 1980;4(3):188–193. doi:10.1111/j.1753-6405.1980.tb00296.x
10. Cooper BA, Branley P, Bulfone L, et al. The Initiating Dialysis Early and Late (IDEAL) study: study rationale and design. Perit Dial Int. 2004;24(2):176–181. doi:10.1177/089686080402400209
11. Australian and New Zealand Dialysis and Transplant Registry (ANZDATA). 46th Annual Report: summary of data collected to December 31st, 2022; 2023. Available from: https://www.anzdata.org.au/report/anzdata-46th-annual-report-2023-data-to-2022/.
12. Australian Bureau of Statistics (ABS). Census 2021; 2023. Available from: https://www.abs.gov.au/census.
13. Kidney Health Australia (KHA). State of the Nation - 2016 Kidney Health Week; 2016. Available from: https://kidney.org.au/uploads/resources/state-of-the-nation-2015.pdf.
14. Kennard A, Glasgow N, Rainsford S, Talaulikar G. Frailty in chronic kidney disease: challenges in nephrology practice. A review of current literature. Internal Med J. 2023;53(4):465–472. doi:10.1111/imj.15759
15. Xue QL. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27(1):1–15. doi:10.1016/j.cger.2010.08.009
16. Fraser SDS, Roderick PJ, May CR, et al. Correction to: the burden of comorbidity in people with chronic kidney disease stage 3: a cohort study. BMC Nephrol. 2020;21(1):543. doi:10.1186/s12882-020-02205-w
17. Clare M, Stewart WM, Bruce G, David H. Comorbidity in chronic kidney disease: a large cross-sectional study of prevalence in Scottish primary care. Br J Gen Pract. 2021;71(704):e243. doi:10.3399/bjgp20X714125
18. Chowdhury R, Peel NM, Krosch M, Hubbard RE. Frailty and chronic kidney disease: a systematic review. Arch Gerontol Geriatrics. 2017;68:135–142. doi:10.1016/j.archger.2016.10.007
19. Hubbard RE, Peel NM, Smith M, et al. Feasibility and construct validity of a Frailty index for patients with chronic kidney disease. Australas J Ageing. 2015;34(3):E9–E12. doi:10.1111/ajag.12231
20. George CR. History of home haemodialysis in Australia. Nephrology. 2005;10(3):215–221. doi:10.1111/j.1440-1797.2005.00390.x
21. Sharif MU, Elsayed ME, Stack AG. The global nephrology workforce: emerging threats and potential solutions! Clin Kidney J. 2016;9(1):11–22. doi:10.1093/ckj/sfv111
22. Agar JWM, Hawley CM, George CRP, Mathew TH, McDonald SP, Kerr PG. Home haemodialysis in Australia - is the wheel turning full circle? Med j Aust. 2010;192(7):403–406. doi:10.5694/j.1326-5377.2010.tb03565.x
23. Mendu ML, Divino-Filho JC, Vanholder R, et al. Expanding utilization of home dialysis: an action agenda from the First International Home Dialysis Roundtable. Kidney Med. 2021;3(4):635–643. doi:10.1016/j.xkme.2021.04.004
24. Wilkie M. Home dialysis—an international perspective. NDT Plus. 2011;4(suppl_3):iii4–iii6. doi:10.1093/ndtplus/sfr129
25. United States Renal Data System. 2023 USRDS Annual Data Report: epidemiology of kidney disease in the United States; 2023. Available from: https://adr.usrds.org/2023.
26. Apel C, Hornig C, Maddux FW, Ketchersid T, Yeung J, Guinsburg A. Informed decision-making in delivery of dialysis: combining clinical outcomes with sustainability. Clin Kidney J. 2021;14(Suppl 4):i98–i113. doi:10.1093/ckj/sfab193
27. NSW Health. Metropolitan Local Health Districts; 2023, Available from: https://www.health.nsw.gov.au/lhd/Pages/default.aspx.
