Back to Journals » Journal of Experimental Pharmacology » Volume 15
Acute and Sub-Acute Toxicity Evaluation of the Crude Methanolic Extract of Justicia schimperiana Leaf in Wistar Albino Rats
Authors Jegnie M , Abula T, Woldekidan S, Chalchisa D, Asmare Z, Afework M
Received 12 October 2023
Accepted for publication 9 November 2023
Published 18 November 2023 Volume 2023:15 Pages 467—483
DOI https://doi.org/10.2147/JEP.S441273
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Mihretu Jegnie,1 Teferra Abula,2 Samuel Woldekidan,3 Dinkenesh Chalchisa,4 Zemen Asmare,5 Mekbeb Afework1
1Department of Anatomy, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia; 2Department of Pharmacology, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia; 3Traditional and Modern Drug Research Directorate, Ethiopian Public Health Institute, Addis Ababa, Ethiopia; 4National Reference Laboratory for Immunohematology, Ethiopian Public Health Institute, Addis Ababa, Ethiopia; 5Department of Pathology, College of Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia
Correspondence: Mihretu Jegnie, Addis Ababa University, P.O. Box 9086, Addis Ababa, Ethiopia, Tel +251 984 079 816, Email [email protected]
Purpose: This study evaluates the acute and sub-acute toxicity of 80% methanolic extracts of the leaves of Justicia schimperiana in Wistar albino rat models.
Methods: Dried powdered leaves of Justicia schimperiana were macerated in 80% methanol. The experiment was conducted in accordance with the Organization for Economic Co-operation and Development guideline 423 for acute and 407 for sub-acute toxicity testing. A single dose of 5000 mg/kg extract was orally administered to three female rats for the acute toxicity study. The plant extract was administered orally for 28 days in daily dosages of 250, 500, and 1000 mg/kg for the sub-acute study. Animals in a control group were given distilled water. A total of 40 rats (5 rats/group/sex) were used for the sub-acute toxicity testing. Daily food intake and weekly body weight measurements were done. The rats were sacrificed at the end of the 28-day treatment period for hematological, biochemical, and histopathological tests. One-way analysis of variance and Kruskal–Wallis tests were employed for the analysis.
Results: The single-dose oral administration of the plant resulted in no deaths or serious morbidity. The median lethal dose was > 5000 mg/kg. The 28-day oral treatment of the plant extract had no significant effect on general behavior, food intake, organ weight, biochemical parameters, or the majority of the hematological markers, with the exception of the decrease in hemoglobin and hematocrit in the 1000 mg/kg extract-treated groups compared to the controls. Both sexes experienced significant weight increases at all dosage levels. With the exception of minor alterations in a few of the organs, no significant histological change was identified.
Conclusion: It is concluded that the single-dose and repeated-dose 28-day oral administration of the methanolic leaf extract of Justicia schimperiana is relatively safe.
Keywords: acute toxicity, sub-acute toxicity, Justicia schimperiana, rats, crude extract
Introduction
Over 95% of traditional medical preparations come from plants.1 According to Robert and John’s research findings, the extract of medicinal plants is used to produce approximately 25% of modern medications.2 Yet, developing countries made too little of an effort in conducting biomedical investigations and promoting public acceptability.3 The Ethiopian health authorities have only recently4 demonstrated dedication to promoting and strengthening it.5
Justicia schimperiana (Hochst. ex Nees) T. Anderson, with alternative names of Adhatoda schimperiana Hochst. ex Nees, and Gendarussa schimperiana Hochst, is known locally as “dhumuugaa” in Afaan Oromoo, and “sensel” or “simiza” in Amharic. It belongs to the Acanthaceae family, which includes approximately 250 genera and 2500 species.6 With close to 600 species, Justicia is the most important genus of the family Acanthaceae.7 It is a classic shrub that thrives in the Dry and Moist Weyna Dega agroclimatic zones of Tigray, Gondar, Gojjam, Sidamo, Kefa, Ilubabor, Shoa, Harerge, and Addis Ababa in the altitude range of 1300–2800 meters.6
In several parts of Ethiopia, the plant J. schimperiana is used traditionally for a variety of illnesses like jaundice, rabies, anthrax, asthma, common cold, stomachache, diarrhea, tapeworm infestation, wound, external parasite, and skin irritation.8–11 Phytochemical analysis of various parts of J. schimperiana showed the presence of major secondary metabolites such as Terpenoids,12,13 Phenols,13–15 Tannins,12–14,16,17 Steroids,12,18 Flavonoids13–15,18 7), Saponins,13–15,18,19 and Alkaloids.15,18,19 Methyl elaidate, methyl oleate, methyl hexadecanoate, and many other chemical compounds were isolated from J. schimperiana.20 Several biologically active chemical compounds, such as apigenin, naringenin, phytol, etc., were also identified in the plant of the same genus, Justicia gendarussa Burm.21 J. schimperiana demonstrated antibacterial,18,22 antidiarrheal,13 antimalarial,15,23,24 anti-diabetic,25 hepatoprotective,26 anti-asthmatic,27–29 wound healing,17 antifungal,14,22,30 and anti-rabies16,31 activities.
