Back to Journals » Integrated Pharmacy Research and Practice » Volume 4
A prescription survey about combined use of acetylcholinesterase inhibitors and anticholinergic medicines in the dementia outpatient using electronic medication history data from community pharmacies
Authors Kurata K, Taniai E , Nishimura K, Fujita K, Dobashi A
Received 15 April 2015
Accepted for publication 28 July 2015
Published 1 October 2015 Volume 2015:4 Pages 133—141
DOI https://doi.org/10.2147/IPRP.S86661
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Jonathan Ling
Kaori Kurata,1 Eitarou Taniai,2 Kanae Nishimura,3 Kenji Fujita,3 Akira Dobashi1
1Education and Research Institute of Information Science, Tokyo University of Pharmacy and Life Sciences, Hachioji, Tokyo, Japan; 2Informational Headquarters, Yakuju Corporation, Minato-ku, Tokyo, Japan; 3General Incorporated Foundation Social University, Minato-ku, Tokyo, Japan
Purpose: We investigated prescriptions regarding the combined use of donepezil hydrochloride (DPZ) and anticholinergics for elderly outpatients in Japan to determine the impact that combination therapy has on decreasing their cognitive functions.
Methods: Using electronic medication records from 142 community pharmacies, outpatients older than 40 years of age taking DPZ, with or without other prescription medicines, were assessed over 6 years, beginning in 2007. We estimated the number of medicines administered along with DPZ, the number of anticholinergics administered along with DPZ, and the medicines' anticholinergic cognitive burden (ACB) scale cumulative score based on data from the top four pharmacies that filled the highest number of prescriptions for DPZ for outpatients with dementia in 2010. Data were gathered from records of 431 patients; only three patients were younger than 60 years.
Results: There was a 1.94-fold increase in the number of prescriptions including DPZ over 6 years. The proportion of patients to whom other medicines were administered along with DPZ was 65.6% (n=283) and the proportion of those taking at least one anticholinergic agent was 24.1% (n=104). The mean number of medicines among subjects taking at least one anticholinergic was 5.7, and the mean cumulative ACB score for anticholinergics contained in these medicines was 2.6. Among 104 patients to whom the anticholinergics were administered along with DPZ, two outpatients taking urologic medicines such as oxybutynin hydrochloride or tolterodine tartrate were found.
Conclusion: Our findings suggest that it is necessary to pay attention to a decline in cognitive function when prescribing multiple medicines, especially to elderly patients who have already been prescribed DPZ.
Keywords: dementia, donepezil hydrochloride, anticholinergic medicines, outpatients' medication, prescription survey, prescription cascade
Introduction
The criteria for dementia in the elderly who are eligible to receive long-term care insurance were established by the Ministry of Health, Labour and Welfare in Japan.1 For elderly with dementia, the degree of independent daily living is classified into five categories: I, II, III, IV, and M.
Category I represents light dementia, with subjects still capable of living independently in their home and community. Category II represents dementia that has progressed compared with Category I, and subjects have some disabilities associated with their condition, activities of daily living, and communication with others, but are capable of living independently under the guide of their families or caregivers. Category III requires long-term care or support, and Category IV represents a continuous decrease in living functions. Category M requires explicit medical care with health care professionals because the patient’s disability has significantly increased in terms of severe physical, mental, and behavioral problems.