28. Riaz P, Caskey F, McIsaac M, et al. Workforce capacity for the care of patients with kidney failure across world countries and regions. BMJ Glob Health. 2021;6(1):e004014. doi:10.1136/bmjgh-2020-004014
29. Polkinghorne KR, Kerr PG, Boudville N. Response to COVID-19 infection in hemodialysis patients: an Australian Perspective. Kidney360. 2020;1(8):829–833. doi:10.34067/KID.0002492020
30. Liu N, Kim J, Jung Y, et al. Remote monitoring systems for chronic patients on home hemodialysis: field test of a copresence-enhanced design. JMIR Hum Fact. 2017;4(3):e21. doi:10.2196/humanfactors.7078
31. Baumgart A, Manera KE, Johnson DW, et al. Meaning of empowerment in peritoneal dialysis: focus groups with patients and caregivers. Nephrol Dial Transplant. 2020;35(11):1949–1958. doi:10.1093/ndt/gfaa127
32. Dominello A, Howell M, Craig JC, et al. Equity in national policies for Australians with kidney disease. Aust N Z J Public Health. 2021;45(4):370–375. doi:10.1111/1753-6405.13096
33. Kidney Health Australia. Chronic Kidney Disease (CKD) Management; 2020. Available from: https://kidney.org.au/uploads/resources/CKD-Management-in-Primary-Care_handbook_2020.1.pdf.
34. Johnson D. When to refer for specialist renal care. Caring for Australia and and New Zealanders with Kidney Impairment; 2012.
35. Rizzolo K, Gonzalez Jauregui R, Barrientos I, et al. Barriers and facilitators to home dialysis among latinx patients with kidney disease. JAMA Network Open. 2023;6(8):e2328944–e2328944. doi:10.1001/jamanetworkopen.2023.28944
36. Schulz PJ, Nakamoto K. The perils of misinformation: when health literacy goes awry. Nat Rev Nephrol. 2022;18(3):135–136. doi:10.1038/s41581-021-00534-z
37. Smith M, e Silva VS, Schick-Makaroff K, et al. Furthering cultural safety in kidney care within indigenous communities: a systematic and narrative review. Kidney Med. 2021;3(6):896–904. doi:10.1016/j.xkme.2021.04.023
38. Rizzolo K, Feldman KE, Dubey M, Powe NR, Cervantes L. The absence of state-wide policy on providing standard dialysis care for undocumented immigrants: challenges and opportunities. Clin J Am Soc Nephrol. 2023;19(1):98–100. doi:10.2215/CJN.0000000000000308
39. Morton RL, Tong A, Webster AC, Snelling P, Howard K. Characteristics of dialysis important to patients and family caregivers: a mixed methods approach. Nephrol Dial Transplant. 2011;26(12):4038–4046. doi:10.1093/ndt/gfr177
40. Manns B, Hemmelgarn B, Lillie E, et al. Setting research priorities for patients on or nearing dialysis. Clin J Am Soc Nephrol. 2014;9(10):1813–1821. doi:10.2215/CJN.01610214
41. Flythe JE, Hilliard T, Castillo G, et al. Symptom prioritization among adults receiving In-center hemodialysis: a mixed methods study. Clin J Am Soc Nephrol. 2018;13(5):735–745. doi:10.2215/CJN.10850917
42. Mehrotra R, Davison SN, Farrington K, et al. Managing the symptom burden associated with maintenance dialysis: conclusions from a Kidney Disease: improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2023;104(3):441–454. doi:10.1016/j.kint.2023.05.019
43. Weisbord SD. Patient-centered dialysis care: depression, pain, and quality of life. Semin Dial. 2016;29(2):158–164. doi:10.1111/sdi.12464
44. Liu FX, Gao X, Inglese G, Chuengsaman P, Pecoits-Filho R, Yu A. A global overview of the impact of peritoneal dialysis first or favored policies: an opinion. Peritoneal Dialysis Int. 2015;35(4):406–420. doi:10.3747/pdi.2013.00204
45. Jeffrey B, Smolonogov T, Kairaitis L. Influence of timing and patient characteristics on outcomes of a comprehensive pre-dialysis programme in Western Sydney.
46. Mudge DW, Boudville N, Brown F, et al. Peritoneal dialysis practice in Australia and New Zealand: a call to sustain the action. Nephrology. 2016;21(7):535–546. doi:10.1111/nep.12731
47. Boudville N, Cho Y, Equinox KL, et al. Teaching peritoneal dialysis in Australia: an opportunity for improvement. Nephrology. 2018;23(3):259–263. doi:10.1111/nep.12992
48. Htay H, Cho Y, Pascoe EM, et al. Center effects and peritoneal dialysis peritonitis outcomes: analysis of a National Registry. Am J Kidney Dis. 2018;71(6):814–821. doi:10.1053/j.ajkd.2017.10.017
49. Jose MD, Johnson DW, Mudge DW, et al. Peritoneal dialysis practice in Australia and New Zealand: a call to action. Nephrology. 2011;16(1):19–29. doi:10.1111/j.1440-1797.2010.01390.x
50. Taskapan H, Tam P, LeBlanc D, et al. Peritoneal dialysis in the nursing home. Int Urol Nephrol. 2010;42(2):545–551. doi:10.1007/s11255-010-9714-y
51. Bethesda M. US renal data system, USRDS annual data report. Atlas Chronic Kidney Dis End-Stage Ren Dis United States Natl Institutes Heal Natl Inst Diabetes Dig Kidney Dis; 2018.