The widely held misconception that herbal remedies are incredibly safe and devoid of side effects is misleading. It has been shown that herbs can cause a wide range of unpleasant or unfavorable reactions, some of which can lead to serious injuries, or even death. As a result, employing animal models to study the harmful effects of medicinal herbs is increasing.32 Acute toxicity tests are short-term tests that are conducted to determine the immediate effects of a substance at a single exposure. It is useful to determine the median lethal dose (LD50). Sub-acute toxicity tests, on the other hand, are designed to evaluate the effects of repeated exposure to a substance.33–35 The substance is often given to the animals on daily basis for 4 weeks.36,37
Prior acute oral toxicity tests of the crude 80% methanolic extract of J. schimperiana leaf (JSLM80) showed inconsistent levels of LD50.13,16,23,25,26,28,29,31 The discrepancy in the LD50 level reported among the previous studies could be due to the variation in toxicity experimental protocol followed and animal characteristics (age, sex, weight, and genetic background). Moreover, the composition of the plant material (geographical location, climate, harvesting time, and storage conditions) and extraction methods can also impact the LD50 level of plant extracts.38
Three studies carried out using Organization for Economic Co-operation and Development (OECD) guideline 42539 (limit test 2000 mg/kg) consistently reported that there was no lethality observed and the LD50 of JSLM80 was >2000 mg/kg.13,23,25 Another study based on OECD guideline 42336 (limit test of 5000 mg/kg) also reported no lethality, making the LD50 >5000 mg/kg.31 However, other studies by Assefa et al (2016),29 Umer et al,26 and Petros et al28 reported the LD50 to be 1286.76 mg/kg, >1000 mg/kg, and 5.01 mg/kg, respectively. These studies did not follow the recommended OECD guidelines, and the mice they used showed variation in sex, age, and weight. All prior investigations were carried out using mice. The current study aims to determine the acute toxicity status in rats, which is the preferred animal by the OECD guideline.36
As to our extensive search of published materials, there is no sub-acute toxicity study on J. schimperiana. The plant has been used in the treatment of various human ailments without scientific proof for toxicity and teratogenicity. Thus, this study aims to evaluate the acute and sub-acute toxicity of JSLM80.
Methods and Materials
Plant Material Collection, Identification and Extract Preparation
Fresh leaves of J. schimperiana were collected in the months of October to January from a rural kebele named Goshu Amba (2300–2500 m altitude above sea level) around Debre Markos town, located in the northwest parts of Ethiopia, which is 320 km far from Addis Ababa. The plant specimen was identified by Melaku Wondafrash, a professional taxonomist at the National Herbarium, Department of Biology, Addis Ababa University and a voucher number MJ-001 was given and deposited in the herbarium for future reference.
According to the plant extraction method by Mekonnen et al, 2018,13 J. schimperiana leaves were washed with distilled water and dried under shade, and then ground to a coarse powder with a mortar and pestle and stored at room temperature. Then, three consecutive days of maceration in 80% methanol at room temperature with occasional stirring were used to extract the powder that had been accurately weighed using an electronic scale. The supernatant was filtered using Whatman filter paper No. 1 after three days. The leftover material was twice similarly macerated and filtered. In a 40°C oven, the mixed filtrate was concentrated. Before the actual study procedures began, the dried extract was weighed, put into a sample bottle, labeled, and stored at −4°C.
Experimental Animals Housing and Feeding
The rats were obtained from the Ethiopian Public Health institute (EPHI) animal breeding laboratory. To identify the rats, a unique number was marked on the tail of each rat using a permanent marker. The rats were kept in a plastic cage in groups according to their dose level. In the acute toxicity study, three rats were housed together. Five rats of the same sex and dose level were housed together for the sub-acute toxicity study. They were placed in a room where there was a 12-hour light/dark cycle, a temperature of 22 ± 3 °C, and a humidity of 50–60% throughout the duration of the experiment. Contaminants were periodically examined in the diet. Prior to the experimental procedures, all experimental animals underwent a 5-day acclimatization period to become accustomed to their surroundings. All of the animals were given unlimited access to food and drinking water during the adaptation period.
Oral Acute Toxicity of JSLM80
The acute toxicity study was conducted based on OECD test guideline 423.36 Healthy, nulliparous, non-pregnant, female rats weighing 220–250 g and ages 8 to 12 weeks, which had not been subjected to previous experimental procedures, were used. The limit test was employed in the acute oral toxicity study since available information suggested that J. schimperiana is likely to be non-toxic, as evidenced by plants of the same genus.36 Moreover, prior toxicity evaluation of the plant in mice did not cause lethality.31 Thus, the current acute toxicity study was conducted as a confirmatory to a prior reported LD50 level of 5000 mg/kg in mice.31 The animals were fasted overnight from food prior to the commencement of dosing of the extract. The animals were weighed on the first day of dosing and then weekly for 2 weeks. A limit dose of 5000 mg/kg was given to a group of three female rats.36 Rats were observed daily for a total of 14 days for signs of toxicity like tremors, convulsions, salivation, diarrhea, lethargy, writhing, edema, opisthotonos, exophthalmos, corneal opacity, eye swelling or reddening, sleep, and coma. Changes in skin and fur, eyes and mucous membranes, respiratory, circulatory, autonomic and central nervous systems, and somatomotor activity and behavior pattern were also noted. Individual bodyweight was measured and recorded before the administration of the extracts and weekly for 2 weeks. Weight changes were also calculated and recorded.