Approximately 1,600,000 elderly Japanese were considered to have Category I dementia regardless of whether they received certification for long-term care insurance in 2010.2 When this population is added into the elderly population with dementia higher than Category II, the prevalence rate of dementia becomes approximately 15% of the Japanese population older than 65 years. Of this population, only 1.3% of the elderly live in a hospital setting.2 Furthermore, approximately 3,800,000 of the elderly are estimated to have mild cognitive impairment (MCI), a number that accounts for approximately 13% of the population older than 65 years of age.3 Elderly subjects with MCI do not always develop dementia, but the rate of shifting from MCI to dementia increases with age. The shifting rate in females is estimated to be 1.4-fold greater than that in males.4
Elderly subjects with dementia generally receive multiple medications, both for the treatment of dementia as well as for the treatment of comorbidities that occur with age. “Safe Pharmacotherapy Guidelines in the Elderly 2005” was published by the Japan Geriatrics Society in 2005 and it listed 45 pharmacologic categories of medicines that require particularly prudent administration in the elderly.5 Attention focuses on “anticholinergic actions” associated with 13 categories of drugs.6 The “Safe Pharmacotherapy Guidelines in the Elderly 2005” was based on Beers criteria published in the USA in 2003.7
Therapeutic medicines used for neuropsychiatric, digestive system, and urologic disorders are typical anticholinergics. An anticholinergic cognitive burden (ACB) scale and an anticholinergic risk scale have been proposed to evaluate the risk for cognitive functional disorders from the use of anticholinergics.8,9 Both scales are rated from 1 to 3, with a higher score being associated with a greater risk of leading to a cognitive functional disorder. Medicines listed in the ACB scale are not only typical anticholinergics but also those with serum anticholinergic activity (SAA) via nonspecific receptor binding, including warfarin potassium, furosemide, and nifedipine.10 Though these three drugs are not clinically classified as anticholinergics, the ACB scale rates these SAA medicines, along with medicines with a weak but definite anticholinergic action, as having a score of 1. In contrast, a score of 3 is given for medicines such as olanzapine and chlorpheniramine maleate for which the potential risk of developing cognitive functional disorders is high. Fox et al8 showed that a dose–response relationship was observed between an increase in cumulative ACB score and a decrease in Mini-Mental State Examination (MMSE) score, which measures individual cognitive function. A cumulative ACB score >4 is significantly associated with a decrease in MMSE score when compared with MMSE scores among patients not receiving anticholinergic medications.8
Donepezil hydrochloride (DPZ) is administered to delay the progression of all types of dementia in general. DPZ is an acetylcholinesterase inhibitor (AChEI) that increases acetylcholine (ACh) levels and can compete against anticholinergics in terms of its pharmacologic actions. Thus, simultaneous administration of DPZ and anticholinergics can affect therapeutic outcome. Through the autonomic nervous system, AChEIs can contribute to urinary incontinence. At the same time, the onset of or worsening of incontinence is commonly seen as part of the natural history of dementia. This fact leads to what is referred to as a prescription cascade.11 The prescription cascade represents what happens when additional medications are added to the patient’s regimen to the point that adverse drug reactions (ADRs) are considered an integral part of the patients’ disease progression or a disease by themselves. Because unintended harmful effects with medicines are similar to the geriatric syndrome that develops with the aging process, other medications are frequently prescribed to treat a disease that is actually an ADR.
We report on the cumulative ACB scores in outpatients receiving DPZ with anticholinergics to determine the impact that combination therapy has on decreasing their cognitive function.
Methods
Filling prescriptions for DPZ in community pharmacies
The number of prescriptions including DPZ, and the number of patients corresponding to these prescriptions, were extracted from a database electronically stored in a community pharmacy chain (Yakuju Corporation, Kanagawa, Japan) from January 1, 2007 to December 31, 2012. The Ethics Committee of Tokyo University of Pharmacy and Life Sciences (Tokyo, Japan) does not require approvals or patient consent for these summarized and anonymized descriptive studies. This corporation had 144 stores (as of December 31, 2012), located in the Japanese Kanto region (eastern half of Japan including Tokyo). Numeric data were collected from all pharmacy stores every year for the 6-year survey period in order to mine the pharmacies that filled the most prescriptions for DPZ until 2010 as well as examine changes in the number of DPZ prescriptions. The criteria for selecting the pharmacies are as follows: 1) filling prescriptions for medicines, not necessarily restricted to DPZ, had been started before December 31, 2012; 2) filling prescriptions for medicines had been continued; and 3) filling prescriptions for DPZ had been made one or more times during the survey period. This survey was carried out for patients older than 40 years who were divided into two groups: 40–59 years and 60 years and older.
Coadministration of DPZ and ACB medicines
Eighty-three ACB medicines have been identified based on generic names listed on the website of the University of East Anglia.12 Among these, 64 medicines containing four types of second-generation antipsychotics (olanzapine, quetiapine fumarate, risperidone, and perospirone hydrochloride) have been approved in Japan and were examined in our survey (Table 1).
To survey the coadministration of DPZ and ACB medicines, we selected the top four pharmacies where the frequency of filling prescriptions for DPZ was the highest from January 1, 2010 to December 31, 2010; these prescriptions were mainly provided by geriatric medical specialists.
As of 2010, the only dementia-related medicine reimbursed by the National Health Insurance in Japan was DPZ. Therefore, our 1-year survey in 2010 can accurately estimate the risk of a decline in cognitive function and/or a prescription cascade possibly brought on by the simple combination of DPZ and anticholinergics.
Medication history data from these pharmacies corresponding to the mentioned period was anonymized in an unlinkable fashion, and 431 patients (178 males and 253 females) who had taken DPZ were extracted from the anonymous data.
