Back to Journals » Local and Regional Anesthesia » Volume 17
A Novel Ultrasound-Guided “Three in One” Approach Plus Interfascial Plane Blocks for the Treatment of Cervicogenic Headache
Authors Ma D, Maimaitimin A, Wang Y
Received 26 October 2023
Accepted for publication 16 January 2024
Published 1 February 2024 Volume 2024:17 Pages 1—8
DOI https://doi.org/10.2147/LRA.S446667
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Stefan Wirz
Danxu Ma,1 Abulaihaiti Maimaitimin,2 Yun Wang3
1Department of Anesthesiology and Pain Medicine, Beijing Chaoyang Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Rheumatology and Immunology, Moyu Uighur Medicine Hospital, Xinjiang, People’s Republic of China; 3Department of Anesthesiology, Beijing Friendship Hospital, Capital Medical University, Beijing, People’s Republic of China
Correspondence: Yun Wang, Department of Anesthesiology, Beijing Friendship Hospital, Capital Medical University, No. 95 Yongan Road, Xicheng District, Beijing, 100050, People’s Republic of China, Email [email protected]
Objective: Cervicogenic headache (CEH) is a condition resulting from upper cervical spine dysfunction and associated structural and soft tissue abnormalities, significantly impacting patients’ quality of life. To acquire better therapeutic results, we presented a novel ultrasound-guided “three in one” approach plus interfascial plane (IFP) blocks for the treatment of CEH. This approach allows for the modulation of C2 dorsal root ganglion (DRG), third occipital nerve (TON), and C3 medial branch with one-point puncture. Additionally, it allows for IFP blocks between the upper neck and occipital muscles within the same scanning plane.
Patients and Methods: We evaluated patients diagnosed with CEH from July 2021 to December 2022 in our pain clinic. We included those who did not respond to conservative treatment and single occipital nerve block, therefore received nerve block or pulsed radiofrequency (PRF) using the “Three in One” approach plus IFP blocks. The accuracy of the ultrasound-guided C2 DRG puncture procedures was confirmed through fluoroscopy with C-arm and the sensory testing of PRF. The therapeutic effect of these interventions was assessed using the numerical rating scale (NRS) scores during telephone follow-ups at 1, 3, and 6 months.
Results: Utilizing the “Three in One” approach, a total of 5 patients diagnosed with CEH underwent nerve block plus IFP blocks, while 2 patients underwent PRF plus IFP blocks. Employing ultrasound-guided C2 DRG puncture procedures, the needle tip’s correct placement was confirmed through both fluoroscopy and sensory testing of PRF. Notably, none of the cases experienced any complications associated with the approach. Subsequent follow-up assessments revealed an improvement in the NRS scores for CEH in all patients.
Conclusion: The ultrasound-guided “Three in One” approach plus IFP blocks may be a potential effective method for the treatment of CEH.
Keywords: cervicogenic headache, interfacial plane blocks, pulsed radiofrequency, C2 dorsal root ganglion, third occipital nerve
Introduction
Cervicogenic headache (CEH) is defined as secondary headache disorders originating from upper cervical spine structures, including cervical facet joints, neurovascular structures and cervical muscles, mainly innervated by the upper cervical spinal nerves. The prevalence of CEH in the general population has been reported at 4.1%.1 Its principle clinical symptom is unilateral or bilateral occipital-distribution pain radiating to frontal regions, aggravated by neck movement, accompanied by neck pain and cervical muscle stiffness.2 It severely affects the quality of patients’ life, and is often associated with conditions such as depression and anxiety disorder.