Oral Sub-Acute Toxicity of JSLM80
The sub-acute toxicity study was conducted in accordance with the OECD guideline 407.37 Young adult male and nulliparous non-pregnant female rats weighing 220–250 g and ages 8 to 12 weeks were used. The animals were randomly divided into four groups using a lottery method, each containing ten rats (5 males and 5 females). The three groups (Group I to III) were treatment groups and gavaged with 250, 500, and 1000 mg/kg body weight per day of the extract for a period of 28 days. The last group was a control group and received distilled water with a dose of 1mL per 100 gram of body weight. The dose levels of the sub-acute toxicity study were determined by the acute toxicity study result (LD50). Given the possibility of cumulative effects from repeated administration of the extract, one-fifth of the doses for the acute toxicity study were used in the repeated dose toxicity study.40 The descending dose levels were then set with twofold intervals according to the recommendation of OECD test guideline number 407.37 The animals were weighed using a digital electronic balance before the first oral administration of the extract and subsequently every week until the last oral administration of the extract. Food intake was measured every 24 hours, starting from the first day of administration to the end of the treatment period. The rats were also observed for the entire experimental period for the presence of mortality, behavioral changes (salivation, fur, lethargy, and sleep) and changes in physical appearance, injuries, pain, and illnesses.
Hematological and Biochemical Analysis
At the end of the treatment period, food but not water was withheld overnight for 12 hours and anesthetized by intraperitoneal injection of 150 mg/kg Pentobarbital. Blood sample was then collected by cardiac puncture into two separate test tubes. The first tube had anticoagulant ethylene diamine tetraacetic acid (EDTA) for hematology, and the second tube was without anticoagulant for blood chemistry. Blood samples in test tubes containing EDTA were immediately processed for hematological parameters using an Automated Hematological Analyzer (Sysmex-XT, 1800i, Japan). The red blood cell count (RBC), hemoglobin concentration (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet count (PLC), and white blood cell count (WBC) with differentials were automatically measured.
For biochemical analysis, the blood samples in the plain test tubes were placed for 3 hours and centrifuged for 15 minutes with a bench-top centrifuge (Humax-K, Human-GmbH, Germany) at 5000 rpm. The plasma was extracted using a micropipette, stored in fresher vials, and then stored in the refrigerator at −20°C until the analysis was done. Finally, an automated clinical chemistry analyzer (Huma Star 80, Germany) was used to determine the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total cholesterol, creatinine, urea, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), Na+, K+, and Cl.
Gross Pathologic Observations and Histopathologic Studies
After blood was collected from each rat, pentobarbital was injected intraperitoneally to cause death. Its entire brain, heart, pancreas, kidneys, adrenal glands, spleen, liver, and seminal vesicles were immediately removed. In addition, the uterus and ovaries in females and the testis and epididymis in males were also removed. These organs underwent a gross pathological evaluation by a pathologist to look for any evident lesions. Finally, slices of each sample were made, and they were all preserved for 24 hours in a 10% neutral buffered formalin fixative solution. Then, an automatic tissue processor (Leica TP 1020, Germany) was used to process the tissue. The automatic tissue processor was set first to dehydrate the fixed tissues with an increasing gradient of alcohol concentration. Following this, the tissue was cleared and impregnated with melted paraffin wax and xylene, respectively. Utilizing a Leica rotary microtome (Leica RM 2125 RT, China, tested in Germany), the blocks were sectioned at a thickness of 5 μm. The tissues were then stained using the hematoxylin and eosin method.
Tissue samples from the treatment and control groups were examined to look for any histopathological alterations. Using an automated digital camera mounted on a microscope (Zeiss Primo Star, Germany), photomicrographs of selected slides from the treated and control groups were taken.
Statistical Analysis
Statistical Software Package for Social Sciences (SPSS) version 26 was used for the analysis. Sub-acute toxicity study parameters that satisfy the normality assumption were analyzed using ANOVA, while parameters that did not meet the assumption were analyzed using the Kruskal–Wallis test. An independent sample t-test was used for the acute toxicity study. Results were presented as mean and standard error of the mean. Results with a p value less than 0.05 were considered statistically significant.
Ethical Consideration
Ethical approval was obtained from the Department of Anatomy Graduate Committee (protocol number: 116/19/ANAT) and the Institutional Review Board of the College of Health Sciences, Addis Ababa University (form AAUMF-03-008) in compliance with the OECD guidelines (407 and 423) for the care and use of laboratory animals.36,37 The animals were humanely kept in the biomedical research laboratory of EPHI. The rats were anesthetised based on the guidelines provided by the American Veterinary Medical Association.41 Finally, the bodies of sacrificed rats were disposed of humanely in following the laboratory standards of EPHI.
