Back to Journals » Breast Cancer: Targets and Therapy » Volume 15
ABCB1 Regulates Immune Genes in Breast Cancer
Authors Chen HK, Chen YL, Wang CY , Chung WP, Fang JH, Lai MD, Hsu HP
Received 8 June 2023
Accepted for publication 12 October 2023
Published 13 November 2023 Volume 2023:15 Pages 801—811
DOI https://doi.org/10.2147/BCTT.S421213
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Robert Clarke
Han-Kun Chen,1 Yi-Ling Chen,2 Chih-Yang Wang,3,4 Wei-Pang Chung,5,6 Jung-Hua Fang,7 Ming-Derg Lai,8,9 Hui-Ping Hsu10
1Department of Surgery, Chi Mei Medical Center, Tainan, Taiwan; 2Department of Health and Nutrition, Chia Nan University of Pharmacy and Science, Tainan, Taiwan; 3PhD Program for Cancer Molecular Biology and Drug Discovery, College of Medical Science and Technology, Taipei Medical University, Taipei, Taiwan; 4Graduate Institute of Cancer Biology and Drug Discovery, College of Medical Science and Technology, Taipei Medical University, Taipei, Taiwan; 5Department of Oncology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, 70403, Taiwan; 6Center of Applied Nanomedicine, National Cheng Kung University, Tainan, Taiwan; 7Laboratory Animal Center, College of Medicine, National Cheng Kung University, Tainan, Taiwan; 8Department of Biochemistry and Molecular Biology, National Cheng Kung University, Tainan, Taiwan; 9Institute of Basic Medical Sciences, College of Medicine, National Cheng Kung University, Tainan, Taiwan; 10Department of Surgery, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
Correspondence: Hui-Ping Hsu, Department of Surgery, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, 138 Sheng-Li Road, Tainan City, 704, Taiwan, Tel +886-6-2353535, ext 5272, Fax +886-6-2766676, Email [email protected]
Background: Resistance to standard chemotherapy is a critical problem for breast cancer patients. The ATP-binding cassette (ABC) superfamily transporters actively pump out drugs and play an important role in chemoresistance. ABCB1 (ABC subfamily B, member 1, also named as multidrug resistance protein 1, MDR1) and suppressive myeloid-derived suppressor cells (MDSCs) potentially involve in chemoresistance of breast cancer. The relationship between ABCB1 and immune genes in breast cancer has not been widely studied.
Methods: Microarray and RNA sequencing data were obtained from The Cancer Genome Atlas Breast Invasive Carcinoma in Genomic Data Commons Data Portal and Gene Expression Omnibus database. A patient-derived xenograft (PDX) model of HER2+ breast cancer was established to investigate the association between ABCB1 and immune genes in breast cancer.
Results: Expression of ABCB1 increased in doxorubicin-selected MCF-7/ADR cells. High expression of ABCB1 mRNA is correlated with lymph-node metastasis and worse overall survival in patients with breast cancer. ABCB1 is positively correlated with IL6, CSF1, CSF3, and PTGS2. In the HER2+ stage IIA breast cancer PDX model, both doxorubicin and paclitaxel suppressed growth of P2 tumors. IL6, CSF1, CSF3, and PTGS2 expression were suppressed by paclitaxel but not doxorubicin. Intrasplenic MDSCs, including CD11b+Ly6G+ and CD11b+Ly6C+ cells, were more abundant than intratumor MDSCs in PDX-carrying nude mice. Clinically, the patient developed cancer recurrence after adjuvant chemotherapy with doxorubicin-based regimen and was well controlled after paclitaxel-trastuzumab combined therapy.
Conclusion: ABCB1 was a poor predictor of HER2+ LN– breast cancer. Regulation of immune genes by ABCB1 contributed to cancer recurrence and treatment effect. The PDX model was suitable for investigation the expression of target genes and expansion of immune cells.
Keywords: breast cancer, ABC transporters, ABCB1, myeloid-derived suppressor cells, patient-derived xenograft
Introduction
Chemoresistance is a major challenge for breast cancer treatment.1 The mechanisms of chemoresistance are complex because of crosstalk between receptor tyrosine kinases and downstream pathways, deregulation of cell-cycle and apoptosis regulators, and modulation of tumor-infiltrating immune cells.2 The ATP-binding cassette (ABC) superfamily is one of the largest families of membrane-bound transport proteins in humans. ABC transporters use the energy from ATP hydrolysis to actively pump out substrates through the cell membrane. ABC transporters also function in cell apoptosis, energy metabolism, and material transport. In cancer cells, ABC transporters participate in detoxification and drug resistance.1 Multidrug resistance protein 1 (MDR1; gene: ABC subfamily B, member 1, ABCB1), multidrug resistance-associated protein 1 (MRP1; gene: ABC subfamily C, member 1, ABCC1), and breast cancer resistance protein (BCRP; gene: ABC subfamily G, membrane 2, ABCG2) have been studied in chemoresistant breast cancer. Increased expression of ABCB1 has been reported in recurrent breast cancer, and patients with ABCB1-positive cancer fail to respond to chemotherapy.3 ABCB1 is also a metastatic marker for triple-negative breast cancer.4 ABCB1 is overexpressed in doxorubicin-resistant MCF7 breast cancer cells.5 Theoretically, inhibition of ABC transporters during cancer therapy should improve the sensitivity of cancer cells to cytotoxic agents. Several clinical trials of ABCB1-reversing agents combined with chemotherapeutic drugs are currently ongoing in cancer patients, but the results have not been satisfactory. No effective ABCB1-reversing drug without significant toxicity has been approved.6,7 Treatment with doxorubicin also induces ABCB1 expression and enhances drug efflux potential through FOXO3a signaling in K562 leukemia cells.8 Non-synergistic effects of chemotherapy agents and ABC-reversing drugs are possible because the downstream pathway of ABCB1 is complicated. Further study of ABCB1 in cancer is needed to aid development of potential therapeutic agents.