Treatment options for CEH should be pursued in a gradual, step-wise fashion, beginning with conservative management, including oral/transdermal medication, manual therapy and physical therapy.3 If the conservative therapeutics are not effective, minimally invasive intervention treatment can be chosen, and surgical interventions could be reserved for select patient populations who have failed all other conservative and minimally invasive options.4,5 Cervical 2 dorsal root ganglion (C2 DRG), third occipital nerve (TON) and C3 medial branch block or pulsed radiofrequency (PRF), are optional options for minimally invasive treatment.6 Traditionally, these interventional procedures are usually carried on under the guidance of C-arm or Computed Tomography (CT), which cannot directly render real-time vision of the needle tip for interventionalists during operative procedures, and the risk of spinal cord and vertebral artery injury cannot be completely avoided. Recent studies have highlighted the use of ultrasound in CEH, for its convenience, without radiation, guided in real time, the visibility of vertebral artery, the posterior complex (ligamentum flavum and dura mater), and even the target nerves, such as TON.7,8 There were few studies reported ultrasound-guided C2 DRG block or PRF, either the “short axis scanning” or “oblique axial scanning” with an in-plane technique method was selected.7,9–11 However, we doubt that the ultrasonic beam can always just enter the atlantoaxial joint cavity and find C2 DRG, at this scanning angle. Moreover, we consider that the risk of spinal cord injury still cannot be completely avoided, by using its puncture target and needle insertion method. Furthermore, it is worth mentioning that, for the existence of neural interconnections and multiple nerves involved in CEH,4,12 the effect of single nerve modulation was often unsatisfactory, and modulation of multiple nerves was required.13
Here, we introduced the technical considerations of an ultrasound-guided “Three in One” approach plus interfascial plane (IFP) blocks for the treatment of CEH. This approach allows for the modulation of C2 DRG, TON and C3 medial branch with one-point puncture, and the IFP blocks between the upper neck and occipital muscles can be performed concurrently within the same scanning plane.
Patients and Methods
Patient Selection
The patients met the following selection criteria were considered for the nerve block or PRF with the “Three in One” approach plus IFP blocks: 1) Patients present with occipital-distribution pain meet the diagnosis of CEH according to the International Classification of Headache Disorders, 3rd Edition (ICHD–3).14 2) Failed with conservative therapy, including oral/transdermal medication, manual therapy, physical therapy.5,15 3) Magnetic Resonance Imaging (MRI) and/or CT of the cervical spine should be performed in order to exclude cervical myelopathy, fracture, and tumors, and which is more important, to evaluate the upper cervical nerve roots and facet joints. Demonstration of a lateral atlanto-axial (AA) joint disorder on imaging may suggest the lateral AA joint as the pain generator, and support the indication of C2 DRG block or/and radiofrequency ablation. Classically, the imaging manifestations of lateral AA joint osteoarthrosis are cartilage loss, subchondral sclerosis and subarticular bone marrow edema. Posterior osteophyte formation may further narrow the C1–C2 foramen and impinge on the C2 DRG, resulting in CEH.16,17 In some cases, C2-C3 facet joint disorders can also be shown in the imaging. 4) Failed with empirical ultrasound-guided single occipital nerve block using a 2 mL solution (a mixture of 0.5% lidocaine, 1mg/mL dexamethasone, and 0.9% normal saline). This included the greater occipital nerve (GON) block between the inferior oblique muscle (IOM) and the semispinalis capitis (SSC), the lesser occipital nerve (LON) block between the sternocleidomastoid muscle, the trapezius muscle (TZ) and the splenius capitis muscle (SC), or the TON block above the C2-C3 facet joint. A block failure was defined as a reduction of less than 20% in characteristic pain at 1 week following a single occipital nerve block. 5) No signs of infection at the systemic and local puncture points.
This study was approved by the Institutional Review Board (IRB) of Beijing Chaoyang Hospital and the study was conducted in accordance with the Helsinki Declaration. Written informed consent was obtained from all patients included in this study.
Technique
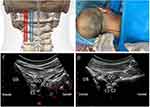
Patients should be placed in the prone position on the procedure table with a chest cushion, the forehead positioned on a contoured foam pillow to ensure a constant position and neck flexion. The surgical procedure can be performed under conscious sedation for patient comfort and for PRF sensory testing. The posterior midline of cervical spine was marked on the patients’ skin (Figure 1B). All ultrasound scans were performed using an ultrasound system (Sonimage HS1 Plus, Konica Minolta, Tokyo, Japan) and a curved array probe (C5-2 MHz frequency). Ultrasound gel was applied between the probe and the skin of the upper cervical region for optimal coupling and the scout scan was performed. The transducer was held in the non-dominant hand of the operator and was positioned 1–2 cm lateral to the spine midline (Figure 1A, blue rectangle), with its orientation marker directed cranially (paramedian sagittal scanning), and the ultrasound beam was insonated into the spinal canal through the interlaminar space. On the sonogram, the important structures such as posterior and anterior complex, C1 arch, the upper cervical laminae, occipital bone, and muscles group could be identified (Figure 1C). Then, the transducer was shifted laterally (Figure 1A, red rectangle) until the hyperechoic posterior and anterior complex just disappeared and the lateral AA joint appeared on the sonogram (Figure 1D). The transducer was finally positioned over the lateral AA joint, and the position of the transducer was marked on the patient’s back using a skin-marking pen.