Result
Oral Acute Toxicity of JSLM80
The single-dose 5000 mg/kg oral administration of JSLM80 in Wistar albino rats showed no mortality or significant morbidity. There were no dizziness, piloerection or lethargy. Loss of appetite and diarrhea were observed in one rat within 30 minutes of dosing that lasted only for two days (Table 1). There was no significant body weight difference between the experimental and control groups both in the first and second week measurements (Table 2). As shown in Figure 1, a steady weight gain was noticed both in the control and treated rats. There was no significant organ weight difference between the experimental and control groups in all of the internal organs measured (p > 0.05) (Table 3). The liver, kidney, adrenal gland, and spleen were observed for possible gross pathological problem. When compared to control rats, observation of the gross appearance of the mentioned internal organs in experimental rats did not reveal any abnormal changes in texture, shape, size, or color. In all categories, there were no lesions found in these organs.
 |
Table 1 Acute Effects of JSLM80 on the General Behavior of Rats as Compared to the Controls |
 |
Table 2 Acute Effects of JSLM80 on the Body Weight of Rats as Compared to the Controls |
 |
Table 3 Acute Effects of JSLM80 on Selected Internal Organ Weights of Rats as Compared to the Controls |
 |
Figure 1 Effects of single dose oral administration of J. schimperiana leaves extract on body weight change in female rats. |
Oral Sub-Acute Toxicity of JSLM80
Effects of JSLM80 on Food Consumption, Body and Organ Weight
The body weight increased in both sexes in all groups in the course of the four-week administration of the plant extract and the vehicle. In male rats, the control group showed significant body weight gain difference compared to the 500 mg/kg and 1000 mg/kg treated groups dose-dependently. In female rats, the rate of weight gain was significantly slower in the rats treated with 250 mg/kg (p = 0.046), 500 mg/kg (p = 0.001), and 1000 mg/kg (p < 0.001), in a dose-dependent manner compared to the control (Table 4). There was also statistically significant weight gain difference based on sex of the rats. Although the rats in the control group consumed more food, there was no statistically significant difference existed in the daily food intake between the control and the treatment groups both in the male (p > 0.05) and female rats (p > 0.05) (Table 5). There was no significant organ weight difference seen between the treatment and control group in both sexes (Table 6).
 |
Table 4 Effect of 28 Days Oral Administration of JSLM80 on Body Weight |
 |
Table 5 Effect of 28 Days Oral Administration of JSLM80 on Mean Daily Food Intake as Compared to Controls |
 |
Table 6 Effects of 28 Days Oral Administration of JSLM80 on Organ Weight of Rats |
Effect of JSLM80 on Hematological Parameters
As illustrated in Table 7, twenty-eight days oral treatment of the extract did not significantly affect majority of the blood parameters. Compared to the control group, the HGB and HCT levels significantly decreased in a dose-dependent manner in the 250 mg/kg (p = 0.01), 500 mg/kg (p = 0.002), and the 1000 mg/kg dose (p = 0.001) groups. Further analysis of the blood parameters by sex showed that male rats had higher platelet, neutrophil, lymphocyte, monocyte, and eosinophil than female rats.
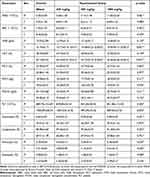 |
Table 7 Effects of 28 Days Oral Administration of JSLM80 on Hematological Parameters of Wistar Albino Rats |
Effect of JSLM80 on Biochemical Parameters
The effect of sub-acute oral administration of JSLM80 on clinical chemistry results is shown in Table 8. There were no statistically significant differences between the control and the treatment groups except the presence of trends of increment or decrement in some of the parameters. Liver enzymes (ALT and ALP) showed non-significant dose-dependent increment in the rats treated with the extract compared to the control. Creatinine in females and urea in males, also showed non-significant dose-dependent increment in the rats treated with the extract compared to the control. Further analysis by sex of the rats showed statistically significant variations in the majority of the biochemical parameters (AST, Urea, Cholesterol, Creatinine, ALP, HDL, K+, and Cl−).
 |
Table 8 Effects of 28 Days Oral Administration of JSLM80 on Biochemical Parameters of Wistar Albino Rats |
Effects of JSLM80 on Gross Pathology and Histopathology
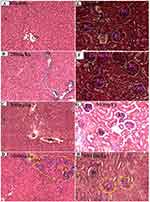
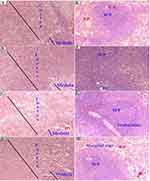
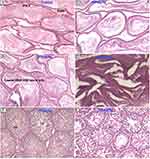
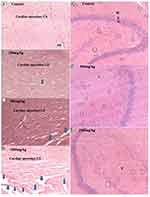
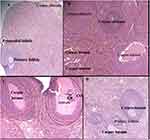
The histopathological analysis found that there were no cellular differences between the treated groups and controls in most of the organs. The extract treated groups revealed no signs of toxicity according to the histopathological slides view under microscope in the majority of the organs such as spleen, adrenal gland, heart, testis, epididymis, seminal vesicles, pancreas, uterus, and ovary (Figures 2–6). However, minor histopathological changes were observed in few rats in organs such as liver (Figure 2), kidney (Figure 2), and brain (Figure 5). The liver of one rat in the 1000 mg/kg administered group showed peri-portal lymphocytic infiltrations. There was an architectural distortion of the glomerulus in the kidney of one rat that received 1000 mg/kg extract. Multiple cytoplasmic vacuolations and few necrotic changes were observed in the majority of the rats’ brain at all levels.