The ability to avoid immune surveillance is important for cancer cells, which they achieve by secreting specific cytokines to recruit and activate suppressive immune cells in the tumor microenvironment, including regulatory T cells, myeloid-derived suppressor cells (MDSCs) and M2 tumor-associated macrophages.9 In the literature, 16 genes of immune factors are expressed by human solid-tumor cell lines, including transforming growth factor beta (gene TGFB1), interleukin-1 beta (IL-1β, gene IL1B), IL-4, IL-6, and IL-10; granulocyte-macrophage colony-stimulating factor (gene CSF2); macrophage colony-stimulating factor (gene CSF1); indoleamine 2.3-dioxygenase (gene IDO); fms-related tyrosine kinase 3 ligand (gene FLT3L); c-kit ligand (gene KITLG); inducible NO synthase (gene NOS2); arginase-1 (gene ARG1); TNF-alpha (gene TNF); cyclooxygenase 2 (gene PTGS2); vascular endothelial growth factor (gene VEGFA), and granulocyte colony-stimulating factor (gene CSF3).10,11 Cancer cells may regulate suppressive MDSCs through these cytokines.
A patient-derived xenograft (PDX) is created by engrafting tumor tissues in immunodeficient mice. The tissue structure, cell morphology, genetic features, and molecular biology are similar to those of surgical specimens. Gradual infiltration of host immune cells has been detected after 3–5 passages.12 Currently, the PDX model is used to identify therapeutic agents, study carcinogenesis, investigate cancer heterogenicity, or develop precision medicine.13 The engraftment rate of PDX depends on the tumor burden and cancer characteristics and ranges from 20% to 90%. Advanced cancers with malignant potential have the highest establishment rate; for example, colorectal, pancreatic, head and neck, and ovarian cancer. Breast cancer has the relatively lowest success rate of PDX engraftment (21–37%).14 Triple-negative breast cancer or HER2+ subtypes have higher success rates than estrogen receptor (ER) positive cancers.15 PDX tumors have comparable therapeutic responses to corresponding clinical observations.16 PDX models have shown superior predictive value in cell-line xenografts or genetically engineered mouse models.17
For the present study, we hypothesized that ABCB1 predicts poor prognosis of patients with breast cancer and regulates secretion of cytokines. To test this hypothesis, we studied the RNA sequencing data from The Cancer Genome Atlas Breast Invasive Carcinoma in Genomic Data Commons Data Portal (GDC TCGA-BRCA) and examined the gene expressions of ABC transporters and cytokine genes. In addition, a PDX model from one patient with ER–, progesterone receptor (PR)+, and HER2+ breast cancer was used to test ABCB1, immune genes, and tumor-infiltrating immune cells in breast cancer.
Methods
Bioinformatics
The Illumina platform was used to sequence the RNA of 1217 breast cancer samples, and raw data were downloaded from the GDC TCGA-BRCA.18 The latest Human Genome Assembly hg38 was used to re-analyze the results, which were re-organized by the University of California Santa Cruz Xena team.19 The upper quartile of the fragments per kilobase of transcript per million mapped reads (HTSeq-FPKM-UQ) was selected. The expressions of ABCB1, ABC transporters, and MDSC-associated genes were extracted for further analysis. Survival status was also obtained.
The cBioPortal platform collects multidimensional cancer genomics and datasets.20,21 A total of 15 breast cancer datasets with 10,928 samples were selected from cBioPortal, including those of primary and metastatic cancer. Breast fibroepithelial tumors, xenografts of breast cancer, metaplastic breast cancer, juvenile papillomatosis, or adenoid cystic breast cancer were excluded because of different tumor pathophysiologies. Amplification, mutation, or deletion of ABCB1 genes were explored.