“Three-in-One” Block
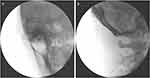
The skin of the occipital and the upper cervical region was disinfected with iodophor and draped with sterile towel. The gel was then applied to the ultrasound transducer, and then the transducer and cable were covered with a sterile plastic sleeve. Iodophor served as the coupling agent. Then, the transducer was placed on the marked position. The lateral AA joint was visualized under the US scan and was kept in the center of the US image. The C2 DRG can be visualized in the axial plane just dorsal to the lateral AA joint. One percent lidocaine 2 mL was injected at 1 cm caudal to the transducer for local infiltration. A 22-gauge, 3.5-inch Quincke-type spinal needle (TuoRen Medical Instrument Finty, Zhengzhou, China) was next inserted in the plane of the transducer such that its tip was oriented towards the C2 DRG (Figure 1B). The needle angle was optimized while in the trapezius muscle, and the needle was then slowly advanced into the target DRG along the C2 laminae and inferior aspect of OCI muscle until the needle tip reached the target (Figure 1D). Fluoroscopy with a C-arm was performed to confirm the position of the needle tip in the anteroposterior (Figure 2A), and lateral view (Figure 2B). Then, the syringe was withdrawn to ensure that no blood return occurred, and 2 mL solution (mixture of 0.5% lidocaine + 1mg/mL dexamethasone + 0.9% normal saline) was slowly instilled through the needle. Care should be taken to avoid advancing the needle medial to the DRG, given the close proximity to the dura. If blood is seen in the hub, the needle may be carefully repositioned, since there is a rich venous plexus surrounding the C2 DRG.
On the sonogram, C2-C3 facet joint could be identified, and TON was founded as a hypoechoic oval shape encircled by a hyperechoic halo above the articulation of C2-C3. And the C3 medial branch was just at the deepest point between the articulations of C2-C3 and C3-C4 (Figure 3A). Therefore, the TON and C3 medial branch could be blocked after completing the C2 DRG block, without changing the puncture site.
“Three-in-One” Pulsed Radiofrequency (PRF)
For PRF, the authors recommend a 22-gauge, 10-cm-length cannula with a 5-mm uninsulated tip. Following the steps described above, the spinal needle was substituted by the PRF cannula (Beijing Neo Science Co., Ltd). Once the appropriate position was confirmed on the sonogram, the style was removed and the radiofrequency probe was inserted. Impedance levels should be checked and are typically <300 Ω. Sensory testing was then performed at 50 Hz, a tingling or prickling sensation should be elicited in the occipital region similar to the regular pain location with voltage 0.3–0.5 V. Motor testing was performed at 2 Hz, mild local contraction of the paraspinous musculature would be evoked with voltage 0.6 ~ 0.8V. Then, a fluoroscopy was obtained with the PRF probe in place for final confirmation of positioning. PRF was then performed at 42°C for 600 seconds. Following PRF, the probe was removed and 2 mL solution as described above could be injected through the cannula to decrease post-procedural inflammation and discomfort. Similarly, the PRF of TON and C3 medial branch could be performed, without changing the puncture site.
Interfascial Plane (IFP) Blocks
Finally, all patients received IFP blocks, the solutions as described above were injected into the interfascial plane between the OCI and SSC, the SSC and TZ, the SSC and SC, or the SC and TZ, sequentially, with a dosage of 1–2 mL per fascia plane (Figure 3B).
