Back to Journals » Drug Design, Development and Therapy » Volume 14
Activation of PI3K/Akt/HIF-1α Signaling is Involved in Lung Protection of Dexmedetomidine in Patients Undergoing Video-Assisted Thoracoscopic Surgery: A Pilot Study
Authors Zhu L , Zhang Y , Zhang Z, Ding X , Gong C, Qian Y
Received 12 August 2020
Accepted for publication 11 November 2020
Published 24 November 2020 Volume 2020:14 Pages 5155—5166
DOI https://doi.org/10.2147/DDDT.S276005
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Linjia Zhu, Yang Zhang, Zhenfeng Zhang, Xiahao Ding, Chanjuan Gong, Yanning Qian
Department of Anesthesiology and Perioperative Medicine, First Affiliated Hospital with Nanjing Medical University, Nanjing 210029, People’s Republic of China
Correspondence: Yanning Qian; Chanjuan Gong
Department of Anesthesiology and Perioperative Medicine, First Affiliated Hospital with Nanjing Medical University, Nanjing 210029 Jiangsu, People’s Republic of China
Tel +86 13951701214
; +86 15951893101
Email [email protected]; [email protected]
Background: Lung resection and one lung ventilation (OLV) during video-assisted thoracoscopic surgery (VATS) may lead to acute lung injury. Dexmedetomidine (DEX), a highly selective α2 adrenergic receptor agonist, improves arterial oxygenation in adult patients undergoing thoracic surgery. The aim of this pilot study was to explore possible mechanism related to lung protection of DEX in patients undergoing VATS.
Patients and Methods: Seventy-four patients scheduled for VATS were enrolled in this study. Three timepoints (before anesthesia induction (T0), 40 min after OLV (T1), and 10 min after two-lung ventilation (T2)) of arterial blood gas were obtained. Meanwhile, lung histopathologic examination, immunohistochemistry analysis (occludin and ZO-1), levels of tumor necrosis factor (TNF)-α and interleukin (IL)-6 in lung tissue and plasma, and activation of phosphoinositide-3-kinase (PI3K)/AKT/hypoxia-inducible factor (HIF)-1α signaling were detected. Postoperative outcomes including duration of withdrawing the pleural drainage tube, length of hospital stay, hospitalization expenses, and postoperative pulmonary complications (PPCs) were also recorded.
Results: Sixty-seven patients were randomly divided into DEX group (group D, n=33) and control group (group N, n=34). DEX improved oxygenation at T1 and T2 (group D vs group N; T1: 191.8 ± 49.8 mmHg vs 159.6 ± 48.1 mmHg, P = 0.009; T2: 406.0 mmHg [392.2– 423.7] vs 374.5 mmHg [340.2– 378.2], P = 0.001). DEX alleviated the alveolar capillary epithelial structure damage, increased protein expression of ZO-1 and occludin, inhibited elevation of the expression of TNF-α and IL-6 in lung tissue and plasma, and increased protein expression of p-PI3K, p-AKT and HIF-1α. Dex administered had better postoperative outcomes with less risk of PPCs and hospitalization expenses as well as shorter duration of withdrawing the pleural drainage tube and length of hospital stay.
Conclusion: Activation of PI3K/Akt/HIF-1α signaling might be involved in lung protection of DEX in patients undergoing VATS.
Keywords: lung protection, dexmedetomidine, occludin, ZO-1, PI3K, Akt, HIF-1α
Introduction
In 2018, lung cancer was the most prevalent cancer and the leading cause of cancer-related death worldwide.1 Thoracic surgery for lung cancer has evolved to video-assisted thoracoscopic surgery (VATS).2 With increasing worldwide use since the 1990s, VATS is an advanced minimally invasive technique that could provide precision in thoracic surgical oncology.3 Owing to its multi-factor, to achieve the best results from “precision surgery”, there is the necessity to improve the treatment of a single individual patient with thoracic cancer.3 Several causative mechanisms can trigger lung injury in VATS, including oxidative and capillary shear stress in the dependent lung and duration-dependent ischemia-reperfusion injury due to re-expansion, as well as surgical resection, of the independent lung.4 Increased levels of pulmonary inflammatory markers in both the dependent and non-dependent lungs contribute to postoperative pulmonary complications after lung resection surgery.5
Lung resection during VATS lobectomy could cause endothelial glycocalyx layer degradation, destruction of normal vascular permeability, and eventual acute lung injury (ALI) appearance.6 Furthermore, one-lung ventilation (OLV), which has become necessary for improving surgical exposure with increased VATS use, can also contribute to ALI development.7 Upregulation of lung proinflammatory mediators and neutrophils may cause epithelial barrier breakdown due to decreased occludin and ZO-1 protein expression with increased leakiness, which induces pulmonary edema and subsequent pathological changes.8,9 ALI-related pulmonary edema and the resulting intrinsic inflammatory process are the leading mortality cause after thoracic surgery and significantly reduces the 1-year survival rate.10
Dexmedetomidine (DEX) is a highly selective α2 adrenergic receptor agonist with current clinical use. DEX has pulmonary protective benefits in lung injury cases through its effect on pulmonary vascular ischemia-reperfusion injury and inflammatory cytokine release.11 Further, DEX improves arterial oxygenation during OLV and decreases the recovery phase duration and postoperative complications in adult patients undergoing thoracic surgery.12–14 However, the underlying molecular mechanism of DEX-related protective effects on post-VATS lung injury remains unclear. In a rat model of thoracic surgery, DEX was found to inhibit interleukin (IL)-6 and tumor necrosis factor (TNF)-α production in pulmonary inflammation development and progression.15 Furthermore, in an obstructive jaundice rat model, DEX can reduce TNF-α and IL-6 levels, as well as attenuate lung injury through the phosphatidylinositol 3-kinase (PI3K)/Akt/hypoxia-inducible factor (HIF)-1α signaling pathway.16 Based on these findings, we hypothesized that DEX can improve intraoperative oxygenation and hospital outcomes by inhibiting excessive inflammatory response, ameliorating lung injury, and increasing tight junction protein expression in patients undergoing VATS.