Gene expression data on breast cancer were collected from the GEO database (http://www.ncbi.nlm.nih.gov/geo/). Doxorubicin-selected MCF-7/ADR cells were compared with parental MCF-7 cells in the GSE24460 dataset to study ABC transporters in chemoresistant cells.22 The Affymetrix Human Genome U133A 2.0 Array was used to examine gene expression. Raw data were obtained and normalized according to the robust multichip average (RMA). The RMA signal was computed for gene-level probe set summaries using the Affymetrix Expression Console (version 1.3) (Affymetrix, Santa Clara, CA, USA) and R (version 3.2.0) (www.r-project.org). A heatmap of ABC transporters was drawn. ABCB1-overexpressing human mammary epithelial cells were compared with parental cells.23 High-throughput RNA sequencing was performed (Illumina, San Diego, CA, USA), and 162 genes were differentially expressed. We uploaded the raw data into the Gene Set Enrichment Analysis platform and used the BIOCARTA pathways for analysis.24 RNA sequencing data from HER2+ patients were download from the GSE43837 dataset. The levels of ABCB1 and ERBB2 mRNA were collected.
The Kaplan–Meier Plotter platform was used to correlate the results of the mRNA microarray and recurrence-free survival of patients with breast cancer.25 The Affymetrix gene chip (Santa Clara, California, United States) using JetSet probe for ABCB1 and 201873_s_at was used to examine mRNA expression. The cutoff point of low or high expression of ABCB1 was based on the median mRNA level. Redundant samples and biased arrays were excluded. The Kaplan–Meier method was used to estimate survival curves and compared by performing the Log rank test. Hazard ratios with 95% confidence intervals (CIs) were used to measure the association between the expression of ABCB1 and survival.
Patients and PDX
One PDX was obtained from a 67-year-old female patient with invasive ductal carcinoma after curative resection in 2018. The characteristics of the patient are presented in Table 1. Written informed consent was obtained from the patients, and the study was approved by the Institutional Review board of the National Cheng University Hospital (NCKUH IRB no. A-ER-106-157). The clinical outcomes were also recorded.
 |
Table 1 Patient Characteristics |
Animal procedures and experimental protocols, such as animal housing and care, were approved by the Laboratory Animal Center and Institutional Animal Care and Use Committee (IACUC, No. NLAC(TN)-104-M-028-R2/R3 & 107-M-001-R1/R2, from Apr 13, 2016 to Apr 30, 2020) of the National Laboratory Animal Center of National Applied Research Laboratories, Tainan, Taiwan, and the Laboratory Animal Center (approval No. NCKU-107226 and 108114, from Aug 1, 2018 to Dec 31, 2022), College of Medicine, National Cheng Kung University. Animal studies and welfare were carried out following the Guide for the Care and Use of Laboratory Animals (eighth edition).26 Fresh samples from 10 breast cancer patients were collected immediately after surgery. The cancer tissue was minced into small pieces and subcutaneously transplanted into the flanks of NOD.Cg-Prkdcscid Il2rgtm1Wjl /YckNarl mice. These mice were maintained at the National Laboratory Animal Center, Tainan, Taiwan. Tumor growth was assessed by measuring two perpendicular diameters once a week. Tumor volumes were calculated as the length × width2 / 2. The relative tumor volume of each tumor was calculated as the ratio of the current volume in relation to the initial volume. The take rate was 10% in 10 breast cancer patients. The first passage (P1) of PDX was established in three mice. After tumor volumes approached 1000 mm3, the PDX cancer tissues were removed. The P1 tumor with highest growth rate was re-implanted into flanks of 20 NOD.Cg-PrkdcscidIl2rgtm1Wjl/YckNarl mice to get P2 mice. Twenty P2 PDX mice were transported to the Laboratory Animal Center, College of Medicine, National Cheng Kung University. Next generation of PDX mice was established by implanting cancer tissue into the subcutaneous fat of non-obese diabetic/severe combined immunodeficiency disorder (NOD/SCID) mice. The PDX mice were maintained until passage 13.
Seventeen P2 PDX mice were treated intraperitoneally with normal saline (n = 3), doxorubicin (18 mg/kg, titrate to 12 mg/kg, n = 5), paclitaxel (10 mg/kg, n = 3), trastuzumab (30 mg/kg, n = 3), or AZD8055 (mTOR inhibitor, 20 mg/kg, n = 3) twice a week for four doses. Tumor size was recorded, and the mice were sacrificed at Day 12 after the first treatment dose. Cancer specimens were obtained for other experiments. Two passage 7 (P7) and two P9 PDXs were established in athymic nude mice. Athymic nude mice lack of T or B cells with preserved innate immunity. Tumor and spleen were collected and minced into small pieces. Intratumor and intrasplenic immune cells were isolated. Flow cytometry was performed to identify cell types. CD4+CD25+ and CD8+ lymphocytes were identified as negative control. Innate immunity cells were analyzed, including CD11b+Ly6C+ and CD11b+Ly6G+ monocytes, and F4/80+ macrophages.