Results
Between July 2021 and December 2022, A total of 7 patients with CEH who came to our hospital pain clinic were enrolled into this study, and the patients’ characteristics are shown in Table 1. Utilizing the “Three in One” approach, with 5 cases underwent nerve blocks plus IFP blocks, while 2 patients underwent PRF plus IFP blocks.
 |
Table 1 Patient Basic Information and Clinical Parameters |
After ultrasound-guided C2 DRG puncture, radiographs demonstrated the accurate positioning of the needle tip for C2 DRG. The anteroposterior fluoroscopy view indicated that the needle tip was situated in the middle of the lateral AA joint (Figure 2A), while the lateral fluoroscopy view revealed that the needle was positioned on the surface of the lateral AA joint (Figure 2B). During the process of PRF, the placement of the needle tip was further validated through sensory testing of PRF, which elicited a tingling or prickling sensation in the occipital region of these two patients, resembling the typical pain location at a voltage of 0.3–0.5 V.
All the procedures went well, and there were no complication associated with the “Three in One” approach in any of the cases. Except for Case 4, no patients underwent invasive treatment subsequent to the treatment procedure. The additional treatment modalities administered to these patients are detailed in Table 1. Telephone follow-up assessments conducted at 1, 3, and 6 months indicated a noteworthy improvement in the numeric rating scale (NRS) scores of all patients, which are also provided in Table 1.
Discussion
The multitude of pain generators makes the diagnosis and treatment of CEH difficult, and the application of the “three in one” approach plus IFP blocks for the treatment of CEH is based on a “full coverage” treatment concept. This study substantiates the practicability of the aforementioned method, 7 patients received this treatment method without any notable adverse effects. All patients have experienced an uneventful clinical course after the procedure and reported a discernible alleviation of pain.
It is known that the most common pain generators of CEH are the lateral AA joint, C2-C3 and C3-4 facet joint.6,18 Thus, their corresponding innervation nerves, such as C2 DRG, TON and C3 medial branch, are often selected as the target of interventional therapy. Among them, ultrasound-guided C2 DRG puncture has the highest difficulty and risk coefficient. Wu et al and Fadayomi et al7,11 believed that C2 DRG is just located below the OCI muscle, and an oblique axis scanning was adopted, the probe was placed along the OCI muscle. Li et al9,10 introduced a short-axis scanning method, the spinous process of C2 was first located, then the probe shifted laterally to find the space between C1 and C2. After the location, they all use the in-plane technique to advance the needle. However, we think that although ultrasound allows real-time needle visualisation, it does not guarantee that the tip of the needle is strictly in the plane, and there is still a risk of spinal cord injury. The “Three in One” approach proposed by us choose the long-axis scanning, the transducer was shifted laterally until the hyperechoic posterior and anterior dura maters just disappeared and the lateral AA joint appeared on the sonogram, so as to avoid spinal cord injury during puncture. Moreover, it can be seen from Figure 1D that the lateral AA joint occlusion presents with certain angle, the long-axis scanning is benefit for the ultrasonic beam entering the joint cavity. Additionally, we found that some patients with CEH have severe tension spasms of the OCI muscle, and there might be a sharp pain caused by the needle penetrating the OCI muscle. In our “Three in One” approach, the needle was advanced along the C2 laminae and the inferior aspect of OCI muscle, so as to minimise the patient’s discomfort.
The next problem to be solved is that the modulation of single nerve in CEH patients is often not favorable. For example, Lee et al19 carried out a retrospective analysis of 45 patients with CEH who received PRF at the C2 DRG, only 18/45 (40.0%) of patients had pain reduction effects of more than 50% at 6 months after the procedure. Govind et al20 performed radiofrequency neurotomy of TON on 49 patients with third occipital headache, 43 (88%) achieved complete relief of pain for at least 90 days. A potential cause of failed pain interventions for CEH was the existence of Cruveilhier plexus, the communicating neural loops between the upper cervical dorsal rami.12,21 A previous anatomical study demonstrated interconnections between C1–C2–C3 (54–65.4%) are more frequently observed, and 36.4% articular branches to the C2–3 facet joint could arise from communicating branches between the TON and C2 dorsal ramus.12 For this reason, multiple upper cervical nerves need to be modulated in CEH treatment. Undoubtedly, our “Three in One” method provides a convenient approach for the modulation of multiple upper cervical nerves.