Patients and Methods
Patient Ethics and Enrollment
In accordance with the CONSORT statement, the present prospective randomized controlled trial was approved by the ethics committee of the First Affiliated Hospital of Nanjing Medical University (2019-SR-514), and written informed consent was provided by all patients. The trial was registered prior to patient enrollment in the Chinese Clinical Trial Registry (ChiCTR2000031101). A total of 74 adult patients undergoing VATS for right lung cancer between April 1, 2020 and June 30, 2020 were included in the study. Inclusion criteria: (1) patients between 18~65 years old; (2) patients with American Society of Anesthesiology (ASA) Physical Status I~II; (3) patients with body mass index (BMI) between 18.5~28 kg/m2. Patients who met any of the following criteria were excluded from the study: (1) patients declined to participate in this research; (2) patients with known allergy to the study drugs; (3) patients with severe cardiac disease, including history of myocardial infarction, arrhythmias, bradycardia [heart rate (HR)<45 beats/min], second- and third-degree atrioventricular block; (4) hypertensive patients on treatment with α2 agonists; (5) patients with severe neuropsychiatric disease, long-term alcohol, opioid, or sedative hypnotic drug addiction and dependency history; (6) patients with serious renal, hepatic, or pulmonary diseases. Furthermore, patients who met any of the following criteria were excluded from the statistical analysis: (1) patients who finally received thoracotomy; (2) patients with pulse oxygen saturation (SPO2) less than 90% during OLV, two-lung ventilation was reinstated; (3) patients who had blood loss >1000 mL during surgery; (4) patients who died within 24 h after surgery.
Randomization and Masking
Enrolled patients were randomly allocated to the control group (group N) or the DEX group (group D) using a randomized sequence generated by a computer. These patients received saline solution or DEX infusion, respectively. A concealed envelope for random allocation was sent to the anesthesia nurses who prepared the saline or DEX. Therefore, the anesthesiologists, nurses, surgeons, and patients were blinded to the treatment allocation. 200 μg of DEX was diluted in 50 mL of saline before infusion. In group D, infusion of DEX (1.0 μg/kg loading dose for a 10-min infusion before anesthesia induction, followed by continuous infusion of 0.5 μg·kg−1·min−1 maintenance until 30 min before the end of operation) was administered. The patients in group N received saline at the same infusion rate.
Anesthesia
After entering the operating room, the radial artery and peripheral vein of the upper arm, opposite to the operation side, were accessed by an anesthesiologist and nurse staff. Intraoperative monitoring, including electrocardiogram, heart rate (HR), SpO2, invasive blood pressure (IBP), bispectral index (BIS, Aspect Medical systems, USA) and end-tidal carbon dioxide tension (etCO2) measurements, was standardized for all patients before induction of anesthesia. These indices were measured using a Mindray T6 monitor (Mindray Inc., Shenzhen, China).
All patients received 5 L/min oxygen using a simple facemask. Anesthesia was induced with intravenous midazolam 0.05 mg/kg, fentanyl 4 μg/kg, etomidate 0.3 mg/kg, and cis-atracurium 0.2 mg/kg. A left-side double-lumen tube was inserted. Correct position was assured by auscultation and fiberoptic bronchoscopy before and after the patient was set in the lateral decubitus position. Intermittent positive pressure ventilation was used during OLV with a tidal volume of 6 mL/kg. The fraction of inspired oxygen was set at 0.8 during operation. An additional 4 μg/kg fentanyl was injected before the incision. The respiratory rate was adjusted to maintain an etCO2 of 35–45 mmHg. During maintenance of anesthesia, all patients were given a total-intravenous infusion of remifentanil 6–12 μg·kg−1·h−1, propofol 4–6 mg−1·kg·h−1, cis-atracurium 0.1–0.2 mg·kg−1·h−1 to maintain the BIS between 40 and 60. Flurbiprofen (50 mg) and azasetron (10 mg) were administered, followed by skin infiltration of 0.75% ropivacaine at the incision sites. The double-lumen tube was removed after the patients fully recovered at the post-anesthesia care unit.
The systolic blood pressure levels of patients were kept higher than 100 mmHg. If these levels dropped below 100 mmHg, vasopressors were administered at the discretion of the anesthesiologist. If HR was lower than 50 bpm, atropine was administered. Patients with a hemoglobin concentration lower than 8 g/dL were given red blood cell transfusions.