Quantitative Real-Time Polymerase Chain Reaction (qPCR)
The fresh cancer tissues from P2 PDX mice after drug treatment were obtained. A RNeasy® Mini kit was used to extract total RNA according to the manufacturer’s instructions. Single-stranded complementary DNA (cDNA) was synthesized from 2 μg of total RNA using M-MuLV reverse transcriptase (Roche) and oligo-dT random primers. The cDNA was amplified with specific genes, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the endogenous control. Primers were designed to be specific for the following genes: IL6 (NM_00600.5), colony-stimulating factor 1 (CSF1, NM_000757.6), CSF3 (NM_000759.4), prostaglandin-endoperoxide synthase 2 (PTGS2, NM_000963.4), vascular endothelial growth factor A (VEGFA, NM_001025366.3), and GAPDH (NM_002046.3) (Table S1). SYBR green fluorescence was added. The PCR parameters were as follows: 95°C for 10 min; 95°C for 15s, 60°C for 1 min and total 40 cycles. Fluorescence increase of fluorescein was automatically measured during PCR. All samples were amplified in duplicate, and the CT value was recorded. The 2-delta-delta CT value was calculated following GAPDH normalization.
Flow Cytometry
Fresh cancer tissues and spleen from nude mice were finely minced into small pieces, then ground, filtered, and centrifugated. Detached cells were washed with PBS and incubated with commercially available anti-CD4, anti-CD25, anti-CD8, anti-CD11b, anti-Ly6G, or anti-Ly6C antibodies (BD Biosciences) under 4°C overnight. Then, 1 mL of staining buffer was added, and cells were analyzed by flow cytometry (BD Biosciences). Data analysis was performed using WinMDI 2.9.
Statistical Analysis
All statistical analyses were performed using STATA version 16.0 (StataCorp LLC, Texas, USA). Continuous variables with normal distribution were compared between two groups by performing Student’s t-test. Results are expressed as the mean ± standard deviation (SD). The nonparametric Wilcoxon rank-sum or Kruskal–Wallis test was performed for two or more groups of non-normally distributed continuous variables. Correlation between two continuous variables was calculated by Spearman’s method and expressed as rho (ρ) with a 95% confidence interval (95% CI). Survival curves were estimated using the Kaplan–Meier method and compared between groups by performing the Log rank test. The median split was used for turning a continuous variable into a binary variable to visualize the difference between groups in the Kaplan–Meier method. A P value <0.05 was considered to be indicative of statistical significance.
Results
ABC Transporters in Breast Cancer
There are a total of 48 ABC transporters in seven suprafamilies.1 RNA sequencing data of the 48 ABC transporters were obtained from TCGA datasets and compared between survivors (censored) and non-survivors (expired). Lower expressions of ABCA3, ABCA7, ABCB2, ABCB8, ABCF3, and ABCG1 were detected in non-survivors. In contrast, the expressions of ABCA1, ABCA5, ABCA6, ABCA8, ABCA9, ABCA10, ABCB1, ABCB5, ABCB7, ABCC2, ABCC9, ABCD2, ABCE1, ABCG2, and ABCG4 were higher in non-survivors than in survivors (Figure S1). We also obtained consistent data and found that compared with the MCF-7 parental cells, ABCB1 had the highest expression of ABC transporter genes in doxorubicin-selected MCF-7/ADR cells from the GSE24460 dataset (Figure S2).
In these ABC transporters, ABCB1 is the most well-known transporter related to drug resistance. The genetic mutation rate of ABCB1 is 1.7% in the cBioPortal database. Amplification of the ABCB1 gene is the most common form of gene alteration in breast cancer (Figure S3). RNA sequencing data from 1217 breast cancer samples, and the corresponding patients’ outcomes were obtained from the TCGA. The patients with lymph-node metastasis had a higher level of ABCB1 mRNA (mean ± SD = 13.9 ± 0.1, median 13.9, range 8.0–17.6) than those without lymph-node metastasis (mean ± SD = 13.7 ± 0.1, median 13.8, range 7.6–17.4) (Figure 1A). Overall survival tended to be worse in the patients with higher ABCB1 expression than in patients with lower ABCB1 expression (Figure 1B). Details of clinical or pathological factors were absent from the TCGA database. Therefore, we used the Kaplan–Meier Plotter, a public database, to analyze survival of patients with breast cancer. The overall survival rates of patients with high or low mRNA levels of ABCB1 were similar (Figure S4A). The patients with high ABCB1 mRNA had worse post-progression survival (P = 0.015, Figure S4B). We selected patients with HER2+and ESR1– breast cancer according to the St. Gallen molecular subtypes. The overall survivals of high and low ABCB1 were similar between the patients with HER2+ breast cancer and those receiving systemic treatment (Figure S4C and D). However, high ABCB1 mRNA was correlated with worse prognosis of patients without lymph-node metastasis and with better prognosis in those with lymph-node metastasis (Figure S4E and F). ABCB1 was a better predictor of poor prognosis for the HER2+ and ESR1– patients without lymph-node metastasis. We further collected RNA sequencing data from HER2+ patients in the GSE43837 dataset. The expressions of ABCB1 and ERBB2 mRNA were compared, and positive correlation was showed in Figure S5.