Interfascial plane (IFP) blocks have been a research hotspot in recent years, due to its safety, simplicity, and effectiveness. Conveniently, patients with CEH are often accompanied by muscle tension of OCI, SSC, TZ and SC. Thus, it is possible for these muscular investments could serve as a source of compression or irritation of the GON and TON.22,23 Therefore, decompression of GON and TON with IFP blocks is effective for some patients, and may result in enduring pain relief.24–26 With this treatment modalities, we injected the solution into the interfascial plane between the OCI and SSC, the SSC and TZ, the SSC and SC, or the SC and TZ concurrently (Figure 3B), to relieve the muscle tension, to release the entrapment place of the peripheral nerve, in order to achieve better therapeutic effect.
Absolutely, the aggravation and relief of CEH are influenced by multiple factors,27 including age, psychopathology, genetics, sleep problems, obesity, sedentary lifestyle, trauma, back pain, and poor general health. For example, Case 6 was a computer engineer and spent most of his time in front of the screen. Case 3 was a baby sitter, and she had difficulty in sleeping at night. Case 2 had undergone lumbar spine surgery and femoral head replacement surgery in the past, and Case 3 had mild thoracic scoliosis, they all experienced back pain. The above factors exacerbate the pain of CEH and may have impact the long-term effects of our treatment. However, paying attention to posture and lifestyle habits, as well as engaging in physical training, such as Case 5 and 7, can alleviate CEH.3 Anyway, both from the NRS scores and the use of analgesics, all of our cases have shown some degree of pain reduction, which demonstrates the effectiveness of our treatment.
Conclusions
In conclusion, this initial study demonstrated that the “Three in One” plus IFP blocks is a potential effective method for the treatment of CEH. However, additional randomized controlled clinical studies are necessary to compare the effectiveness of this approach with other interventional treatments for CEH.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sjaastad O, Bakketeig LS. Prevalence of cervicogenic headache: vaga study of headache epidemiology. Acta Neurol Scand. 2008;117(3):173–180. doi:10.1111/j.1600-0404.2007.00962.x
2. Ng A, Wang D. Cervical facet injections in the management of cervicogenic headaches. Curr Pain Headache Rep. 2015;19(5):484. doi:10.1007/s11916-015-0484-1
3. Jull G. Cervicogenic headache. Musculoskelet Sci Pract. 2023;66:102787. doi:10.1016/j.msksp.2023.102787
4. Raposio G, Raposio E. Surgical therapy of occipital (Arnold) neuralgia: a case series. Ann Med Surg Lond. 2022;80:104237. doi:10.1016/j.amsu.2022.104237
5. Barmherzig R, Kingston W. Occipital neuralgia and cervicogenic headache: diagnosis and management. Curr Neurol Neurosci Rep. 2019;19(5):20. doi:10.1007/s11910-019-0937-8
6. Nagar VR, Birthi P, Grider JS, Asopa A. Systematic review of radiofrequency ablation and pulsed radiofrequency for management of cervicogenic headache. Pain Physician. 2015;18(2):109–130.
7. Wu B, Yue L, Sun F, Gao S, Liang B, Tao T. The feasibility and efficacy of ultrasound-guided C2 nerve root coblation for cervicogenic headache. Pain Med. 2019;20(6):1219–1226. doi:10.1093/pm/pny227
8. Siegenthaler A, Schliessbach J, Curatolo M, Eichenberger U. Ultrasound anatomy of the nerves supplying the cervical zygapophyseal joints: an exploratory study. Reg Anesth Pain Med. 2011;36(6):606–610. doi:10.1097/AAP.0b013e3182286af5
9. Li J, Yin Y, Ye L, Zuo Y. Pulsed radiofrequency of C2 dorsal root ganglion under ultrasound-guidance and CT confirmed for chronic headache: follow-up of 20 cases and literature review. J Pain Res. 2020;13:87–94. doi:10.2147/JPR.S229973