Discussion
This study was carried out to evaluate the acute and sub-acute toxic effects of J. schimperiana methanolic crude extract of its leaf (JSLM80) on Wistar albino rat models. The absence of death and serious morbidity following a limit test administration of 5000 mg/kg extract to the rats implies that the LD50 value is >5000 mg/kg. The study reveals that the acute oral administration of J. schimperiana is practically non-toxic, as per the chemical toxicity classification by Hodge and Sterner.42 This result is consistent with the finding of a related study done by Zewde et al.31 Our finding is also comparable with the studies of Tesfaye et al,25 Bobasa et al,23 and Mekonnen et al,13 who consistently reported the LD50 of JSLM80 to be >2000 mg/kg. The discrepancy in the LD50 level could only be due to the limit test dose selection variation between the current and the previous three studies. However, the LD50 reported by Assefa et al29 (LD50=1286.76 mg/kg), and Umer, Asres, and Veeresham (2010)26 (LD50>1000 mg/kg) is lower than the present study. The observed variation could be attributed to the non-adherence of the previous studies to the OECD guideline, mainly the variation in animal selection (age, sex, and weight).
A significant loss of body weight of more than 10% from its initial weight following exposure to a hazardous substance is typically a straightforward and sensitive indicator of toxicity.43 There was no significant body weight difference between the experimental and control groups both in the first and second week measurements. The body weight of the rats increased both in the control and experimental group without significant difference which is in line with a similar study.31
Daily administration of the crude JSLM80 for 28 days in Wistar albino rats did not result in toxicity-related death or morbidity. Rats from all the treatment and control groups gained weight throughout the course of the study period. However, rats in the control group gain more weight than in the treatment groups. There was a significant difference in animal weight gain between the control and the treatment (500 mg/kg and 1000 mg/kg) dose groups in both sexes. As there was no statistically significant treatment-related effect on food consumption, the body weight gain difference may imply that the plant possesses an active compound that could help in controlling weight gain. Therefore, the plant may contain bioactive compounds that could have pharmacological effects on the rats’ metabolism, such as nutrient absorption, energy expenditure, or fat metabolism. Essentially, a drug’s toxicity nature may cause deviations in body weight.44 A decrease in body weight may be a sign of severe toxicity, but a reduction in body weight gain may simply be a minor kind of intoxication.45,46 Thus, the observed weight gain difference may not be toxicologically significant due to its inconsistency with other parameters like organ weights, biochemical markers, or histopathological analysis.
There was no statistically significant organ weight difference between the control and treatment groups in our study. This finding is in harmony with the work of Akintimehin et al47 who also reported the absence of significant difference in the relative weights of kidney and liver following a repeated dose administration of a plant of similar genus, Justicia carnea.
Blood profiles typically offer crucial insights on how the body reacts to trauma, lesions, deprivation, and stress.48 The primary roles of WBCs and their differentials are to build immunity and defend the body against microbes and toxic insults. The administered doses did not interfere with the differentiation of hematopoietic stem cells into these parameters, as indicated by the non-significant difference in WBC count and its differentials between the treatment and control groups in the current study. The most effective markers for the diagnosis of anemia are red blood cell indices like the mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC).49 In our study, in all treatment groups as compared to the control group, the HGB and HCT values significantly decreased in female rats. However, it is important to note that despite this decrease, the HGB and HCT levels in the treatment group still fall within the normal range for rats of the same sex, age and strain.50 The observed lower HGB and HCT levels in the extract administered group compared to the control could not be attributed to a decreased production or increased destruction of RBCs as there was no significant reduction in RBCs in the extract-administered groups. Rather, the decrease in HGB and HCT could be the result of hemodilution or extract interference with iron absorption, which needs further research to ascertain. The lack of prior sub-acute toxicity studies on the plant extract limits the comparison of the results of this study. The fact that none of the doses employed in this study significantly affected the platelet count suggests that the extract does not affect coagulation.
Serum biochemical measurements are essential for assessing alterations caused by a toxicant.51 Intragastric administration of J. schimperiana extract to the rats did not significantly affect any of the biochemical parameters. Increased serum levels of the liver’s enzymes or the kidney’s nitrogenous waste products may be a sign that tissue necrosis has caused spilling into the circulation.52 Liver function tests comprise a number of serum chemistries that indicate the condition of liver function. The most popular serum liver chemistry tests are the transaminases (alanine aminotransferase (ALT) and aspartate aminotransferase (AST)). These enzymes can escape from damaged liver cells and accumulate to higher levels in the blood.53 In our study, ALT non-significantly increased by 8.3%, 13.7%, and 45.0% in a dose-dependent manner in male rats administered with 250 mg/kg, 500 mg/kg, and 1000 mg/kg, respectively, compared to the control group. Similarly, AST non-significantly increased by 42.2%, 40.1%, and 26.5% in a dose-dependent manner in male rats treated with 250 mg/kg, 500 mg/kg, and 1000 mg/kg, respectively.