In summary, expression of ABCB1 increased in non-survivors and in doxorubicin-selected MCF-7/ADR cells. Amplification of ABCB1 genes was the most common type of genetic alteration and correlated with ERBB2 level. High expression of ABCB1 correlated with worse post-progression survival and overall survival in patients with HER2+, ESR1–, and LN– breast cancer.
Expression of Immune Genes in ABCB1high Breast Cancer
RNA sequencing data of ABCB1 and immune genes were obtained from the TCGA dataset. Spearman’s rank-order correlation coefficient (ρ) was calculated to express correlations. The results showed that ABCB1 was positively correlated with IL6 and PTGS2 with stringent association by ρ values >0.4 (Figures 2A and B). Weak association between CSF1/CSF3 and ABCB1 was also detected by ρ values 0.3 – 0.4 (Figures 2C and 2D). A negative correlation between ABCB1 and VEGFA was found as shown by a ρ value <–0.3 (Figure 2E). ABCB1 mRNA was not correlated with other cytokines (TGFB1, IL1B, IL4, IL10, CSF2, FLT3, KITLG, NOS2, ARG1, IDO1, and TNF) (Figure 2F–P).
Since ABCB1 showed high expression in doxorubicin-selected breast MCF-7/ADR cells, we then performed BIOCARTA pathway analysis for the upregulated genes in ABCB1-overexpressing human mammary epithelial cells in the GSE173411 dataset. The data indicated that cytokines and inflammatory response-related genes, including IL6, CSF1, and CSF3, were significantly associated with ABCB1-overexpressing cells (Figure S6).
Clinical Course of a Human Patient and Establishment of a PDX Mouse Model
We used a PDX mouse model to assess the significance of ABCB1 and immune genes in breast cancer. The postoperative pathological report of a 67-year-old female patient with invasive ductal carcinoma who underwent a total mastectomy and sentinel lymph-node biopsy in 2018 showed that she had ER–, PR+, and HER2+ stage IIA cancer without lymph-node metastasis. She was treated with adjuvant therapy with cyclophosphamide-epirubicin-5-fluorouracil (500–75–500 mg/m2) every 3 weeks for six courses, followed by adjuvant endocrine therapy with letrozole. After her mastectomy, a left-upper lung nodule was detected at 23 months, and a right-lower lung nodule was detected at 28 months by chest computed tomography. Bilateral lung nodules were resected by video-assisted thoracoscopic surgery. The pathological report confirmed a HER2–enriched metastatic carcinoma of breast origin. Salvage therapy with docetaxel–pertuzumab–trastuzumab (60 mg/m2–840 mg–6 mg/m2) every 3 weeks for six courses and pertuzumab–trastuzumab for a total of 1 year. The patient was maintained on trastuzumab therapy through ≥43 months after mastectomy with partial response (Table 1).
The take rate of 10 breast cancer samples was 10% in NOD.Cg-PrkdcscidIl2rgtm1Wjl/YckNarl mice. The first passage (P1) of PDX was established in three mice at Day 50 after transplantation (Table S2). The mice were maintained at the National Laboratory Animal Center, Tainan, Taiwan. The P1 tumor with highest growth rate was re-implanted into back of 20 NOD.Cg-PrkdcscidIl2rgtm1Wjl/YckNarl mice to get P2 mice. Twenty P2 PDX mice were transferred to the Laboratory Animal Center, College of Medicine, National Cheng Kung University. Three P2 mice were maintained until Day 49 after implantation for further passage. Next, the mice were sacrificed, and cancer tissues were re-implanted into the subcutaneous fat of NOD/SCID mice to establish the next passage of PDX. The growth rate was better for P2–P6 than for P7–P12 PDX (Figure 3A).
Phosphoinositide 3-kinases/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) signaling is downstream of HER2 signaling.27 We treated the other 17 P2 mice with standard chemotherapy drugs (paclitaxel and doxorubicin) and anti-HER2 agents (trastuzumab and AZD8055) (Figure 3B). AZD8055 is a selective ATP-competitive mTOR kinase inhibitor. There was no effect on tumor growth by treatment with AZD8055 (blue line in Figure 3B) and only a minimal tumor suppressive effect by treatment with trastuzumab (green line in Figure 3B). Treatment with doxorubicin suppressed tumor growth after post-transplantation Day 43 (red line in Figure 3B). However, significant body weight loss (>10%) was recorded in the doxorubicin group, and survival was shorter. Treatment with paclitaxel successfully suppressed the P2 xenograft growth at post-implantation Day 43 and after Day 48 (orange line in Figure 3B). The mice were sacrificed, and the xenografts were removed at post-implantation Day 62 (12 days after the first dose). The cancer tissues were collected for qPCR analysis.
