Back to Journals » Journal of Asthma and Allergy » Volume 17
Abrocitinib Improved Dupilumab-Resistant Severe Atopic Dermatitis with Comorbid Mild Alopecia Areata in a 12-Year-Old Boy: A Case Report with 1-Year Follow-Up
Received 25 January 2024
Accepted for publication 27 March 2024
Published 2 April 2024 Volume 2024:17 Pages 305—311
DOI https://doi.org/10.2147/JAA.S458684
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Luis Garcia-Marcos
Xiaohan Liu,1– 3,* Biao Song,1– 3,* Hongzhong Jin1– 3
1Department of Dermatology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China; 2State Key Laboratory of Complex Severe and Rare Diseases, Beijing, People’s Republic of China; 3National Clinical Research Center for Dermatologic and Immunologic Diseases, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hongzhong Jin, Department of Dermatology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, No. 1 Shuaifuyuan, Wangfujing, Dongcheng District, Beijing, 100730, People’s Republic of China, Email [email protected]
Abstract: Atopic dermatitis (AD) may sometimes be comorbid with alopecia areata (AA). However, traditional treatments for AA show limited efficacy. New treatment options, such as dupilumab and Janus kinase inhibitors, have proven efficacy in addressing both AD and AA. This article highlights the challenging case of a 12-year-old boy experiencing severe refractory AD and comorbid AA treated with oral abrocitinib after dupilumab failure with 1-year follow-up. After 3 months of treatment, his skin manifestations improved and the hair completely regenerated. No adverse reactions were observed during the 1-year follow-up period. This case provides evidence of the efficacy and safety of using abrocitinib to treat pediatric patients with both AD and AA.
Keywords: atopic dermatitis, alopecia areata, Janus kinase inhibitors, dupilumab
Introduction
Atopic dermatitis (AD) is a common chronic inflammatory skin disease in childhood. Children with AD often have a personal or family history of atopic diseases such as food allergies, allergic rhinitis, and asthma. AD affects approximately 15% to 20% of children, 5% to 20% of adolescents, and 1% to 3% of adults.1 It is characterized by recurrent eczematous skin lesions accompanied by intense itching, significantly impacting patients’ quality of life.2 Additionally, moderate to severe AD is associated with increased rates of anxiety, depression, and sleep disturbances, affecting patients’ mental health and contributing to a considerable public health burden.3,4
Alopecia areata (AA) is a condition characterized by hair loss without scarring and affects approximately 2% of people worldwide.5 Because of its aesthetic impact, patients with AA often experience psychological distress. AD can also sometimes be comorbid with AA, and a bilateral association between AD and AA has been reported.6–8 AD is considered a classic Th2 inflammatory disease, whereas the precise pathophysiology of AA remains unclear. Both Th1 and Th2 inflammation may contribute to the development of AA.9 Their pathogenesis involves various cytokines, such as interleukins (ILs) and interferons, which mediate inflammatory signaling through the Janus kinase/signal transducer and activator of transcription (JAK-STAT) pathway.10
Several biologics and JAK inhibitors are available for moderate to severe AD in adolescents, including dupilumab (IL-4/IL-13 inhibitor), tralokinumab (IL-13 inhibitor), lebrikizumab (IL-13 inhibitor), nemolizumab (IL-31 inhibitor), abrocitinib (JAK1 inhibitor), and upadacitinib (JAK1 inhibitor).11–16 However, conventional treatments for AA in adolescents show limited efficacy, and there is still insufficient evidence regarding the safety and efficacy of biologics and JAK inhibitors.17
We herein share our experience of treating a 12-year-old boy with abrocitinib. This therapeutic strategy was utilized to manage severe and unresponsive AD and AA following an unsuccessful attempt with dupilumab.