Outcome Measures
These outcomes included the following: (1) Three timepoints of arterial blood gas (ABG) samples were obtained, they are before anesthesia induction (T0), 40 min after OLV (T1), and 10 min after two-lung ventilation (T2), respectively; (2) lung histological analysis and protein expression of occludin and ZO-1; (3) levels of inflammatory response markers (IL-6 and TNF-α) in blood and lung tissue; (4) PI3K/Akt/HIF-1α signaling pathway-related proteins; (5) postoperative outcomes including duration of withdrawing the pleural drainage tube, length of hospital stay, hospitalization expenses, and postoperative pulmonary complications (PPCs). PPCs included purulent sputum, low fever, prolonged air leakage, and pulmonary embolism. All patients were followed-up until discharge.
Histopathologic Examination
About 40 min after the operation, right lobe tissues collected from the surgical resection of lung tissue away from cancerous position (more than 2 cm) were sampled in 12 randomly selected patients within each group. The tissue was immersed in 4% paraformaldehyde overnight to be fixed. Then, it was embedded in paraffin and sectioned (5 μm slices). The sections were stained with hematoxylin and eosin (H&E) at 22°C for 30 sec, and then evaluated with a microscope by a pathologist who was blinded to the groups. The lung histological injury manifested as the intra-alveolar congestion, intra-alveolar hemorrhage, intra-alveolar, and interstitial infiltration of leukocytes and the thickness of the alveolar wall/hyaline membrane. The severity of lung injury was quantified by a 4-point scoring system provided by Kozian et al.17 Scoring standards were: 0, no change or very mild; 1, slightly changes; 2, moderate changes; and 3, severe changes. The summation of four scores was lung injury score. All slides were observed and imaged under a Nikon eclipse 80i light microscope (Nikon Corporation, Japan).
Immunohistochemistry
Lung sections were deparaffinized and rehydrated in an ethanol series. Endogenous peroxidase activity was blocked by 3% H2O2 for 30 min. Sections were incubated at 4°C overnight with primary antibodies, including rabbit polyclonal antibodies against occludin (1:100, Abcam, USA) or ZO-1 (1:100, Abcam, USA). After a few washes, a biotinylated goat anti-rabbit antibody (1:100) was added, and samples were incubated at 37°C for 30 min before incubated with an ABC kit treatment. Freshly prepared 3,3-diaminobenzidine (DAB) (Golden Bridge International, Mukilteo, WA) was used to visualize the antigen–antibody reaction. Brown granules represented positive cells and were analyzed by two independent observers who were blinded to the experiment. Ten high-magnification fields were randomly selected at × 200 magnification within each field.
Enzyme-Linked Immunosorbent Assay
After plasma had been collected at three time points as well as supernatant from homogenized lung tissue, concentration analyses of IL-6 and TNF-α were detected by ELISA kits (Nanjing, Yfxbio Biotechnology, Nanjing, China). All samples from each patient were detected strictly according to the manufacturer’s instructions by the same investigator blinded to randomization. The Lung tissue supernatants from each group were collected after pretreatment with phosphate-buffered saline (PBS) centrifuged at 3000 round per minute for 20 min to extract total protein. The absorbance was measured at 450 nm. Protein concentrations in the experimental samples were extrapolated from a standard curve.
Western Blot Analysis of Lung Tissue
The lung tissue obtained from two groups, frozen in liquid nitrogen and stored at −80°C, were homogenized in 1% PMSF and RIPA lysis buffer containing protease inhibitors. Then, samples were centrifuged at 12,000×g (4°C) for 20 min, and the supernatants were harvested. Protein concentrations were measured by a bicinchoninic acid assay (BCA) kit. Proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Then, the protein bands were transferred onto a polyvinylidene difluoride (PVDF) microporous membrane (Millipore, USA). After being blocked with 5% non-fat milk for 1 h at room temperature, the membrane was incubated overnight at 4°C with primary antibodies: polyclonal rabbit anti-phospho-PI3K (1:500), polyclonal rabbit anti-human HIF-1α (1:1000), polyclonal rabbit anti-human phospho-Akt (1:1000). An antibody against GAPDH (1:5000) was administered as an internal standard. The membranes were incubated with the corresponding secondary antibody (Cell Signaling Technology, USA) (1:8000) for 1 h at room temperature. The immunoblots were detected via standard ECL (Millipore, USA) and the band intensity was quantified in Image J software (Version 1.44p, National Institutes of Health, Bethesda, MD, USA).
Statistical Analysis
According to the pilot experiment with a sample size of 10 patients in each group, we obtained the arterial oxygen concentration at T2 (the mean difference between two groups: 39.5 mmHg). For two-tailed statistical analysis, 28 patients were needed in each group with a risk of type-I error of 0.05 and power of 0.8. To compensate for 20% of the possible dropouts approximately, 70 patients were enrolled in this study. Continuous data were expressed as mean ± standard deviation (SD) or medians with 95% confidence interval (CI) after checking the normality with the Shapiro–Wilk test. The chi-square test or Fisher exact test was employed to analyze dichotomous data. The Student’s t-test was used for normally distributed continuous data. The Mann–Whitney U-test was used for skewness ordinal data. Statistical significance was assumed as P<0.05. All statistical analyses were performed using the SPSS 22.0 statistical software.