52. Mehrotra R, Khawar O, Duong U, et al. Ownership patterns of dialysis units and peritoneal dialysis in the United States: utilization and Outcomes. Am J Kidney Dis. 2009;54(2):289–298. doi:10.1053/j.ajkd.2009.01.262
53. Gorham G, Howard K, Zhao Y, et al. Cost of dialysis therapies in rural and remote Australia – a micro-costing analysis. BMC Nephrol. 2019;20(1):231. doi:10.1186/s12882-019-1421-z
54. Mathew A, McLeggon J-A, Mehta N, et al. Mortality and hospitalizations in intensive dialysis: a systematic review and meta-analysis. Can J Kidney Health Dis. 2018;5:2054358117749531. doi:10.1177/2054358117749531
55. Morita PP, Huynh K, Zakir A, et al. Supporting the establishment of new home dialysis programs through the explore home dialysis program. Kidney Int Rep. 2019;4(2):293–300. doi:10.1016/j.ekir.2018.10.019
56. Aydede SK, Komenda P, Djurdjev O, Levin A. Chronic kidney disease and support provided by home care services: a systematic review. BMC Nephrol. 2014;15(1):118. doi:10.1186/1471-2369-15-118
57. Rosner MH, Lew SQ, Conway P, et al. Perspectives from the Kidney Health Initiative on Advancing Technologies to facilitate remote monitoring of patient self-care in RRT. Clin J Am Soc Nephrol. 2017;12(11):1900–1909. doi:10.2215/CJN.12781216
58. Davenport A. Portable and wearable dialysis devices for the treatment of patients with end-stage kidney failure: wishful thinking or just over the horizon? Pediatr Nephrol. 2015;30(12):2053–2060. doi:10.1007/s00467-014-2968-3
59. Anna Jónsdóttir A, Firestone S, Kessler L, Kim J-E. Human factors considerations in designing a portable dialysis device: understanding patients’ and care partners’ needs for increased mobility. Proceed Human Fact Ergon Soc Ann Meet. 2021;65(1):520–524. doi:10.1177/1071181321651151
60. Jónsdóttir AA, Kessler LG, Rim S-Y, Kim J-E. What patients and care partners want in a wearable dialysis device: a mixed-methods study. IISE Trans Healthc Syst Eng. 2022;12(2):101–110. doi:10.1080/24725579.2021.1958273
61. Flythe JE, Forfang D, Gedney N, et al. Development of a Patient Preference Survey for wearable kidney replacement therapy devices. Kidney360. 2022;3(7):1197–1209. doi:10.34067/KID.0001862022
62. Gedney N, Sipma W, Sondergaard H. Innovations in dialysis: the user’s perspective. Nat Rev Nephrol. 2020;16(10):544–545. doi:10.1038/s41581-020-0292-7
63. Dorough A, Forfang D, Mold JW, Kshirsagar AV, DeWalt DA, Flythe JE. A person-centered interdisciplinary plan-of-care program for dialysis: implementation and preliminary testing. Kidney Med. 2021;3(2):193–205.e1. doi:10.1016/j.xkme.2020.11.010
64. González AM, Gutman T, Lopez-Vargas P, et al. Patient and caregiver priorities for outcomes in CKD: a Multinational Nominal Group Technique Study. Am J Kidney Dis. 2020;76(5):679–689. doi:10.1053/j.ajkd.2020.03.022
65. Melles M, Albayrak A, Goossens R. Innovating health care: key characteristics of human-centered design. Int J Qual Health Care. 2021;33(Supplement_1):37–44. doi:10.1093/intqhc/mzaa127
66. Australian Commission on Safety and Quality in Health Care (ACSQHC). Patient-centred care: improving quality and safety by focusing care on patients and consumers; 2011. Available from: https://www.safetyandquality.gov.au/sites/default/files/migrated/PCC_Paper_August.pdf.