Case Presentation
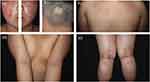
The patient was a 12-year-old boy who weighed 101 kg and had a history of AD since childhood. He presented with recurrent erythema, scales, and severe itching [Pruritus‐Numeric Rating Scale (P-NRS) score of 8/10] on his face, trunk, and extremities 18 months previously and a round patch of hair loss on the scalp 16 months prior. Previous treatment with oral Chinese herbal medicine, oral antihistamines, and dupilumab for 4 months (initial dose of 600 mg, followed by 300 mg every 2 weeks) resulted in minimal improvement. Physical examination showed extensive symmetrical red patches and papules with scales on his face, trunk, and extremities, notably accompanied by significant yellow crusts and exudates on his face [Investigator’s Global Assessment (IGA) score of 5, Eczema Area Severity Index (EASI) score of 34] (Figure 1a, c–e). The occipital scalp showed a coin-sized patch of hair loss (Figure 1b). Routine laboratory tests showed an increased immunoglobulin E level (510 kU/L). Other laboratory values, including complete blood counts, liver and kidney function tests, tumor markers, hepatitis markers, and screening tests for tuberculosis, were normal. The patient was diagnosed with severe AD and treated with oral abrocitinib (200 mg/day). After 12 weeks of treatment, his skin manifestations improved significantly, and hair regrowth occurred in the affected area of the scalp [P-NRS score of 2/10, IGA score of 1, EASI score of 2.2] (Figure 2a–e). The dose of abrocitinib was then reduced to 100 mg once daily, and the patient remained under treatment and follow-up. More than 1 year after commencement of therapy, the patient had experienced complete resolution of his AA symptoms with only mild relapse of the AD lesions [IGA score of ≤2] during the follow-up period, and no adverse events had been observed.
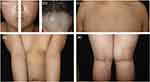 |
Figure 2 Clinical photographs after 12 weeks of abrocitinib treatment. (a, c-e) The skin manifestations improved significantly, and (b) hair regrowth occurred in the affected area of the scalp. |
Discussion
This case provides information on the use of abrocitinib in a pediatric patient with both AD and AA after dupilumab failure with 1-year follow-up.
Systemic treatments for severe AD with AA may involve glucocorticoids, immunosuppressants, dupilumab, and JAK inhibitors.18 Because of long-term safety concerns in pediatric patients, we avoided glucocorticoids and immunosuppressants in the present case. Dupilumab is expected to have a therapeutic effect on AD and AA by down-regulating Th2 inflammation through the blockade of IL-4/13 signaling. However, drug resistance and potential AA exacerbation have been reported.6 JAK inhibitors affect both Th1-dominant and Th2-dominant states by targeting interferon-γ/IL-15 in addition to IL-4/13.10 Only ritlecitinib (JAK3 inhibitor) has been approved for the treatment of AA in patients aged ≥12 years, but indications for its use in the treatment of AD are lacking.19 Most reports on the use of other JAK inhibitors in pediatric patients with AA are case reports, and the long-term safety remains unclear. Kołcz et al18 conducted a comprehensive literature review on the use of JAK inhibitors for treating pediatric AA, including tofacitinib (JAK1/3 inhibitor, with minimal JAK2 inhibition), baricitinib (JAK1/2 inhibitor), and ruxolitinib (JAK1/2 inhibitor). Side effects include mild headaches, upper respiratory tract infections, mild elevation of liver enzymes, diarrhea, and others.18 Based on this review, we have summarized the latest reports of JAK inhibitor treatment for pediatric AA in the past 2 years (Table 1).18,20–35 Six cases involved the use of upadacitinib (selective JAK1 inhibitor) and abrocitinib (selective JAK1 inhibitor) in treating pediatric patients with AA, showing good tolerability.18,31–35 Upadacitinib and abrocitinib have been approved for severe AD in children aged ≥12 years.36 Selective JAK1 inhibitors have potential safety advantages because of their specific pathway targeting, offering greater JAK2-related hematopoietic function preservation than non-selective inhibitors.
 |
Table 1 Summary of Cases of AA in Pediatric Patients Treated with Systemic JAK Inhibitors in the Most Recent 2 Years |
We also conducted an in-depth review of cases in which selective JAK1 inhibitors were used in pediatric patients with comorbid AD and AA. To our knowledge, there has been no prior report of successful treatment of comorbid AD and AA by switching to abrocitinib after dupilumab failure (Table 2).18,31–35 Throughout our 1-year follow-up, we observed a favorable response to abrocitinib with no adverse reactions, suggesting its safety in treating such conditions in children.
 |
Table 2 Summary of Comorbid Cases of AD and AA in Pediatric Patients Treated with Selective JAK1 Inhibitors |
This case report had two main limitations. First, the patient had relatively mild AA. Although abrocitinib might be effective in patients with severe AD and severe AA, further confirmation is still required. Second, whether the outcome in our case was due to a delayed response to dupilumab treatment or spontaneous remission of AA remains unknown. Additional reports are needed to confirm the efficacy of abrocitinib for AA.
Conclusion
Our report demonstrates that abrocitinib may offer an effective and safe treatment option for pediatric patients with both AA and AD, especially when dupilumab results in an inadequate response. Further research is needed to confirm its efficacy in treating AA.