Results
Characteristics of the Study Participants
Figure 1 shows the CONSORT diagram of the inclusion of patients. Seventy-four patients met the inclusion criteria; among them, four patients declined to participate. Among the remaining 70 patients, three were excluded due to surgical plan changes. We analyzed data from 33 patients in group D and 34 patients in group N. There was no significant between-group difference in the baseline characteristics (Table 1).
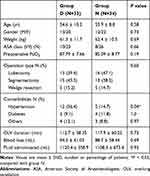 |
Table 1 Demographics and Intraoperative Profiles of Participants in the Two Groups |
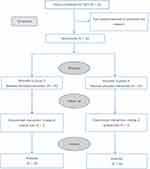 |
Figure 1 Patients flow chart for the study. |
Further, there were no between-group differences in the demographic characteristics and intraoperative profiles, including surgical type, OLV duration, blood loss amount, and administered fluid amount; however, patients in group D had a higher hypertension rate (12, 36.4% vs 5, 14.7%; P = 0.04) (Table 1).
DEX Improves Intraoperative Oxygenation
Figure 2 shows the results of blood gas analysis in three stages. Based on consensus baseline profiles, we found that oxygenation, expressed as PaO2, was more favorable at 40 post-OLV min (T1) in group D than that in group N (191.8 ± 49.8 mmHg vs 159.6 ± 48.1 mmHg, P = 0.009). Compared with group N, group D showed a significant improvement in the PaO2 at 10 min after two-lung ventilation (T2) (406.0 mmHg [392.2–423.7] vs 374.5 mmHg [340.2–378.2], P = 0.001).
 |
Figure 2 PaO2 changes at three different timepoints in the two groups. |
DEX Protects Against Lung Tissue Morphological Injuries
In group N, lung tissue exhibited breakage of pulmonary epithelial barrier (PEB) integrity, erythrocyte exudation and inflammatory cell infiltration in the interstitial and alveolar space, edema, and thickened alveolar wall (Figure 3B). Intraoperative DEX administration ameliorated lung tissue damage as indicated by better aerated alveoli; decreased neutrophil infiltration; and reduced intra-alveolar congestion, exudates, and hemorrhage (Figure 3A). As shown in Figure 3C, the lung injury scores were 1.88 ± 0.64 and 1.25 ± 0.46 in group N and D, respectively (P = 0.045).
 |
Figure 3 DEX treatment ameliorated pathological changes in lung tissue. |
DEX Increases Occludin and ZO-1 Protein Expression
TJs, mainly occludin and ZO-1, are crucially involved in PEB integrity. As shown in Figure 4, VATS induced a decrease in occludin (Figure 4C) and ZO-1 (Figure 4D) levels as indicated by light brown pathological stains. Group D showed increased occludin (Figure 4A) and ZO-1 (Figure 4B) levels, which were shown as increased brown granules that made the image color dark brown and indicated more pronouncedly positive cells. These results suggested that DEX infusion reversed VATS-induced reduction in pulmonary occludin and ZO-1 expression.
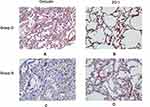 |
Figure 4 DEX increased expression of occludin and ZO-1 proteins in pulmonary epithelial barrier. |
DEX Decreases IL-6 and TNF-α Protein Expression in Plasma and Lung Tissues
Pro-inflammatory cytokines, including IL-6 and TNF-α, are critically involved in PEB integrity disruption. As shown in Figure 5A and B, IL-6 and TNF-α levels in group N were significantly higher than those in group D at T1 (IL-6: 7.25 ± 1.86 pg/mL vs 5.34 ± 0.91 pg/mL, P = 0.004; TNF-α: 46.8 pg/mL [40.6–57.7] vs 36.9 pg/mL [31.7–44.1], P = 0.02) and T2 (IL-6: 7.03 pg/mL [6.00–8.43] vs 4.82 pg/mL [4.11–5.04], P < 0.01; TNF-α: 46.9 pg/mL [40.6–56.3] vs 32.8 pg/mL [26.9–40.6] pg/mL, P = 0.002). However, there was no significant between-group difference in IL-6 and TNF-α levels at T0 (P>0.05). These findings suggest that VATS significantly increased plasma IL-6 and TNF-α levels at T1 and T2, which was attenuated by treatment with DEX.
 |
Figure 5 DEX decreased inflammatory cytokine production in plasma and lung tissue. |
As shown in Figure 5C and D, pulmonary IL-6 and TNF-α protein levels were significantly decreased in group D (IL-6: 8.70 ± 1.62 pg/mL vs 12.18 ± 2.20 pg/mL, P = 0.003; TNF-α: 59.55 ± 7.85 pg/mL vs 78.48 ± 11.44 pg/mL, P = 0.002, respectively), which were consistent with the plasma results. These results suggest that DEX infusion could ameliorate the production of pro-inflammatory factors in local lung tissue.
DEX Promotes p-PI3K, p-Akt, and HIF-1α Expression
PI3K/Akt/HIF-1α signaling pathway has been shown to suppress the inflammatory response.16 Figure 6A shows the Western blotting images of p-PI3K, p-Akt, and HIF-1α expression in the lung tissues from 16 patients. We found that treatment with DEX increased p-PI3K and p-Akt protein levels (Figure 6A–C, all P<0.05).