67. Beach MC, Sugarman J, Johnson RL, Arbelaez JJ, Duggan PS, Cooper LA. Do patients treated with dignity report higher satisfaction, adherence, and receipt of preventive care? Ann Fam Med. 2005;3(4):1544–1717. Electronic.
68. Arbuthnott A, Sharpe D. The effect of physician–patient collaboration on patient adherence in non-psychiatric medicine. Patient Educ Couns. 2009;77(1):60–67. doi:10.1016/j.pec.2009.03.022
69. Glickman SW, Boulding W, Manary M, et al. Patient satisfaction and its relationship with clinical quality and inpatient mortality in acute myocardial infarction. Circulation. 2010;3(2):188–195. doi:10.1161/CIRCOUTCOMES.109.900597
70. Stone S. A retrospective evaluation of the impact of the planetree patient-centered model of care on inpatient quality outcomes. HERD. 2008;1(4):55–69. doi:10.1177/193758670800100406
71. Sweeney L, Halpert A, Fau - Waranoff J, Waranoff J. Patient-centered management of complex patients can reduce costs without shortening life. Am J Manag Care. 2007;13(2):1936–2692.
72. Charmel PA, Frampton SB. Building the business case for patient-centered care. Healthc Financ Manag. 2008;62(3):0732–0735.
73. Crail S, Walker R, Brown M; Renal Supportive Care working g. Renal supportive and palliative care: position statement. Nephrology. 2013;18(6):393–400. doi:10.1111/nep.12064
74. So S, Brennan FP, Li KC, Brown MA. End-stage kidney disease: the last 12 months. Aust J Gen Pract. 2021;50(4):193–198. doi:10.31128/AJGP-11-20-5736
75. Siriwardana AN, Hoffman AT, Brennan FP, Li K, Brown MA. Impact of renal supportive care on symptom burden in dialysis patients: a Prospective Observational Cohort Study. J Pain Symptom Manage. 2020;60(4):725–736. doi:10.1016/j.jpainsymman.2020.04.030
76. Sobels E, Best M, Chadban S, Pais R. End stage kidney disease patient experiences of renal supportive care in an Australian Teaching Hospital - a qualitative study. J Pain Symptom Manage. 2022;63(5):737–746. doi:10.1016/j.jpainsymman.2021.12.024
77. Chia XX, Johnston R, Aggarwal R, et al. Renal supportive care programs: an observational study assessing impact on hospitalization and survival outcomes. Nephrology. 2021;26(6):522–529. doi:10.1111/nep.13869
78. Roumelioti M-E, Steel JL, Yabes J, et al. Rationale and design of technology assisted stepped collaborative care intervention to improve patient-centered outcomes in hemodialysis patients (TĀCcare trial). Contemp Clin Trials. 2018;73:81–91. doi:10.1016/j.cct.2018.09.002
79. Johnson JA, Al Sayah F, Buzinski R, et al. A cluster randomized controlled trial for the Evaluation of routinely Measured PATient reported outcomes in HemodialYsis care (EMPATHY): a study protocol. BMC Health Serv Res. 2020;20(1):731. doi:10.1186/s12913-020-05557-z
80. Unruh M, Cukor D, Rue T, et al. Sleep-HD trial: short and long-term effectiveness of existing insomnia therapies for patients undergoing hemodialysis. BMC Nephrol. 2020;21(1):443. doi:10.1186/s12882-020-02107-x
81. Greenham L, Bennett PN, Dansie K, et al. The Symptom Monitoring with Feedback Trial (SWIFT): protocol for a registry-based cluster randomised controlled trial in haemodialysis. Trials. 2022;23(1):419. doi:10.1186/s13063-022-06355-0
82. Australia D. Home dialysis; 2023. https://www.dialysisaustralia.com.au/home-dialysis.html.
83. Nel H, Debbie F, Narelle H, Sean R, Aron C. A retrospective clinical and economic analysis of an assisted automated peritoneal dialysis programme in Western Australia. Perit Dial Int. 2023;8968608231190772. doi:10.1177/08968608231190772
84. Gautier N, Ficheux M, Henri P, Lanot A, Béchade C, Allard B. Innovations en hémodialyse à domicile: innovations in home hemodialysis. Néphrologie Thérapeutique. 2022;18(5, Supplement 1):5S2–5S11. doi:10.1016/S1769-7255(23)00004-4
85. Gogoi S. Nurse-assisted home hemodialysis with NxStage system one portable machine: an Experience in the Gulf Cooperation Council (GCC); 2023.