Informed Consent
Written informed consent was obtained from the patient’s mother to publish the details of this case, including publication of the images.
Acknowledgments
We thank the patient’s mother for granting permission to publish this information.
Funding
This work was supported by the National High-Level Hospital Clinical Research Funding [2022-PUMCH-B-092] and the National Key Clinical Specialty Project of China.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Silverberg JI, Barbarot S, Gadkari A, et al. Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol. 2021;126(4):417–428.e2. doi:10.1016/j.anai.2020.12.020
2. Mastorino L, Viola R, Panzone M, et al. Dupilumab induces a rapid decrease of pruritus in adolescents: a pilot real-life study. Dermatol Ther. 2021;34(6):e15115. doi:10.1111/dth.15115
3. Miniotti M, Ribero S, Mastorino L, et al. Long-term psychological outcome of patients with moderate-to-severe atopic dermatitis continuously treated with Dupilumab: data up to 3 years. Exp Dermatol. 2023;32(6):852–858. doi:10.1111/exd.14786
4. Barbarot S, Silverberg JI, Gadkari A, et al. The Family Impact of Atopic Dermatitis in the Pediatric Population: results from an International Cross-sectional Study. J Pediatr. 2022;246:220–226.e5. doi:10.1016/j.jpeds.2022.04.027
5. Lee HH, Gwillim E, Patel KR, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82(3):675–682. doi:10.1016/j.jaad.2019.08.032
6. McKenzie PL, Castelo-Soccio L. Dupilumab therapy for alopecia areata in pediatric patients with concomitant atopic dermatitis. J Am Acad Dermatol. 2021;84(6):1691–1694. doi:10.1016/j.jaad.2021.01.046
7. Sun R, Kong D. Bilateral Association Between Atopic Dermatitis and Alopecia Areata: a Systematic Review and Meta-Analysis. Dermatitis. 2023. doi:10.1089/derm.2023.0114
8. Chen W, Li S, Cai X, et al. Association between alopecia areata and atopic dermatitis: current evidence. J Eur Acad Dermatol Venereol. 2023;13:44. doi:10.1111/jdv.19044
9. Kageyama R, Ito T, Hanai S, et al. Immunological Properties of Atopic Dermatitis-Associated Alopecia Areata. Int J Mol Sci. 2021;22(5):2618.
10. Muddebihal A, Khurana A, Sardana K. JAK inhibitors in dermatology: the road travelled and path ahead, a narrative review. Expert Rev Clin Pharmacol. 2023;16(4):279–295. doi:10.1080/17512433.2023.2193682
11. Stingeni L, Bianchi L, Antonelli E, et al. Moderate-to-severe atopic dermatitis in adolescents treated with dupilumab: a multicentre Italian real-world experience. J Eur Acad Dermatol Venereol. 2022;36(8):1292–1299. doi:10.1111/jdv.18141
12. Paller AS, Flohr C, Cork M, et al. Efficacy and Safety of Tralokinumab in Adolescents With Moderate to Severe Atopic Dermatitis: the Phase 3 ECZTRA 6 Randomized Clinical Trial. JAMA Dermatol. 2023;159(6):596–605. doi:10.1001/jamadermatol.2023.0627
13. Silverberg JI, Guttman-Yassky E, Thaçi D, et al. Two Phase 3 Trials of Lebrikizumab for Moderate-to-Severe Atopic Dermatitis. N Engl J Med. 2023;388(12):1080–1091. doi:10.1056/NEJMoa2206714
14. Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396(10246):255–266. doi:10.1016/s0140-6736(20)30732-7
15. Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151–2168. doi:10.1016/s0140-6736(21)00588-2
16. Kabashima K, Matsumura T, Komazaki H, Kawashima M. Nemolizumab plus topical agents in patients with atopic dermatitis (AD) and moderate-to-severe pruritus provide improvement in pruritus and signs of AD for up to 68 weeks: results from two Phase III, long-term studies. Br J Dermatol. 2022;186(4):642–651. doi:10.1111/bjd.20873
17. Barton VR, Toussi A, Awasthi S, Kiuru M. Treatment of pediatric alopecia areata: a systematic review. J Am Acad Dermatol. 2022;86(6):1318–1334. doi:10.1016/j.jaad.2021.04.077