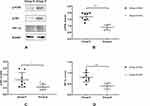 |
Figure 6 DEX promoted the expressions of p-PI3K, p-Akt, and HIF-1α. |
HIF-1α is the initiating factor for the endogenous protective mechanism and a downstream PI3K/Akt target. Compared with group N, group D showed a significant increase in HIF-1α expression (Figure 6A and D, P < 0.05).
Postoperative Outcomes
As shown in Table 2, group D showed more favorable hospital outcomes, including a decreased duration of withdrawing the pleural drainage tube (group D vs group N; 2 d [2.31–2.44] vs 3 d [2.78–2.99], P < 0.001) and length of hospital stay (group D vs group N; 7.0 d [6.76–7.14] vs 8.0 d [7.71–8.13], P < 0.001). Meanwhile, group N had a higher incidence of PPCs than group D (35.3% [12 of 34] vs 12.1% [4 of 33], P = 0.03). Among them, 23.5% of patients in group N developed purulent sputum compared with 6% in group D (P = 0.096). In addition, two patients in both groups developed low fever (P = 1.00). No patients in group D appeared prolonged air leakage and pulmonary embolism, while the incidence rates in group N were 2.9% (P = 1.00).
 |
Table 2 Postoperative Outcomes |
Discussion
Inflammatory responses, which are characterized by neutrophil infiltration and alveolar structural damage, are the main lung injury cause.18 Excessive upregulation of proinflammatory mediators and neutrophil infiltration into lungs is a progressive injury process, which can progress from ALI to systemic inflammatory response syndrome and multiple organ dysfunction syndrome.19,20 TNF-α, which is an initiating factor in ALI, is involved in the induction and perpetuation of inflammatory reactions. This subsequently enhances leukocyte recruitment to the inflammation site.21 Lung recruitment after complete lung collapse leads to increased TNF-α and IL-6 expression in the collapsed lung. Lung re-expansion and re-ventilation after 1 or 3 h of OLV in a rat model were found to induce cytokine release, neutrophil recruitment into the alveolus, and protein extravasation.22 Previous animal model studies have reported that DEX has a lung-protective effect involving IL-6 inhibition and endothelin-induced inflammatory reaction.23–25 We observed that DEX decreases the plasma and lung tissue levels of IL-6 and TNF-α during the perioperative period.
DEX partially attenuates lung injury by activating PI3K/Akt/HIF-1α signaling pathway and significantly reduces serum inflammatory cytokine levels in rat models of obstructive jaundice.16 Prolonged infiltration of inflammatory factors damages TJ function, which increases alveolar capillary wall permeability, plasma protein exudation, and diffuse alveolar damage. This eventually causes pulmonary edema.26 Our results suggest that DEX may decrease inflammatory cytokine expression and improve PEB integrity by activating the PI3K/Akt/HIF-1α pathway.
PI3K/Akt signaling pathway, which is ubiquitous in cells, regulates the inflammatory microenvironment and prevents infiltration of inflammatory cells in vivo.27,28 It plays a major role in regulating HIF-1α accumulation through epidermal growth factor receptor and PI3K activation.29 HIF-1α subunit degradation is inhibited in a hypoxic environment, which promotes HIF-1 transfer into the nucleus to regulate the transcription of multiple genes. Further, HIF-1α could allow continuous cell differentiation and promote angiogenesis under hypoxia, which is important for the vascular system. Therefore, HIF-1α can maintain tissue and cell stability under hypoxic conditions. Studies have shown that increased HIF-1α levels can dampen the inflammatory response and reduce tissue damage in animal models.30,31 DEX treatment can alleviate lung ischemia-reperfusion lung injury in rats by activating the PI3K/Akt signaling pathway at the transcriptional level.32 Further, DEX could protect organs by activating multiple signaling pathways as follows: a) activation of cell survival kinase; b) regulation of cell death by apoptosis; and c) regulation of inflammation reaction and oxidative stress.33,34 In the present study, we found that DEX infusion activated the PI3K/Akt/HIF-1α pathway, which contributes to inhibition of the inflammatory responses.
TJs located between the alveoli and fluid-filled tissue, including cytoplasmic and transmembrane proteins, are responsible for intercellular sealing and regulation of alveolar epithelial permeability.35 ZO-1 and occludin are two characteristic proteins within TJs that are crucially involved in maintaining barrier function and integrity. During inflammatory responses, several inflammatory factors, including IL-6 and TNF-α, stimulate neutrophil activation, which stimulates elastase (NE) and myeloperoxidase (MPO) release. Further, prolonged inflammatory factor infiltration can destroy TJ function and alveolar epithelial barrier, which causes plasma protein exudation and eventually pulmonary edema.36,37 Damage to the alveolar epithelial barrier could induce the above-mentioned pathogenesis.37–39 Further, hypoxia-related inflammatory cytokines may contribute to the development of high-altitude pulmonary edema.40 A mouse model study reported that DEX significantly increased ZO-1 expression; further, it remarkably attenuated lung injury and pulmonary microvascular hyper-permeability induced by renal ischemia-reperfusion.41 We found that without DEX administration, pulmonary injury pathogenesis is characterized by increased pulmonary permeability and intra-alveolar congestion, exudates, hemorrhage, diffuse infiltration of various inflammatory cells, and pulmonary atelectasis. Furthermore, infusion with DEX increased occludin and ZO-1 expression, as well as partially maintained PEB integrity.