10. Li J, Yin Y, Ye L, Zuo Y. Pulsed radiofrequency of C2 dorsal root ganglion under ultrasound guidance for chronic migraine: a case report. J Pain Res. 2018;11:1915–1919. doi:10.2147/JPR.S172017
11. Fadayomi O, Kendall MC, Nader A. Ultrasound-guided pulsed radiofrequency of C2 dorsal root ganglion as adjuvant treatment for chronic headache disorders: a case report. A a Pract. 2019;12(11):396–398. doi:10.1213/XAA.0000000000000942
12. Tubbs RS, Mortazavi MM, Loukas M, D’Antoni AV, Shoja MM, Cohen-Gadol AA. Cruveilhier plexus: an anatomical study and a potential cause of failed treatments for occipital neuralgia and muscular and facet denervation procedures. J Neurosurg. 2011;115(5):929–933. doi:10.3171/2011.5.JNS102058
13. Li SJ, Feng D. Pulsed radiofrequency of the C2 dorsal root ganglion and epidural steroid injections for cervicogenic headache. Neurol Sci. 2019;40(6):1173–1181. doi:10.1007/s10072-019-03782-x
14. Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders. Cephalalgia. 2018;38(1):1–211. doi:10.1177/0333102417738202
15. Xiao H, Peng BG, Ma K, et al. Expert panel’s guideline on cervicogenic headache: the Chinese association for the study of pain recommendation. World J Clin Cases. 2021;9(9):2027–2036. doi:10.12998/wjcc.v9.i9.2027
16. Chazen JL, Roytman M, Yoon ES, Mullen TK, Lebl DR. CT-Guided C2 dorsal root ganglion radiofrequency ablation for the treatment of cervicogenic headache: case series and clinical outcomes. AJNR Am J Neuroradiol. 2022;43(4):575–578. doi:10.3174/ajnr.A7471
17. Chazen JL, Ebani EJ, Virk M, Talbott JF, Shah V. CT-guided block and radiofrequency ablation of the C2 dorsal root ganglion for cervicogenic headache. AJNR Am J Neuroradiol. 2019;40(8):1433–1436. doi:10.3174/ajnr.A6127
18. Mehnert MJ, Freedman MK. Update on the role of z-joint injection and radiofrequency neurotomy for cervicogenic headache. PM R. 2013;5(3):221–227. doi:10.1016/j.pmrj.2013.01.001
19. Lee HJ, Cho HH, Nahm FS, Lee PB, Choi E. Pulsed radiofrequency ablation of the C2 dorsal root ganglion using a posterior approach for treating cervicogenic headache: a retrospective chart review. Headache. 2020;60(10):2463–2472. doi:10.1111/head.13759
20. Govind J, King W, Bailey B, Bogduk N. Radiofrequency neurotomy for the treatment of third occipital headache. J Neurol Neurosurg Psychiatry. 2003;74(1):88–93. doi:10.1136/jnnp.74.1.88
21. Baek IC, Park K, Kim TL, J O, Yang HM, Kim SH. Comparing the injectate spread and nerve involvement between different injectate volumes for ultrasound-guided greater occipital nerve block at the C2 level: a cadaveric evaluation. J Pain Res. 2018;11:2033–2038. doi:10.2147/JPR.S1726910.2147/JPR.S172692
22. Son BC, Kim DR, Lee SW. Intractable occipital neuralgia caused by an entrapment in the semispinalis capitis. J Korean Neurosurg Soc. 2013;54(3):268–271. doi:10.3340/jkns.2013.54.3.268
23. Kim HS, Shin KJ, Kwon HJ, Lee M, Yang HM, Yang H-M. Stereotactic topography of the greater and third occipital nerves and its clinical implication. Sci Rep. 2018;8(1):870. doi:10.1038/s41598-018-19249-6
24. Blake P, Burstein R. Emerging evidence of occipital nerve compression in unremitting head and neck pain. J Headache Pain. 2019;20(1):76. doi:10.1186/s10194-019-1023-y
25. Arici T. Ultrasound-guided interfascial blocks of the trapezius muscle for cervicogenic headache: a report of two cases. Agri. 2021;33(4):278–281. doi:10.14744/agri.2020.08831
26. Gfrerer L, Hansdorfer MA, Ortiz R, Chartier C, Nealon KP, Austen WG. Muscle fascia changes in patients with occipital neuralgia, headache, or migraine. Plast Reconstr Surg. 2021;147(1):176–180. doi:10.1097/PRS.0000000000007484
27. Cohen SP, Hooten WM. Advances in the diagnosis and management of neck pain. BMJ. 2017;358:j3221. doi:10.1136/bmj.j3221
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.