These patients were divided into three groups: group A included patients who received DPZ alone; group B included patients who had received one or more ACB medicines in addition to DPZ; and group C included patients who had received medications other than ACB medicines in addition to DPZ.
The maximum number of concomitant medicines that individual patients received along with DPZ was counted for group B and group C during the 1-year survey period; this number was defined as the “coadministration number”. The coadministration number of each patient was accumulated and averaged in each group. In group B, the score of ACB medicines was totaled to determine the “cumulative ACB score” for each patient.
Statistical analyses in older patients were carried out after further dividing these patients into two age groups: 60–79 years and 80–99 years. Only three patients were in the younger age group (40–59 years); they were excluded from the 1-year analysis of the four pharmacies because their number was so small. Statistical tests were performed using PASW Statistic 18 (IBM, Chicago, IL, USA). Fisher’s exact test (P<0.05) was applied for differences in the proportion of patients in groups B and C to that of all patients between males and females and that between patients aged 60–79 and 80–99 years and those corresponding to the proportion of the number in group B to that of the number in groups B and C. Mann–Whitney U test (P<0.05) was used for identifying differences in the mean number of medicines between sex, between the two elderly age groups, and between groups B and C.
Results
Annual change in the number of prescriptions for DPZ
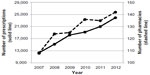
The number of prescriptions, including DPZ, had a tendency to increase 1.94-fold from 12,363 prescriptions in 2007 to 23,992 in 2012 (Figure 1). A Japanese formal prescription can include multiple medicines on one prescription page; thus, a “prescription including DPZ” is defined as one on which either DPZ as the generic name or its brand name such as Aricept (Eisai Co Ltd, Tokyo, Japan) is written or printed, even if other medications appear on the sheet.
During this 6-year survey, the number of community pharmacies filling DPZ prescriptions increased 1.31-fold from 108 stores in 2007 to 142 in 2012. The number of patients older than 60 years increased 1.94-fold from 1,826 people in 2007 to 3,550 in 2012. In contrast, the number of patients with ages ranging from 40 to 59 years was considerably smaller than that of the previous generation in each year and decreased 0.73-fold from 26 people in 2007 to 19 in 2012 (Figure 2).
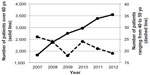  | Figure 2 Annual changes in the number of patients prescribed DPZ by age. |
Coadministration of DPZ and ACB medicines
Profile of 431 patients older than 40 years from four pharmacies
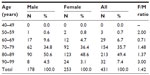
The mean age of 431 patients from the four pharmacies was 80.0±6.9 (SD) years; the mean age in males was 79.1±6.8 years and the mean age in females was 80.6±6.9 years. The number of patients by decade was the highest in the age range of 80–89 years (Table 2). The number of female patients was greater than that of male patients in every age range greater than 70 years: 1.48-fold greater for 70–79 years, 1.37-fold greater for 80–89 years, and threefold greater for 90–99 years.
Burden of concomitant use with DPZ
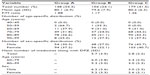
As shown in Table 3, 148 patients (34.3%) were in group A. One hundred four patients in group B had taken one or more ACB medicines (24.1%). Two hundred eighty-three patients (65.7%, coadministration rate) were in groups B (n=104) and C (n=179), who were administered a combination of one or more medicines in addition to DPZ. The proportion of patients in group B to that of groups B and C (ACB medicine-coadministration rate) was 36.7% (104/283).
The mean age of group B patients was 79.6±7.9 years, which was younger than that of group A (80.1±6.9 years) or group C (80.2±6.3 years). Approximately 21.1% of the patients aged 80–89 years were in group B, which was smallest of all the age groups (Table 3).
The proportion of patients in groups B and C to that of all patients (coadministration rate) calculated for the age ranges of 60–79 and 80–99 years was 67.2% and 73.0%, respectively. These values were not significantly different. The proportion of patients in group B to that of groups B and C (ACB medicine-coadministration rate) for the two age ranges was 37.4% and 31.8%, respectively. There were no significant differences by age.
The coadministration rates corresponding to groups B and C were 69.7% for males and 62.8% for females. The ACB medicine-coadministration rates by sex in group B were 38.7% and 35.2%, respectively. There were no significant differences by sex.