Regarding the beneficial effects of intraoperative DEX infusion on inflammatory responses and oxygenation in patients undergoing VATS, there were significant between-group differences in the postoperative outcomes. According to our results, patients treated with DEX showed an improved duration of withdrawal of the pleural drainage tube and length of hospital stay. Notably, therapy with DEX could decrease the risks of PPCs hospitalization expenses. Previous literatures also pointed out that continuous infusion of DEX could decrease the occurrence of PPCs during the first seven postoperative days and shorten the length of hospital stay.42,43 Although the average length of hospital stay was 7–8 days in the present study, these patients usually needed 3–4 days approximately to schedule indispensable preoperative examinations; thus, most patients could discharge within 1 day or so after the removal of the drain.
This study has several limitations. First, this study only included patients with ASA I–II. Therefore, these findings cannot be generalized to patients with ASA III–IV, especially those with pulmonary dysfunction. Moreover, compared to the traditional procedure in intubated general anesthesia, VATS lung metastasectomy in non-intubated anesthesia may have a lesser impact on both immunological and inflammatory response.44 Herein, our further research will focus on the effects of DEX on perioperative immunity and stress response in awake patients with non-intubated anesthesia for VATS lung metastasectomy. Second, given the general cancer-promoting activity of catecholamines, DEX can be postulated to exhibit carcinogen-initiated effects. This has been reported by laboratory studies on animal and human cancer cells exposed to α2 agonists, which showed pro-tumoral effects and detrimental effects on innate immune system cells.45,46 Although the majority of laboratory evidence is suggestive of the detrimental effect of α2 agonists in cancer surgery, their use could be allowed to reduce patient exposure to volatile anesthetics and opioids, which are both implicated in cancer recurrence.47,48 It remains unclear whether these factors are balanced with respect to clinical outcomes; however, clinical studies have not reported beneficial effects on the recurrence risk. This study did not use long-term follow-up data to investigate the correlation between DEX use during the perioperative period and the tumor recurrence risk. Third, we did not employ another group with a specific inhibitor of the PI3K/Akt signaling pathway to confirm whether the lung protection mechanism of DEX is only dependent on the activation of this signaling pathway. However, given the possible trauma to human self-protection function, this method is only restricted to animal trails. Finally, this was a single-center randomized controlled clinical research; therefore, there is a need for further studies to address the above-mentioned issues.
In conclusion, DEX administration can protect the alveolar epithelium barrier, optimize the maintenance of the oxygenation function of OLV patients, and eventually improve the hospital outcomes by inhibiting inflammation response in our pilot study which demonstrate the feasibility of a large study to confirm its lung-protective effects. Further, it amplifies occludin and ZO-1 expression possibly by activating the PI3K/p-Akt/HIF-1α signaling pathway.
Data Sharing Statement
The data used to support the findings of the study are available from the corresponding author upon request.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Funding
This work was supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Disclosure
The authors have no conflicts of interest to declare.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Abbas AE. Surgical management of lung cancer: history, evolution, and modern advances. Curr Oncol Rep. 2018;20(12):98.
3. Migliore M, Halezeroglu S, Mueller MR. Making precision surgical strategies a reality: are we ready for a paradigm shift in thoracic surgical oncology? Future Oncol. 2020;16(16s):1–5.
4. Lohser J, Slinger P. Lung injury after one-lung ventilation: a review of the pathophysiologic mechanisms affecting the ventilated and the collapsed lung. Anesth Analg. 2015;121(2):302–318. doi:10.1213/ANE.0000000000000808
5. de la Gala F, Piñeiro P, Garutti I, et al. Systemic and alveolar inflammatory response in the dependent and nondependent lung in patients undergoing lung resection surgery: a prospective observational study. Eur J Anaesthesiol. 2015;32(12):872–880. doi:10.1097/EJA.0000000000000233
6. Wang J, Wu A, Wu Y. Endothelial glycocalyx layer: a possible therapeutic target for acute lung injury during lung resection. Biomed Res Int. 2017;130:5969657. doi:10.1155/2017/5969657
7. Okahara S, Shimizu K, Suzuki S, Ishii K, Morimatsu H. Associations between intraoperative ventilator settings during one-lung ventilation and postoperative pulmonary complications: a prospective observational study. BMC Anesthesiol. 2018;18(1):13. doi:10.1186/s12871-018-0476-x
8. Kim BG, Lee PH, Lee SH, Park CS, Jang AS. Impact of ozone on claudins and tight junctions in the lungs. Environ Toxicol. 2018;33(7):798–806. doi:10.1002/tox.22566
9. Hua T, Yang M, Zhou Y, Chen L, Wu H, Liu R. Alda-1 prevents pulmonary epithelial barrier dysfunction following severe hemorrhagic shock through clearance of reactive aldehydes. Biomed Res Int. 2019;2019:2476252. doi:10.1155/2019/2476252
10. Wang Y, Lin L, Ji Y, et al. Prognostic value of the advanced lung cancer inflammation index in early-stage non-small cell lung cancer patients undergoing video-assisted thoracoscopic pulmonary resection. Ann Palliat Med. 2020;9(3):721–729. doi:10.21037/apm.2020.03.18
11. Bao N, Tang B. Organ-protective effects and the underlying mechanism of dexmedetomidine. Mediators Inflamm. 2020;2020:6136105. doi:10.1155/2020/6136105
12. Huang SQ, Zhang J, Zhang XX, et al. Can dexmedetomidine improve arterial oxygenation and intrapulmonary shunt during one-lung ventilation in adults undergoing thoracic surgery? A meta-analysis of randomized, placebo-controlled trials. Chin Med J (Engl). 2017;130(14):1707–1714. doi:10.4103/0366-6999.209891
13. Asri S, Hosseinzadeh H, Eydi M, Marahem M, Dehghani A, Soleimanpour H. Effect of dexmedetomidine combined with inhalation of isoflurane on oxygenation following one-lung ventilation in thoracic surgery. Anesth Pain Med. 2020;10(1):e95287. doi:10.5812/aapm.95287
14. Kar P, Durga P, Gopinath R. The effect of epidural dexmedetomidine on oxygenation and shunt fraction in patients undergoing thoracotomy and one lung ventilation: a randomized controlled study. J Anaesthesiol Clin Pharmacol. 2016;32(4):458–464.