Because of their high rate of blood perfusion and ability to concentrate a variety of substances in the tubular lumen, kidneys are particularly vulnerable to toxic agents. Following administration of the crude J. schimperiana extract, a non-significant increase by 11.4%, 22.9%, and 20.0% in the level of creatinine was observed in rats treated with 250 mg/kg, 500 mg/kg, and 1000 mg/kg compared to the control, respectively. Similarly, urea non-significantly increased by 4%, 1.17%, and 8.5% in rats treated with 250 mg/kg, 500 mg/kg, and 1000 mg/kg compared to the control, respectively. The concentration of sodium, potassium, and chloride ions was not significantly affected by extract administration at any of the doses in this study.
There was no significant treatment related histopathological abnormality noted in internal organs such as liver, kidney, spleen, adrenal gland, heart, pancreas, testis, ovary, uterus, epididymis, and brain. Apart from a sporadic small number of focal peri-portal lymphocytic cell infiltration in the 1000 mg/kg treated rat, no gross or microscopic lesions were found in the liver after the extract was given for 28 days. Homogenous pinkish stroma was seen in the brain of one rat that received 1000 mg/kg extract which is a sign of an early phase of necrosis. Multiple cytoplasmic vacuolations and few necrotic changes were observed in the majority of the rats’ brain. This finding is less likely to result from the treatment; rather, it could be related to a hypoxic injury as it is present both in the control and treatment groups. There was also cellular architectural disruption in the 500 mg/kg and 1000 mg/kg dose treatment groups.
Conclusion
The findings of this study demonstrated that acute oral administration of J. schimperiana in Wistar albino rats is practically non-toxic. The repeated dose 28-day oral administration of the plant extract is relatively safe. Further safety evaluations of the plant for longer durations (sub-chronic and chronic toxicity studies) are needed. Its effect on animal fetuses and pregnant animals shall also be studied to ensure sufficient toxicity profiles for its use in humans.
Abbreviations
ALT, alanine aminotransferase; ALP, Alkaline phosphatase; AST, aspartate aminotransferase; Cl−, Chlorine; EPHI, Ethiopia Public Health Institute; mg, milligram; mg/kg, milligram/kilogram; HCT; hematocrit; HGB, hemoglobin; HDL, High-density lipoprotein; H&E, Hematoxylin and eosin; JSLM80, 80% methanolic extract of Justicia schimperiana leaf, K+, Potassium; LD50-Lethal dose 50%; LDL, Low-density lipoprotein; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean cell volume; Na+, Sodium; OECD/OCDE, organization for Economic Co-operation and Development; RBC, red blood cells; SEM, Standard error of means; WBC, white blood cells.
Data Sharing Statement
All the data sets used in this article can be accessed from the corresponding author on a reasonable request.
Acknowledgment
The authors are thankful for the financial support provided by the School of Graduate Studies of Addis Ababa University (AAU) and Debre Tabor University. The authors also would like to thank the staff of the Traditional and Modern Medicine Research Directorate at EPHI and Departments of Anatomy, Pharmaceutical Chemistry and Pharmacognosy, and Pathology at AAU for their assistance during the study.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Abebe D. Traditional medicine in Ethiopia: the attempts being made to promote it for effective and better utilization. SINET. 1986;9:61–69.
2. Bannerman RH, Burton J. Traditional medicine and health care coverage. J Mod Afr Stud. 1984;22(4):695–696. doi:10.1017/S0022278X00056421
3. Desta B. Ethiopian traditional herbal drugs: potentiality and appropriate utilization. In:
4. Abebe W. Traditional pharmaceutical practice in Gondar region, northwestern Ethiopia. J Ethnopharmacol. 1984;11(1):33–47. doi:10.1016/0378-8741(84)90094-1
5. Abebe D. Proceedings of the workshop on development and utilization of herbal remedies in Ethiopia. Addis Ababa: Ethiopian Health and Nutrition Research Institute; 1996.
6. Bekele-Tesemma A. Useful Trees and Shrubs of Ethiopia: Identification, Propagation and Management for 17 Agroclimatic Zones; 2007.
7. Corrêa GM, de Alcântara AFC. Chemical constituents and biological activities of species of Justicia: a review. Rev Bras Farmacogn. 2012;22(1):220–238. doi:10.1590/S0102-695X2011005000196
8. Abera B. Medicinal plants used in traditional medicine by Oromo people, Ghimbi District, Southwest Ethiopia. J Ethnobiol Ethnomed. 2014;10(1):40. doi:10.1186/1746-4269-10-40
9. Yineger H, Kelbessa E, Bekele T, Lulekal E. Plants used in traditional management of human ailments at Bale Mountains National Park, Southeastern Ethiopia. J Med Plants Res. 2008;2(6):132–153.