18. Kołcz K, Żychowska M, Sawińska E, Reich A. Alopecia Universalis in an Adolescent Successfully Treated with Upadacitinib-A Case Report and Review of the Literature on the Use of JAK Inhibitors in Pediatric Alopecia Areata. Dermatol Ther. 2023;13(3):843–856. doi:10.1007/s13555-023-00889-0
19. King B, Zhang X, Harcha WG, et al. Efficacy and safety of ritlecitinib in adults and adolescents with alopecia areata: a randomised, double-blind, multicentre, phase 2b-3 trial. Lancet. 2023;401(10387):1518–1529. doi:10.1016/s0140-6736(23)00222-2
20. Ma Y, Wang W, Shi D. Tofacitinib treatment in a severe pediatric alopecia areata: a case report and a literature review. Skin Res Technol. 2024;30(1):e13553. doi:10.1111/srt.13553
21. Zhou J, Yang Y, Xu M, Lyu Z, Wu X. Efficacy, safety, and Pharmacoeconomics of Three Common Strategies for Pediatric Alopecia Areata Patients: a Retrospective Cohort Study. Clin Cosmet Invest Dermatol. 2023;16:2947–2956. doi:10.2147/ccid.S425534
22. Youssef S, Bordone LA. Clinical response to oral tofacitinib in pediatric patients with alopecia areata. JAAD Case Rep. 2023;31:83–88. doi:10.1016/j.jdcr.2022.08.024
23. Huang J, Li T, Tan Z, et al. Effectiveness of Tofacitinib in Pre-adolescent Alopecia Areata: a Retrospective Case Series and Literature Review. Acta Derm Venereol. 2023:
24. Wagh N. Tofacitinib: a Promising Treatment for Adolescent Alopecia Areata. Int J Trichol. 2023;15(3):113–114. doi:10.4103/ijt.ijt_90_23
25. Geng SL, Gong T, Ji C, Su HH. Oral tofacitinib for successful treatment of refractory alopecia areata in preschool children. J Eur Acad Dermatol Venereol. 2022;36(12):e1055–e1057. doi:10.1111/jdv.18447
26. Bhokare A. Recovery of Resistant Alopecia Areata Treated with Tofacitinib: an 8-Year-Old Child’s Case Report. Int J Trichol. 2022;14(4):135–137. doi:10.4103/ijt.ijt_15_22
27. Moussa A, Eisman S, Kazmi A, et al. Treatment of moderate-to-severe alopecia areata in adolescents with baricitinib: a retrospective review of 29 patients. J Am Acad Dermatol. 2023;88(5):1194–1196. doi:10.1016/j.jaad.2022.12.033
28. Zhan J, Cao J, Chen F, Jin Y, Huang C. Real-data on the use of baricitinib in adolescents with severe alopecia areata. J Eur Acad Dermatol Venereol. 2023;17. doi:10.1111/jdv.19121
29. Asfour L, Bokhari L, Bhoyrul B, et al. Treatment of moderate-to-severe alopecia areata in pre-adolescent children with baricitinib. Br J Dermatol. 2023;189(2):248–250. doi:10.1093/bjd/ljad118
30. Rotaru S, Common M, Mahé E. Severe inflammatory tinea capitis in a child receiving baricitinib therapy for alopecia areata. Ann Dermatol Venereol. 2023;150(2):160–161. doi:10.1016/j.annder.2023.02.001
31. Yu D, Ren Y. Upadacitinib for Successful Treatment of Alopecia Universalis in a Child: a Case Report and Literature Review. Acta Derm Venereol. 2023;103:adv5578. doi:10.2340/actadv.v103.5578
32. Ha GU, Kim JH, Jang YH. Improvement of severe alopecia areata in an adolescent patient on upadacitinib. Pediatr Dermatol. 2023. doi:10.1111/pde.15504
33. Bourkas AN, Sibbald C. Upadacitinib for the treatment of alopecia areata and severe atopic dermatitis in a paediatric patient: a case report. SAGE Open Med Case Rep. 2022;10:2050313x221138452. doi:10.1177/2050313x221138452
34. Zhao J, Liu L. A case of atopic dermatitis with alopecia universalis in a patient treated with abrocitinib. JAAD Case Rep. 2022;22:99–100. doi:10.1016/j.jdcr.2022.02.027
35. Huang J, Liu O. Effective treatment of refractory alopecia areata in pediatric patients with oral abrocitinib. J Cosmet Dermatol. 2024;23(1):348–349. doi:10.1111/jocd.15896
36. Wollenberg A, Kinberger M, Arents B, et al. European guideline (EuroGuiDerm) on atopic eczema: part I - systemic therapy. J Eur Acad Dermatol Venereol. 2022;36(9):1409–1431. doi:10.1111/jdv.18345
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.