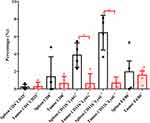
The expressions of immune genes in the P2 xenografts were detected by qPCR and compared with the expression of GAPDH. The ΔCT values were recorded and adjusted by subtracting those of the normal saline group. The expressions of IL6, CSF1, CSF3, and PTGS2 were increased in the xenograft after treatment with doxorubicin but decreased after treatment with paclitaxel or trastuzumab. The expression of VEGFA was suppressed in all groups. Treatment with AZD8055 inhibited the expressions of IL6, CSF1, CSF3, and VEGFA, but not of PTGS2 (Figure 4).
 |
Figure 4 Expression of ABCB1 in cancer samples from PDX mice after drug treatment. ABCB1, ABC subfamily B, member 1; PDX, patient-derived xenograft. |
We also established a PDX model in nude mice to examine innate immunity in cancer-bearing mice. Intrasplenic and intratumor immune cells were analyzed by flow cytometry. The levels of CD4+CD25+, CD8+, and F4/80+ cells were low with large standard deviation in the spleen and xenograft (Figure 5). Mouse MDSCs include CD11b+Ly6G+ and CD11b+Ly6C+ cells. Intrasplenic CD11b+Ly6G+ and CD11b+Ly6C+ cells were higher than intratumor MDSCs (Figure 5).
Discussion
Early recurrence is one of the critical issues in treatment of patients with breast cancer. The PDX model was established to test drugs and study the mechanism of carcinogenesis or cancer heterogenicity. In our data, the expression of ABCB1 was correlated with lymph-node metastasis and poor survival in 1217 patients with breast cancer in the GDC TCGA-BRCA database. Increased expression of ABCB1 gene was detected in doxorubicin-resistant MCF-7/ADR cells. High expression of ABCB1 was correlated with increased IL6, CSF1, CSF3, and PTGS2 levels and decreased VEGFA level. Cytokine- and inflammatory response-related genes, including IL6, CSF1, and CSF3, were significantly associated with ABCB1-overexpressing cells. We also used a PDX model to study the expressions of ABCB1 and cytokine genes. Treatment with doxorubicin and paclitaxel suppressed growth of a PDX tumor; however, monotherapy with anti-HER2 monoclonal antibodies (trastuzumab) or mTOR inhibitor (AZD8055) failed to suppress tumor growth. The expression of VEGFA was repressed after treatment with doxorubicin, paclitaxel, trastuzumab, and AZD8055. The expressions of IL6, CSF1, CSF3, and PTGS2 increased after treatment with doxorubicin, but decreased after treatment with paclitaxel or trastuzumab. Expansion of splenic MDSCs (CD11b+Ly6G+ and CD11b+Ly6C+ cells) was detected in PDX-implanted nude mice. The human donor for the PDX developed cancer recurrence and lung metastasis 23 months after curative resection and adjuvant chemotherapy with anthracycline-based regimen. The metastatic cancer was well controlled after a taxane-based regimen with anti-HER2 therapy, which was consistent with the results of suppression of ABCB1 and immune genes in the PDX model.
The PDX model was initially established to study cancer heterogenicity and preclinical drug testing.28 The successful rate of PDX engraftment in breast cancer ranges from 21% to 37%, which is lower than that in other kinds of cancer. The take rate is highest in TNBC (51.3%), followed by HER2+ cancer (26.5%).14 Our patient had pT2N0, stage IIA, HER2+ invasive ductal carcinoma. Cancer recurrence with lung metastasis developed after curative surgery and adjuvant anthracycline-based chemotherapy. The mechanism of drug resistance to anthracycline is complicated, and activation of MDSCs to inhibit cytotoxic T cells has been reported.29 An increase in circulating MDSCs has been correlated with cancer stage and treatment with doxorubicin–cyclophosphamide. By contrast, the level of MDSCs has been shown to decrease to pretreatment status after paclitaxel.30 Our findings are consistent with the change in MDSC level in a previous report. Immune genes were increased in the PDX tumors after treatment with doxorubicin and reduced after paclitaxel. Implantation of PDX in nude mice was also useful for studying immune cells in circulation or the tumor microenvironment; the formers were considered to be intrasplenic cells, and the latter were intratumor cells. The level of intrasplenic MDSCs (CD11b+Ly6G+ and CD11b+Ly6C+cells) increased. These results confirmed the usefulness of the PDX model for studying MDSCs and cytokines.
Chemoresistance is a major challenge for the treatment of patients with breast cancer, and the mechanism is complex. Membranous transporters of the ABC superfamily use the energy from ATP hydrolysis to actively pump substrates out through the cell membrane. ABC transporters also function in cell apoptosis, energy metabolism, and material transport. In cancer cells, ABC transporters participate in detoxification and drug resistance.1 MDR1 (gene: ABCB1, also named P-glycoprotein, P-gp) is important in breast cancer chemoresistance. Increased expression of ABCB1 protein has been detected in recurrent breast cancer and patients with ABCB1-positive cancer who were unresponsive to chemotherapy.3 ABCB1 genes are overexpressed in doxorubicin-resistant MCF7 breast cancer cells.4 Other studies have reported that loss of ABCB1 protein in immunohistochemistry staining was correlated with triple-negative breast cancer, lymph-node metastasis, larger tumor size, and poor prognosis of patients.31 In our study, we collected RNA sequencing data from 1217 breast cancer samples, and ABCB1 gene expression was higher in non-survivors and patients with lymph-node metastasis. High expression of ABCB1 mRNA was correlated with post-progression survival of all patients with breast cancer and overall survival of HER2+, ESR1–, and LN– patients. Based on our results, ABCB1 performed as a tumor promoter in HER2+ breast cancer.