10. Chekole G, Asfaw Z, Kelbessa E. Ethnobotanical study of medicinal plants in the environs of Tara-gedam and Amba remnant forests of Libo Kemkem District, northwest Ethiopia. J Ethnobiol Ethnomed. 2015;11(1). doi:10.1186/1746-4269-11-4
11. Teklehaymanot T, Giday M. Ethnobotanical study of medicinal plants used by people in Zegie Peninsula, Northwestern Ethiopia. J Ethnobiol Ethnomed. 2007;3:3. doi:10.1186/1746-4269-3-12
12. Limenew A, Tadesse M. Phytochemical screening and peroxide value determination of methanolic extract of four traditional medicinal plants from Debre Tabor Town, Ethiopia. J Med Plants Res. 2018;12(16):203–208. doi:10.5897/jmpr2018.6579
13. Mekonnen B, Asrie AB, Wubneh ZB. Antidiarrheal Activity of 80% Methanolic Leaf Extract of Justicia schimperiana. Evid Based Complement Alternat Med. 2018;2018:1–10. doi:10.1155/2018/3037120
14. Gizaw A, Marami LM, Teshome I, et al. Phytochemical screening and in vitro antifungal activity of selected medicinal plants against candida albicans and Aspergillus Niger in West Shewa Zone, Ethiopia. Adv Pharmacol Pharm Sci. 2022;2022:1–8. doi:10.1155/2022/3299146
15. Abdela JE, Engidawork E, Shibeshi W. In vivo antimalarial activity of solvent fractions of the leaf of Justicia schimperiana Hochst. Ex Nees (Acanthaceae) against Plasmodium berghei in mice. Ethiop Pharm J. 2014;30(October 2015):95–108. doi:10.4314/epj.v30i2.3
16. Tesera Y, Desalegn A, Tadele A, et al. Phytochemical screening, acute toxicity and anti-rabies activities of extracts of selected Ethiopian traditional medicinal plants. J Pharm Res. 2022;7(150). doi:10.33140/JPR.07.01.06
17. Gebrewoled S, Giorgis G, Ambikar D, Tsegaw A, Belayneh YM. Wound healing activity of 80% methanolic crude extract and solvent fractions of the leaves of justicia schimperiana (Hochst. ex Nees) T. Anderson (Acanthaceae) in Mice. J Exp Pharmacol. 2022. doi:10.2147/JEP.S340177
18. Asfere Y, Zinabu D, Kebede A, Aiello F. In-vitro antimicrobial activities and phytochemical screening of selected plant extracts against some medically and agriculturally important pathogens. European J Med Plants. 2020;31(10):167–189. doi:10.9734/ejmp/2020/v31i1030293
19. Abebe H, Kibret B, Haile A. Phytochemical investigation on leaf extract of Adhatoda schimperiana, Ethiopia. J Med Plants Stud. 2014;2(2):23–31.
20. Tafesse T. Chemical composition and antimicrobial activity study of root extracts of Justicia schimperiana; 2017.
21. Carneiro MRB, Sallum LO, Martins JLR, de Peixoto JC, Napolitano HB, Rosseto LP. Overview of the justicia genus: insights into its chemical diversity and biological potential. Molecules. 2023;28(3):1190. doi:10.3390/molecules28031190
22. Abebe W, Zhang W, Zhang S, Xie G. Chemical composition and antimicrobial activity of essential oil from justicia schimperiana. J Pharmacogn Nat Prod. 2018;04(02):6–8. doi:10.4172/2472-0992.1000154
23. Bobasa EM, Alemu BG, Berkessa ST, et al. Antimalarial activity of selected Ethiopian medicinal plants in mice. J Pharm Pharmacogn Res. 2018;6(1):57–64.
24. Petros Z, Melaku D. In vivo anti-plasmodial activity of Adhatoda schimperiana leaf extract in mice. PhramacologyOnline. 2012;3:95–103.
25. Tesfaye A, Makonnen E, Gedamu S. Hypoglycemic and antihyperglycemic activity of aqueous extract of Justicia Schimperiana leaves in normal and streptozotocin-induced diabetic mice. Int J Pharma Sci Res. 2016;7(2):110–113.
26. Umer S, Asres K, Veeresham C. Hepatoprotective activities of two Ethiopian medicinal plants. Pharm Biol. 2010;48(4):461–468. doi:10.3109/13880200903173593
27. Petros Z, Makonnen E, Debella A. Tracheal relaxant effect of column chromatographic elutes of chloroform fraction of Adhatoda schimperiana leaves in Guinea-pigs. Pharmacogn Mag. 2009;5(17):86.
28. Petros Z, Makonnen E, Debella A. Bronchodilatory and respiratory distress protective effect of fractions isolated from Adhatoda schimperiana in Guinea-pigs. Pharmacologyonline. 2008;2:889–900.
29. Assefa A, Urga K, Melaku D, Guta M, Mekonnen W. Bronchodilator and anti-inflammatory activities of Adhatoda schimperiana. Ethiop J Health Sci. 2016;18(2). doi:10.4314/ejhs.v18i2
30. Seshathri K. Antimicrobial properties of Ethiopian chewing sticks against Candida albicans. J Appl Pharm Sci. 2012;02(01):45–50.