15. Wang J, Yi X, Jiang L, et al. Protective effects of dexmedetomidine on lung in rats with one-lung ventilation. Exp Ther Med. 2019;17(1):187–192.
16. Shi L, Guo C, Xie Y, Liu Y, Wu F. Dexmedetomidine attenuates lung injury in obstructive jaundice rats through PI3K/Akt/HIF-1α signaling pathway. Arch Med Res. 2019;50(5):233–240. doi:10.1016/j.arcmed.2019.08.006
17. Kozian A, Schilling T, Fredén F, et al. One-lung ventilation induces hyperperfusion and alveolar damage in the ventilated lung: an experimental study. Br J Anaesth. 2008;100(4):549–559. doi:10.1093/bja/aen021
18. Long ME, Gong KQ, Eddy WE, et al. MEK1 regulates pulmonary macrophage inflammatory responses and resolution of acute lung injury. JCI Insight. 2019;4(23).
19. Li Y, Huang J, Foley NM, et al. B7H3 ameliorates LPS-induced acute lung injury via attenuation of neutrophil migration and infiltration. Sci Rep. 2016;6:31284. doi:10.1038/srep31284
20. Oda J, Yamashita K, Inoue T, et al. Acute lung injury and multiple organ dysfunction syndrome secondary to intra-abdominal hypertension and abdominal decompression in extensively burned patients. J Trauma. 2007;62(6):1365–1369. doi:10.1097/TA.0b013e3180487d3c
21. Yi L, Zhou Z, Zheng Y, et al. Suppressive effects of GSS on lipopolysaccharide-induced endothelial cell injury and ALI via TNF-α and IL-6. Mediators Inflamm. 2019;2019:4251394. doi:10.1155/2019/4251394
22. Funakoshi T, Ishibe Y, Okazaki N, et al. Effect of re-expansion after short-period lung collapse on pulmonary capillary permeability and pro-inflammatory cytokine gene expression in isolated rabbit lungs. Br J Anaesth. 2004;92(4):558–563. doi:10.1093/bja/aeh101
23. Yang CL, Tsai PS, Huang CJ. Effects of dexmedetomidine on regulating pulmonary inflammation in a rat model of ventilator-induced lung injury. Acta Anaesthesiol Taiwan. 2008;46(4):151–159. doi:10.1016/S1875-4597(09)60002-3
24. Ding D, Xu S, Zhang H, et al. 3-Methyladenine and dexmedetomidine reverse lipopolysaccharide-induced acute lung injury through the inhibition of inflammation and autophagy. Exp Ther Med. 2018;15(4):3516–3522.
25. Zhang Q, Wu D, Yang Y, Liu T, Liu H. Dexmedetomidine alleviates hyperoxia-induced acute lung injury via inhibiting NLRP3 inflammasome activation. Cell Physiol Biochem. 2017;42(5):1907–1919. doi:10.1159/000479609
26. Yanagi S, Tsubouchi H, Miura A, Matsumoto N, Nakazato M. Breakdown of epithelial barrier integrity and overdrive activation of alveolar epithelial cells in the pathogenesis of acute respiratory distress syndrome and lung fibrosis. Biomed Res Int. 2015;2015:573210. doi:10.1155/2015/573210
27. Li Y, Yang W, Quinones-Hinojosa A, et al. Interference with protease-activated receptor 1 alleviates neuronal cell death induced by lipopolysaccharide-stimulated microglial cells through the PI3K/Akt pathway. Sci Rep. 2016;6:38247. doi:10.1038/srep38247
28. Wei Y, Hong H, Zhang X, et al. Salidroside inhibits inflammation through PI3K/Akt/HIF signaling after focal cerebral ischemia in rats. Inflammation. 2017;40(4):1297–1309. doi:10.1007/s10753-017-0573-x
29. Joshi S, Singh AR, Zulcic M, Durden DL. A macrophage-dominant PI3K isoform controls hypoxia-induced HIF1α and HIF2α stability and tumor growth, angiogenesis, and metastasis. Mol Cancer Res. 2014;12(10):1520–1531.