The mean number of medicines administered along with DPZ was 5.7 for group B (n=104) and 2.8 for group C (n=179) (Table 3). When group B was compared with group C, group B had a significantly greater mean number of medicines than group C (P<0.05). The mean number of medicines in group B was estimated for two age ranges: was 6.0 for those aged 60–79 years and 5.3 for those aged 80–99 years. There were no significant differences by age. There were no significant differences by age in group C or by sex in either group.
ACB medicines coadministered in this study
Among the 64 approved ACB medicines, 29 were administered to subjects in group B. These ACB medicines had the following ACB scores: 14 of 32 medicines (43.8%) with a score of 1, two of six (33.3%) with a score of 2, and 13 of 26 (50.0%) with a score of 3. Most of these medicines affected the central nervous system and were used to treat the behavioral and psychological symptoms of dementia. The ACB medicines with a score of 1 included seven SAA medicines: nifedipine, warfarin potassium, furosemide, ranitidine hydrochloride, cimetidine, captopril, and theophylline.
Table 4 shows the number of patients to whom each ACB medicine was administered, their mean age, the mean number of medicines given along with DPZ, and the mean cumulative score of ACB medicines. Among group B subjects taking at least one ACB medicine, the number of patients taking amantadine hydrochloride was the highest (n=24/104; 23.1%). Their mean age was 75.5 years, and the mean number of medicines was 6.4. The number of patients taking risperidone was second highest (n=13; 12.5%); these subjects had a mean age of 77.2 years and took a mean of 4.8 medicines.
  | Table 4 List of 29 ACB medicines administered with DPZ for each patient |
Among the 104 outpatients for whom ACB medicines were prescribed, two patients took oxybutynin hydrochloride or tolterodine tartrate, urologic medicines that have anticholinergic actions. Both patients took only one anticholinergic and a number of other medicines; this number of concomitant medicines was five for one patient (age 71 years, male) prescribed oxybutynin hydrochloride and ten for another patient (age 85 years, female) prescribed tolterodine tartrate.
Amitriptyline hydrochloride was most likely to be prescribed with other medications (mean, 17.0 other medicines) followed by digoxin (12.0 other medicines) and promethazine hibenzate and methylene disalicylate (11.0 other medicines each).
Cumulative scores of the ACB medicines administered to each patient
The mean number of medicines per person administered along with DPZ for 104 patients in group B in this study was 5.7, and the mean number of only ACB medicines per person administered along with DPZ for 104 patients in group B was 1.3 (Table 5). When the scores for the ACB medicines administered to each patient were added together, this total was averaged for 104 patients in group B, and the mean cumulative ACB score was found to be 2.6 (Table 5).
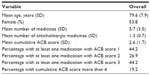  | Table 5 The burden of anticholinergic use among 104 outpatients in group B, as determined by the ACB scale |
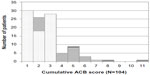
Among the 104 patients in group B, 20 (19.2%) had a cumulative ACB score greater than 4 (Figure 3). Fifteen patients showed cumulative ACB scores ranging from 4 to 6 with a combination of two ACB medicines, and two patients showed a cumulative ACB score of 5 or 7 with the combination of three ACB medicines. Three patients showed a cumulative ACB score of 5, 8, and 11 with the combination of four ACB medicines.
Discussion
This study showed that the number of outpatients who received DPZ at community pharmacies increased 1.94-fold from 2007 to 2012. The relationship between anticholinergic medicines and an increase in MCI was pointed out in the 1970s, and, in 2006, a longitudinal cohort study concluded that long-term continuous use of the anticholinergics lead to MCI in the elderly; the extent of the disability is light, but the frequency is high.13 Bhattacharya et al14 reported that, in the USA in 2006, 42.9% of outpatients with dementia who were older than 65 years had taken anticholinergics. There are only a few surveys related to the administration of anticholinergics among outpatients in Japan.15 The findings of this study showed that the mean concomitant medicines per prescription (not per patient) for dementia patients older than 65 years who also received DPZ was 4.2. The coadministration rate was 32.5% in this study; however, this represents the number of patients who had potentially taken medications other than ACB medicines, because they appeared on the medicine list evaluated with the Beers criteria.
The number of elderly patients with dementia who had received pharmacotherapy treatment in an outpatient setting in our study, based on administration of DPZ, had a clear tendency to increase in our survey period. This increase may be due to an increase in the older population, not due to an increase in the incidence of dementia. We can assume this increase from the fact that, as of the last 2012 in the 6-year survey period, 142 community pharmacies that are located into Kanto region have filled prescriptions including DPZ. It is reasonable to assume that with the growth of the elderly population, the number of elderly with dementia receiving DPZ will continue to increase in Japan.