There is no direct evidence linking expression of ABCB1 with secretion of cytokines except in several related reports. Increased expression of PTGS2 genes has been detected by ABCB1-expressing normal human mammary epithelial cells, and PTGS2 was found to encode cyclooxygenase-2 protein.23 Treatment with paclitaxel has been shown to suppress secretion of VEGF from cancer cell lines.32 In our data, the expressions of IL6, CSF1, CSF3, and PTGS2 increased in the PDX tumor after treatment with doxorubicin but decreased with treatment of paclitaxel or trastuzumab. Expression of VEGFA was suppressed in all groups. An increased number of intrasplenic MDSCs was also detected in PDX-implanted nude mice.
A limitation of this study was the small sample size. The successful rate of PDX engraftment was quite low, and we only had one PDX model. To solve this problem, we used RNA sequencing data from 1217 breast cancer samples to study the correlation between ABCB1 and immune genes. High expression of ABCB1 was correlated with increased levels of IL6, CSF1, CSF3, and PTGS2 and a decreased level of VEGFA. Larger RNA sequencing datasets could provide useful evidence of correlations, and PDX is an operable model for assessing causal relationships. The two experimental methods are complementary.
In summary, we found that ABCB1 mRNA expression was correlated with lymph-node metastasis and poor survival in patients with breast cancer. High expression of ABCB1 was correlated with increased expressions of IL6, CSF1, CSF3, and PTGS2 and decreased expression of VEGFA. Treatment with doxorubicin and paclitaxel suppressed growth of PDX tumors and repressed ABCB1 and VEGF expression. The expression of IL6, CSF1, CSF3, and PTGS2 increased after treatment with doxorubicin, but decreased after treatment with paclitaxel or trastuzumab. The PDX model reflected the clinical course of the donor patient. The combination of big data from RNA sequencing and experimental results from a PDX model represents a foundation for precision medicine.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This study was approved by the Institutional Review Board of National Cheng Kung University Hospital (A-ER-106-157) and was performed according to the Declaration of Helsinki. Written informed consent was obtained from all participants. The animal study was approved by the National Laboratory Animal Center of National Applied Research Laboratories, Tainan, Taiwan (NLAC(TN)-104-M-028-R2/R3 & 107-M-001-R1/R2), and the Laboratory Animal Center, College of Medicine, National Cheng Kung University (NCKU-107226 and 108114).
Acknowledgments
The authors are thankful to the patient who participated in the study. We thank the Laboratory Animal Center, College of Medicine, National Cheng Kung University, National Laboratory Animal Center, NARLabs, Taiwan, and Taiwan Animal Consortium for the technical support. We were blessed with support from the late superintendent, Professor Pin-wen Lin. We also thank Dr Po-Hsien Huang and Miss Ya-Li Hsiao for their support.
Funding
This study was supported by the Ministry of Science and Technology (MOST) of Taiwan (grant No. 109-2314-B-006-018-MY3 to H.P.H.), the National Cheng Kung University Hospital (grant Nos. NCKUH-10902031 & NCKUH-11002013 & NCKUH-11102007 to H.P.H.), and the Chi Mei Medical Center (grant No. CMNKCKU11004).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Ji X, Lu Y, Tian H, Meng X, Wei M, Cho WC. Chemoresistance mechanisms of breast cancer and their countermeasures. Biomed Pharmacother. 2019;114:108800. doi:10.1016/j.biopha.2019.108800
2. Chun KH, Park JH, Fan S. Predicting and overcoming chemotherapeutic resistance in breast cancer. Adv Exp Med Biol. 2017;1026:59–104. doi:10.1007/978-981-10-6020-5_4
3. Trock BJ, Leonessa F, Clarke R. Multidrug resistance in breast cancer: a meta-analysis of MDR1/gp170 expression and its possible functional significance. J Natl Cancer Inst. 1997;89(13):917–931. doi:10.1093/jnci/89.13.917
4. Nedeljković M, Tanić N, Prvanović M, Milovanović Z, Tanić N. Friend or foe: ABCG2, ABCC1 and ABCB1 expression in triple-negative breast cancer. Breast Cancer. 2021;28(3):727–736. doi:10.1007/s12282-020-01210-z
5. Arnason T, Harkness T. Development, maintenance, and reversal of multiple drug resistance: at the crossroads of TFPI1, ABC transporters, and HIF1. Cancers. 2015;7(4):2063–2082. doi:10.3390/cancers7040877
6. Choi YH, Yu AM. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr Pharm Des. 2014;20(5):793–807. doi:10.2174/138161282005140214165212