86. Hamad A, Mohamed M, Elshirbeny M, et al. Assisted home hemodialysis: a game-changer for patients and providers; 2023.
87. Australian Commission on Safety and Quality in Health Care (ACSQHC). Review of key attributes of high-performing person-centred healthcare organisations; 2018. Available from: https://www.safetyandquality.gov.au/sites/default/files/migrated/FINAL-REPORT-Attributes-of-person-centred-healthcare-organisations-2018.pdf.
88. Kalantar-Zadeh K, Pk-t L, Tantisattamo E, et al. Living well with kidney disease by patient and care-partner empowerment: kidney health for everyone everywhere. J Nephrol. 2021;34(2):381–388. doi:10.1007/s40620-021-01000-6
89. Morfín JA, Yang A, Wang E, Schiller B. Transitional dialysis care units: a new approach to increase home dialysis modality uptake and patient outcomes. Semin Dial. 2018;31(1):82–87. doi:10.1111/sdi.12651
90. Blankenship DM, Usvyat L, Kraus MA, et al. Assessing the impact of transitional care units on dialysis patient outcomes: a multicenter, propensity score-matched analysis. Hemodial Int. 2023;27(2):1542–4758. Electronic. doi:10.1111/hdi.13068
91. Soi V, Faber MD, Paul R. Incremental hemodialysis: what we know so far. Int J Nephrol Renovasc Dis. 2022;15:161–172. doi:10.2147/IJNRD.S286947
92. Hegerty K, Jaure A, Scholes-Robertson N, et al. Australian Workshops on patients’ perspectives on hemodialysis and incremental start. Kidney Int Rep. 2023;8(3):478–488. doi:10.1016/j.ekir.2022.11.012
93. Murashima M, Hamano T, Abe M, Masakane I. Combination of once-weekly haemodialysis with peritoneal dialysis is associated with lower mortality compared with peritoneal dialysis alone: a longitudinal study. Clin Kidney J. 2021;14(6):1610–1617. doi:10.1093/ckj/sfaa173
94. Giacomo M, Gaetano A, Francesco F, Riccardo M. Hybrid dialysis: a promising strategy to reduce hospital access during the SARS-CoV-2 pandemic. BMJ Case Rep. 2020;13(10):e236411. doi:10.1136/bcr-2020-236411
95. Chung M-C, T-m Y, M-j W, et al. Is combined peritoneal dialysis and hemodialysis redundant? A nationwide study from Taiwan. BMC Nephrol. 2020;21(1):348. doi:10.1186/s12882-020-01989-1
96. Watanabe Y, Okada H. Effect of combined peritoneal dialysis and hemodialysis on health-related quality of life. Recent Adv Dialysis Therap Japan. 2018. doi:10.1159/000485713
97. Maruyama Y, Yokoyama K, Nakayama M, et al. Combined therapy with peritoneal dialysis and hemodialysis: a multicenter retrospective observational cohort study in Japan. Blood Purificat. 2014;38(2):149–153. doi:10.1159/000368389
98. American Society of Nephrology. End-Stage Kidney Disease: NepSAP. Vol. 22. American Society of Nephrology; 2023. Available from: https://nephsap.org/view/journals/nephsap/22/2/nephsap.22.issue-2.xml. Accessed March 19, 2024.