31. Zewde D, Dawo F, Hurisa B, Mengesha A, Tadele A. Determination of anti-rabies virus activities of crude extracts from some traditionally used medicinal plants in East Wollega, Ethiopia. Int J Basic Appl Virol. 2019;8(2):28–37. doi:10.5829/idosi.ijbav.2019.28.37
32. Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;4. doi:10.3389/FPHAR.2013.00177/ABSTRACT
33. Aydin A, Aktay G, Yesilada E. A guidance manual for the toxicity assessment of traditional herbal medicines. Nat Prod Commun. 2016;11(11):1763–1773. doi:10.1177/1934578x1601101131
34. ScienceDirect Topics. Acute Toxicity Study - an overview. Available from: https://www.sciencedirect.com/topics/medicine-and-dentistry/acute-toxicity-study.
35. Rhiouani H, El-Hilaly J, Israili ZH, Lyoussi B. Acute and sub-chronic toxicity of an aqueous extract of the leaves of Herniaria glabra in rodents. J Ethnopharmacol. 2008;118(3):378–386. doi:10.1016/j.jep.2008.05.009
36. OECD. OECD Guidelines for the Testing of Chemicals: Acute Oral Toxicity - Acute Toxic Class Method-423. OECD; 2002.
37. OECD. OECD Guidelines for the testing of chemicals: repeated Dose 28-day Oral Toxicity Study in Rodents-407. In: OECD Guidelines for the Testing of Chemicals, Section 4. 2008; Paris:OECD Publishing. doi:10.1787/9789264070684-en
38. Cassileth B, Ladenheim D, Mccarthy T, et al. Evaluating the safety of herbal medicines: integrated toxicological approaches. Science. 2014;2014:(S):47–49.
39. OECD. OECD Guidelines for the Testing of Chemicals: Acute Oral Toxicity-Up-and-Down-Procedure-425. OECD; 2001.
40. Oladotun AO, Michael O, Daniyan Gbola O. Biochemical, hematological and histopathological evaluation of the toxicity potential of the leaf extract of Stachytarpheta cayennensis in rats. J Tradit Complement Med. 2020;10(6):544–554. doi:10.1016/j.jtcme.2019.05.001
41. American Veterinary Medical Association (AVMA). AVMA Guidelines for the Euthanasia of Animals: 2020. Schaumburg: American Veterinary Medical Association; 1931. Available from: https://www.avma.org.
42. Hodge A, Sterner B. Toxicity classes in: Canadian Center for occupational health and safety; 2005.
43. Tan PV, Mezui C, Enow-Orock G, Njikam N, Dimo T, Bitolog P. Teratogenic effects, acute and sub chronic toxicity of the leaf aqueous extract of Ocimum suave Wild (Lamiaceae) in rats. J Ethnopharmacol. 2008;115(2):232–237. doi:10.1016/j.jep.2007.09.022
44. Nirogi R, Goyal VK, Jana S, Pandey SK, Gothi A. What suits best for organ weight Analysis: review of relationship between organ weight and body/brain weight for rodent toxicity studies. Int J Pharm Sci Res. 2014;5(4):1525–1532.
45. Michael B, Yano B, Sellers RS, et al. Evaluation of organ weights for rodent and non-rodent toxicity studies: a review of regulatory guidelines and a survey of current practices. Int J Pharm Sci Res. 2007;35(5):742–750. doi:10.1080/01926230701595292
46. Piao Y, Liu Y, Xie X. Change trends of organ weight background data in Sprague Dawley rats at different ages. J Toxicol Pathol. 2013;26(1):29–34. doi:10.1293/tox.26.29
47. Akintimehin ES, Karigidi KO, Omogunwa TS, Adetuyi FO. Safety assessment of oral administration of ethanol extract of Justicia carnea leaf in healthy Wistar rats: hematology, antioxidative and histology studies. Clin Phytoscience. 2021;7(1). doi:10.1186/s40816-020-00234-4
48. A-S OA, E-H TM, A-M AA. Effect of prolonged vigabatrin treatment on hematological and biochemical parameters in plasma, liver and kidney of Swiss albino mice. Sci Pharm. 2002;70(2):135–145. doi:10.3797/scipharm.aut-02-16
49. Akpamu U, Nwaopara AO, Izunya AM, et al. A comparative study on the acute and chronic effect of oral administration of Yaji (a complex Nigerian meat sauce) on some hematological parameters. Br J Pharmacol Toxicol. 2011;2(3):108–112.
50. Vigneshwar R, Arivuchelvan A, Mekala P, Imayarasi K. Sex-specific reference intervals for Wistar albino rats: hematology and clinical biochemistry. Indian J Anim Heal. 2021;60(1):58–65. doi:10.36062/ijah.60.1.2021.58-65
51. Jacobson-Kram D, Keller KA. Toxicological testing handbook: principles, applications and data interpretation. Informa Health Care. 2006;2006:1.
52. Rahman MF, Siddiqui MKJ, Jamil K. Effects of Vepacide (Azadirachta indica) on asp artate and al anine aminotransferase profiles in a subchronic study with rats. Hum Exp Toxicol. 2001;20(5):243–249. doi:10.1191/096032701678227730
53. Thapa BR, Walia A. Liver function tests and their interpretation. Indian J Pediatr. 2007;74:663–671. doi:10.1007/s12098-007-0118-7
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.