7. Tamaki A, Ierano C, Szakacs G, Robey RW, Bates SE, Sharom FJ. The controversial role of ABC transporters in clinical oncology. Essays Biochem. 2011;50(1):209–232. doi:10.1042/bse0500209
8. Hui RC, Francis RE, Guest SK, et al. Doxorubicin activates FOXO3a to induce the expression of multidrug resistance gene ABCB1 (MDR1) in K562 leukemic cells. Mol Cancer Ther. 2008;7(3):670–678. doi:10.1158/1535-7163.MCT-07-0397
9. Labani-Motlagh A, Ashja-Mahdavi M, Loskog A. The tumor microenvironment: a milieu hindering and obstructing antitumor immune responses. Front Immunol. 2020;11:940. doi:10.3389/fimmu.2020.00940
10. Bruno A, Mortara L, Baci D, Noonan DM, Albini A. Myeloid derived suppressor cells interactions with natural killer cells and pro-angiogenic activities: roles in tumor progression. Front Immunol. 2019;10:771. doi:10.3389/fimmu.2019.00771
11. Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010;185(4):2273–2284. doi:10.4049/jimmunol.1000901
12. Fujii E, Kato A, Suzuki M. Patient-derived xenograft (PDX) models: characteristics and points to consider for the process of establishment. J Toxicol Pathol. 2020;33(3):153–160. doi:10.1293/tox.2020-0007
13. Yoshida GJ. Applications of patient-derived tumor xenograft models and tumor organoids. J Hematol Oncol. 2020;13(1):4. doi:10.1186/s13045-019-0829-z
14. Chen C, Lin W, Huang Y, Chen X, Wang H, Teng L. The essential factors of establishing patient-derived tumor model. J Cancer. 2021;12(1):28–37. doi:10.7150/jca.51749
15. Whittle JR, Lewis MT, Lindeman GJ, Visvader JE. Patient-derived xenograft models of breast cancer and their predictive power. Breast Cancer Res. 2015;17(1):17. doi:10.1186/s13058-015-0523-1
16. Zhang X, Claerhout S, Prat A, et al. A renewable tissue resource of phenotypically stable, biologically and ethnically diverse, patient-derived human breast cancer xenograft models. Cancer Res. 2013;73(15):4885–4897. doi:10.1158/0008-5472.CAN-12-4081
17. Murayama T, Gotoh N. Patient-derived xenograft models of breast cancer and their application. Cells. 2019;8(6):621. doi:10.3390/cells8060621
18. Singh JC, Jhaveri K, Esteva FJ. HER2-positive advanced breast cancer: optimizing patient outcomes and opportunities for drug development. Br J Cancer. 2014;111(10):1888–1898. doi:10.1038/bjc.2014.388
19. Izumchenko E, Paz K, Ciznadija D, et al. Patient-derived xenografts effectively capture responses to oncology therapy in a heterogeneous cohort of patients with solid tumors. Ann Oncol. 2017;28(10):2595–2605. doi:10.1093/annonc/mdx416
20. Rong Y, Yuan CH, Qu Z, et al. Doxorubicin resistant cancer cells activate myeloid-derived suppressor cells by releasing PGE2. Sci Rep. 2016;6(1):23824. doi:10.1038/srep23824
21. Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58(1):49–59. doi:10.1007/s00262-008-0523-4
22. Delou JMA, Vignal GM, Índio-do-Brasil V, et al. Loss of constitutive ABCB1 expression in breast cancer associated with worse prognosis. Breast Cancer. 2017;9:415–428. doi:10.2147/BCTT.S131284
23. McQuerry JA, Chen J, Chang JT, Bild AH. Tepoxalin increases chemotherapy efficacy in drug-resistant breast cancer cells overexpressing the multidrug transporter gene ABCB1. Transl Oncol. 2021;14(10):101181. doi:10.1016/j.tranon.2021.101181
24. Podolski-Renić A, Andelković T, Banković J, Tanić N, Ruždijić S, Pešić M. The role of paclitaxel in the development and treatment of multidrug resistant cancer cell lines. Biomed Pharmacother. 2011;65(5):345–353. doi:10.1016/j.biopha.2011.04.015
25. National institute of health, United States National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/home.
26. National Research Council. Guide for the Care and Use of Laboratory Animals.
27. Goldman MJ, Craft B, Hastie M, et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat Biotechnol. 2020;38(6):675–678. doi:10.1038/s41587-020-0546-8
28. Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. doi:10.1158/2159-8290.CD-12-0095
29. Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):11. doi:10.1126/scisignal.2004088
30. Calcagno AM, Salcido CD, Gillet JP, et al. Prolonged drug selection of breast cancer cells and enrichment of cancer stem cell characteristics. J Natl Cancer Inst. 2010;102(21):1637–1652. doi:10.1093/jnci/djq361
31. Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–15550. doi:10.1073/pnas.0506580102
32. Győrffy B. Survival analysis across the entire transcriptome identifies biomarkers with the highest prognostic power in breast cancer. Comput Struct Biotechnol J. 2021;19:4101–4109. doi:10.1016/j.csbj.2021.07.014
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.