30. Eltzschig HK, Bratton DL, Colgan SP. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug Discov. 2014;13(11):852–869. doi:10.1038/nrd4422
31. Wang C, Wang Z, Zhang X, et al. Protection by silibinin against experimental ischemic stroke: up-regulated pAkt, pmTOR, HIF-1α and Bcl-2, down-regulated Bax, NF-κB expression. Neurosci Lett. 2012;529(1):45–50. doi:10.1016/j.neulet.2012.08.078
32. Liang S, Wang Y, Liu Y. Dexmedetomidine alleviates lung ischemia-reperfusion injury in rats by activating PI3K/Akt pathway. Eur Rev Med Pharmacol Sci. 2019;23(1):370–377.
33. Li J, Guo M, Liu Y, et al. Both GSK-3β/CRMP2 and CDK5/CRMP2 pathways participate in the protection of dexmedetomidine against propofol-induced learning and memory impairment in neonatal rats. Toxicol Sci. 2019;171(1):193–210. doi:10.1093/toxsci/kfz135
34. Zhang W, Zhang JQ, Meng FM, Xue FS. Dexmedetomidine protects against lung ischemia-reperfusion injury by the PI3K/Akt/HIF-1α signaling pathway. J Anesth. 2016;30(5):826–833. doi:10.1007/s00540-016-2214-1
35. Yang J, Wang Y, Liu H, Bi J, Lu Y. C2-ceramide influences alveolar epithelial barrier function by downregulating Zo-1, occludin and claudin-4 expression. Toxicol Mech Methods. 2017;27(4):293–297. doi:10.1080/15376516.2017.1278812
36. Tan HT, Hagner S, Ruchti F, et al. Tight junction, mucin, and inflammasome-related molecules are differentially expressed in eosinophilic, mixed, and neutrophilic experimental asthma in mice. Allergy. 2019;74(2):294–307. doi:10.1111/all.13619
37. Wittekindt OH. Tight junctions in pulmonary epithelia during lung inflammation. Pflugers Arch. 2017;469(1):135–147. doi:10.1007/s00424-016-1917-3
38. You K, Xu X, Fu J, et al. Hyperoxia disrupts pulmonary epithelial barrier in newborn rats via the deterioration of occludin and ZO-1. Respir Res. 2012;13(1):36. doi:10.1186/1465-9921-13-36
39. Wang H, Wang T, Yuan Z, et al. Role of receptor for advanced glycation end products in regulating lung fluid balance in lipopolysaccharide-induced acute lung injury and infection-related acute respiratory distress syndrome. Shock (Augusta, Ga). 2018;50(4):472–482. doi:10.1097/SHK.0000000000001032
40. Hartmann G, Tschöp M, Fischer R, et al. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine. 2000;12(3):246–252. doi:10.1006/cyto.1999.0533
41. Chen Q, Yi B, Ma J, et al. α2-adrenoreceptor modulated FAK pathway induced by dexmedetomidine attenuates pulmonary microvascular hyper-permeability following kidney injury. Oncotarget. 2016;7(35):55990–56001. doi:10.18632/oncotarget.10809
42. Gong Z, Long X, Wei H, et al. Dexmedetomidine combined with protective lung ventilation strategy provides lung protection in patients undergoing radical resection of esophageal cancer with one-lung ventilation. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;40(7):1013–1017.
43. Liu Y, Zhu X, Zhou D, Han F, Yang X. Dexmedetomidine for prevention of postoperative pulmonary complications in patients after oral and maxillofacial surgery with fibular free flap reconstruction: a prospective, double-blind, randomized, placebo-controlled trial. BMC Anesthesiol. 2020;20(1):127. doi:10.1186/s12871-020-01045-3
44. Mineo TC, Sellitri F, Vanni G, Gallina FT, Ambrogi V. Immunological and inflammatory impact of non-intubated lung metastasectomy. Int J Mol Sci. 2017;18(7):1466. doi:10.3390/ijms18071466
45. Lavon H, Matzner P, Benbenishty A, et al. Dexmedetomidine promotes metastasis in rodent models of breast, lung, and colon cancers. Br J Anaesth. 2018;120(1):188–196. doi:10.1016/j.bja.2017.11.004
46. Su X, Fan Y, Yang L, et al. Dexmedetomidine expands monocytic myeloid-derived suppressor cells and promotes tumour metastasis after lung cancer surgery. J Transl Med. 2018;16(1):347. doi:10.1186/s12967-018-1727-9
47. Zheng L, Jia R, Zhao J. Dexmedetomidine regulates proliferation, apoptosis, migration, and invasion in ovarian cancer cells via MiR-155-HIF-1α axis. Med Sci Monit. 2019;25:10164–10172. doi:10.12659/MSM.919112
48. Wall T, Sherwin A, Ma D, Buggy DJ. Influence of perioperative anaesthetic and analgesic interventions on oncological outcomes: a narrative review. Br J Anaesth. 2019;123(2):135–150. doi:10.1016/j.bja.2019.04.062
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