Overall, 65.7% of 431 outpatients in our survey had taken another medication in addition to DPZ (24.1% for group B and 41.5% for group C), and 24.1% had taken an anticholinergic medicine in addition to DPZ (group B). In the USA, the coadministration rate of anticholinergics in outpatients aged 65 years and older with dementia was 42.9% in 2006−2007.14 In 2010, Shuto et al16 reported on the number of inpatients taking DPZ along with other medications. The proportion of inpatients taking DPZ in this study as divided by the groups defined in our study was as follows: group A, 12.1%; group B, 45.6%; and group C, 42.2%.16 Among these inpatients, the coadministration rate related to the number of patients in groups B and C, and that in group B was 87.9% and 45.6%, respectively.16 That prescription survey, however, was based on complete medication information for inpatients.
The coadministration rate in inpatients is generally higher than that in outpatients because inpatients have more severe conditions than those observed in outpatients.17,18 Our study found that the proportion of group A dementia outpatients taking DPZ alone was 34.3%, which was higher than the 12.1% observed for inpatients. Even if they may receive other medicines from other community pharmacies, we can consider the situation where elderly outpatients with dementia take DPZ only in Japan.
The 2008 Japanese census reported that 35.7% of outpatients older than 65 years had gone to two or more hospitals and/or clinics to receive medical follow-up treatment. Therefore, the coadministration rate estimated in our study may be considerably lower than the actual rate of the combined use of anticholinergics in elderly outpatients taking DPZ because their prescriptions are not always filled with one single pharmacy.
However, we could estimate the maximum risk for a decline in cognitive function with ACB medicines at least in one pharmacy, as approximately 60% of outpatients examined in our study had no change in the number of medicines that combined to DPZ.
Currently, there is no national database capable of listing all medicines administered to a particular patient in Japan. Our 1-year survey is intended not to follow-up the continuous administration of DPZ for a particular patient (as has been performed for inpatients by Shuto et al16), but to track prescriptions for outpatients to whom DPZ had been prescribed even once.
The combined use of DPZ and ACB medicines was surveyed in this study as of 2010. After 2011, rivastigmine and galanthamine hydrobromide, which are AChEIs, and memantine hydrochloride, which is an NMDA receptor antagonist, were approved for the Japanese market. Therefore, the risk for a decline in cognitive function and/or a prescription cascade brought on by the combination of these newer agents with anticholinergics should be studied in the future to provide updated data.
Our 1-year survey was intended to determine if a patient is receiving specified medicines that may interact and lead to a prescription cascade. We thus used the four pharmacies with the highest number of prescriptions for DPZ to help determine the use of DPZ and ACB medicines in an outpatient setting.
Increasing number of medicines administered at the same time may bring about ADRs, especially in the elderly.19 A combination of DPZ and anticholinergics for elderly patients with dementia may bring about a further decrease in cognitive function, and, in some cases, a prescription cascade, with additional medications being prescribed by mistake.
The average cumulative ACB score was 2.6 in our study. Approximately 19.2% of outpatients for whom DPZ has been prescribed however had the cumulative ACB score >4. Thus, additional administration of prescription medicines to patients should be carefully done, and patients should be followed up for the occurrence or progression of cognitive functional disorders.
Two outpatients taking oxybutynin hydrochloride/tolterodine tartrate along with DPZ were found in 431 outpatients for whom DPZ was prescribed for dementia, probably because of occurrence of urinary symptoms such as urinary incontinence as a result of taking DPZ. A cohort study by Gill et al11 showed that elderly (>65 years) who were diagnosed with dementia, receiving an AChEI, and had no recorded medical history of urinary incontinence still had a 1.66 hazard ratio of being prescribed an anticholinergic for impending urinary incontinence compared with patients not being treated with an AChEI. This finding indicates that patients receiving an AChEI may have a significant risk of experiencing a prescription cascade.
Because 86.4% of dementia patients are cared for at their homes or at nursing and care homes in Japan, we think that it is necessary to understand actual medication usage for dementia on an outpatient basis.2
DPZ may lead to harmful interactions with medicines metabolized by either CYP3A4 or CYP2D6 and those inducing CYP. Thus, prudent monitoring is required for elderly outpatients with dementia taking multiple medications.
As the pharmacotherapy of patients with dementia is often complicated, compared with these patients with other chronic diseases, all community pharmacies in Japan need to understand the potential risks associated with polypharmacy. Appropriate medication for dementia patients, which takes into consideration cognitive function disorder that may be brought about by concomitant anticholinergics, should be provided in all community pharmacies in Japan.