99. Geremia I, Stamatialis D. Innovations in dialysis membranes for improved kidney replacement therapy. Nat Rev Nephrol. 2020;16(10):550–551. doi:10.1038/s41581-020-0293-6
100. Geremia I, Bansal R, Stamatialis D. In vitro assessment of mixed matrix hemodialysis membrane for achieving endotoxin-free dialysate combined with high removal of uremic toxins from human plasma. Acta Biomater. 2019;90:100–111. doi:10.1016/j.actbio.2019.04.009
101. Robinson BM, Akizawa T, Jager KJ, Kerr PG, Saran R, Pisoni RL. Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet. 2016;388(10041):294–306. doi:10.1016/S0140-6736(16)30448-2
102. Ronco C, Cruz D. Hemodiafiltration history, technology, and clinical results. Adv Chronic Kidney Dis. 2007;14(3):231–243. doi:10.1053/j.ackd.2007.04.002
103. Mercadal L, Franck J-E, Metzger M, et al. Hemodiafiltration versus hemodialysis and survival in patients with ESRD: the French Renal Epidemiology and Information Network (REIN) Registry. Am J Kidney Dis. 2016;68(2):247–255. doi:10.1053/j.ajkd.2015.11.016
104. Ok E, Asci G, Toz H, et al. Mortality and cardiovascular events in online haemodiafiltration (OL-HDF) compared with high-flux dialysis: results from the Turkish OL-HDF Study. Nephrol Dial Transplant. 2013;28(1):192–202. doi:10.1093/ndt/gfs407
105. Morena M, Jaussent A, Chalabi L, et al. Treatment tolerance and patient-reported outcomes favor online hemodiafiltration compared to high-flux hemodialysis in the elderly. Kidney Int. 2017;91(6):1495–1509. doi:10.1016/j.kint.2017.01.013
106. Lang T, Zawada AM, Theis L, et al. Hemodiafiltration: technical and medical insights. Bioengineering. 2023;10(2):145. doi:10.3390/bioengineering10020145
107. Peters SAE, Bots M, Canaud B, et al. Haemodiafiltration and mortality in end-stage kidney disease patients: a pooled individual participant data analysis from four randomized controlled trials. Nephrol Dial Transplant. 2016;31(6):978–984. doi:10.1093/ndt/gfv349
108. Blankestijn PJ, Vernooij RWM, Hockham C, et al. Effect of hemodiafiltration or hemodialysis on mortality in kidney failure. N Engl J Med. 2023;389(8):700–709. doi:10.1056/NEJMoa2304820
109. Golper TA. Improving dialysis techniques for patients? N Engl J Med. 2023;389(8):762–763. doi:10.1056/NEJMe2307709
110. Cahill Z, Conway PT, Lim MD. Reducing the risks of home dialysis innovation and uptake: the case for human-centered product design. Clin J Am Soc Nephrol. 2022;17(11):1688–1690. doi:10.2215/CJN.05100422
111. Gura V, Rivara MB, Bieber S, et al. A wearable artificial kidney for patients with end-stage renal disease. JCI Insight. 2016;1(8). doi:10.1172/jci.insight.86397
112. Htay H, Gow SK, Jayaballa M, et al. Preliminary safety study of the Automated Wearable Artificial Kidney (AWAK) in Peritoneal Dialysis patients. Peritoneal Dialysis Int. 2021;42(4):394–402. doi:10.1177/08968608211019232
113. Groth T, Stegmayr BG, Ash SR, et al. Wearable and implantable artificial kidney devices for end-stage kidney disease treatment: current status and review. Artif. Organs. 2023;47(4):649–666. doi:10.1111/aor.14396
114. Ramada DL, de Vries J, Vollenbroek J, et al. Portable, wearable and implantable artificial kidney systems: needs, opportunities and challenges. Nat Rev Nephrol. 2023;19(8):481–490. doi:10.1038/s41581-023-00726-9
115. Fissell WH, Roy S. Innovation in the treatment of uremia: proceedings from the Cleveland Clinic workshop: the implantable artificial kidney. Semin Dial. 2009;22(6):665–670. doi:10.1111/j.1525-139X.2009.00662.x
116. Kim EJ, Chen C, Gologorsky R, et al. Feasibility of an implantable bioreactor for renal cell therapy using silicon nanopore membranes. Nat Commun. 2023;14(1):4890. doi:10.1038/s41467-023-39888-2
117. Barbieri C, Molina M, Ponce P, et al. An international observational study suggests that artificial intelligence for clinical decision support optimizes anemia management in hemodialysis patients. Kidney Int. 2016;90(2):422–429. doi:10.1016/j.kint.2016.03.036
118. Kim HW, Heo S-J, Kim M, et al. Deep learning model for predicting intradialytic hypotension without privacy infringement: a retrospective two-center study. Original Research. Front Med. 2022;9. doi:10.3389/fmed.2022.878858
119. Ayers JW, Poliak A, Dredze M, et al. Comparing physician and artificial intelligence chatbot responses to patient questions posted to a public social media forum. JAMA Intern Med. 2023;183(6):589–596. doi:10.1001/jamainternmed.2023.1838
120. Fayos De Arizón L, Viera ER, Pilco M, et al. Artificial intelligence: a new field of knowledge for nephrologists? Clin Kidney J. 2023:sfad182. doi:10.1093/ckj/sfad182
121. Erickson KF, Warrier A, Wang V. Market Consolidation and Innovation in US dialysis. Adv Chronic Kidney Dis. 2022;29(1):65–75. doi:10.1053/j.ackd.2022.01.002
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.