Disclosure
The authors report no conflicts of interest in this work.
References
[Notice of the Director-General of the Health and Welfare Bureau for the Elderly, Ministry of Health, Labour and Welfare, No 0119001]. Publication day; January 19, 2006. Available from: http://www.pref.mie.lg.jp/CHOJUS/HP/kaisei/other/nintei2.pdf. Accessed February 17, 2015. Japanese. | |
Ministry of Health, Labour and Welfare, Health and Welfare Bureau for the Elderly, Elderly Support Division, Dementia and Abuse Prevention Promotion Office. [Orange plan explanatory material]. Publication day; June 25, 2013. Available from: http://www.mhlw.go.jp/stf/shingi/2r98520000035rce-att/2r98520000035rfx_1_1.pdf. Accessed February 17, 2015. Japanese. | |
Asada T. [Dementia prevalence in urban areas and corresponding to the life dysfunction of dementia. Health, Labour and Welfare Chemical Research Grant, Dementia Counterplan Research Project, Report of Research Project from 2011 to 2012]. 2013. Japanese. | |
Takeda M. [Factors to defend the cognitive decline and factors defining cognitive impairment]. Nou 21. 2012;15(2):117–122. Japanese. | |
Japan Geriatrics Society. [Safe Pharmacotherapy Guideline on Elderly 2005]. 1st ed. Tokyo, Japan: Medical View Co, Ltd; 2005. Japanese. | |
Japan Geriatrics Society. [List of pharmaceutical medicines asking for particularly prudent administration for the elderly, 2005]. Available from: http://www.jpn-geriat-soc.or.jp/proposal/pdf/drug_list.pdf. Accessed February 17, 2015. Japanese. | |
Fick DM, Cooper JW, Wade WE, Waller JL, Ma-clean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163(22):2716–2724. | |
Fox C, Richardson K, Maidment ID, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59(8):1477–1483. | |
Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med. 2008;168(5):508–513. | |
Carnahan RM, Lund BC, Perry PJ, Pollock BG, Culp KR. The anticholinergic drug scale as a measure of drug-related anticholinergic burden; associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46(12):1481–1486. | |
Gill SS, Mamdani M, Naglie G, et al. A prescribing cascade involving cholinesterase inhibitors and anticholinergic drugs. Arch Intern Med. 2005;165(7):808–813. | |
University of East Anglia. Drugs on the Anticholinergic Burden (ACB) scale. Available from: http://www.uea.ac.uk/mac/comm/media/press/2011/June/Anticholinergics+study+drug+list;. Accessed February 17, 2015. | |
Ancelin ML, Artero S, Portet F, Dupuy AM, Touchon J, Ritchie K. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. BMJ. 2006;332(7539):455–459. | |
Bhattacharya R, Chatterjee S, Carnahan RM, Aparasu RR. Prevalence and predictors of anticholinergic agents in elderly outpatients with dementia. Am J Geriatr Pharmacother. 2011;9(6):434–441. | |
Tanaka Y, Onda M, Nanaumi Y, et al. An attempt at objective evaluation of the current situation of concomitant drug use for dementia outpatients at community pharmacies. Jpn J Drug Inform. 2015;15(4):155–164. | |
Shuto H, Miyazu D, Imakyure O, et al. [Survey of combination use of acetyl cholinesterase inhibitors and anticholinergic drugs]. In: Proceeding of 15th Academic Conference in Japanese Society of Drug Informatics; 2012:107. Japanese. | |
Hanlon JT, Schmader KE, Ruby CM, et al. Suboptimal prescribing in older inpatients and outpatients. J Am Geriatr Soc. 2001;49(2):200–209. | |
Brunot A, Lachaux B, Sontag H, et al. [Pharmaco-epidemiological study on antipsychotic drug prescription in French psychiatry: patient characteristics, antipsychotic treatment, and are management for schizophrenia]. Encephale. 2002;28(2):129–138. French. | |
Ministry of Health, Labour and Welfare, Minister ‘s Secretariat, Statistics and Information Department, Social Statistics Division. [Outline of Survey: Survey of Medical Care Activities in Public Health Insurance 2008]. Tokyo, Japan: Ministry of Health, Labour and Welfare. Publication day; June 2009. Available from: http://www.mhlw.go.jp/toukei/saikin/hw/sinryo/tyosa08/. Accessed February 17, 2015. Japanese. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.