Back to Journals » Chronic Wound Care Management and Research » Volume 10
A Moisture-Balancing Hydropolymer Gel Dressing with a Tissue Boost Effect - Experimental and Clinical Evidence
Authors Wiegand C , Wesenberg U, Heggemann J
Received 28 June 2023
Accepted for publication 14 September 2023
Published 5 October 2023 Volume 2023:10 Pages 11—21
DOI https://doi.org/10.2147/CWCMR.S422493
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Marco Romanelli
Cornelia Wiegand,1 Ulrike Wesenberg,2 Jan Heggemann3
1Department of Dermatology, Jena University Hospital, Friedrich Schiller University, Jena, Germany; 2Community Hospital Herdecke, Herdecke, Germany; 3Christian Hospital Melle, Niels Stensen Hospitals, Melle, Germany
Correspondence: Cornelia Wiegand, Department of Dermatology, Jena University Hospital, Am Klinikum 1, Jena, D-07747, Germany, Tel +49 3641 9328878, Fax +49 3641 9328876, Email [email protected]
Purpose: Chronic wounds are impaired in their healing processes and often require a tissue boost to restart healing progression. Dressings of all forms have been used extensively for decades and a variety of dressings was specifically designed for chronic wounds. A dressing based on a hydropolymer gel matrix with a semi-permeable polyurethane backing is thought to provide an optimal balanced wound moisture environment and could hence positively affect the biochemical composition of exudate. However, the mechanism behind the technology is described sparsely.
Patients and Methods: We herein report in vitro data to support the mode of action of the hydropolymer gel dressing concerning fluid management, protease binding and thermal insulation. Moreover, a non-interventional prospective study examined the use of the unique hydropolymer gel dressing on pain levels.
Results: Data demonstrated a possible tissue boost by retaining as well as providing fluid, depending on the exudate amount, and by binding proinflammatory proteases. The dressing was further able to stabilize growth factors and provide thermal insulation, thereby positively supporting keratinocyte proliferation and migration in vitro. Furthermore, a significant reduction of the pain level after application could be observed clinically.
Conclusion: Together, these factors provide a beneficial environment for wound healing and restarting stagnating wounds (or providing optimal conditions for acute wounds). In addition, the dressing appeared to be a good choice for painful wounds.
Keywords: hydrogel, pain relief, cooling effect, thermal insulation, wound healing
Introduction
Wound care is an extensive medical field that has been characterized by rapid development in recent decades. The process of wound healing is distinguished by the superimposed phases: hemostasis, inflammation, proliferation and maturation/remodeling.1 These complex physiological processes involve the interaction of different cell types, growth factors, extracellular matrix components, and proteinases.2 In non-healing or slow-healing chronic wounds, the healing progression is interrupted. These wounds are characterized by an excessive inflammatory phase. Studies on model organisms yielded mechanistic insights into the influence of inflammatory pathways on wound healing.3 They lead to increased infiltration of neutrophils, heightened levels of pro-inflammatory cytokines, and elevated amounts of proteases including matrix metalloproteases (MMPs) and elastase as well as augmented quantities of reactive oxygen and nitrogen species (RONS). In addition, reduced amounts of growth factors lead to impaired cellular functions of fibroblasts and epithelial cells.4 For instance, in chronic wounds, growth factors such as platelet-derived growth factor (PDGF) are affected, which promote chemotactic cell recruitment and proliferation as well as increases angiogenesis.4 New findings from the analysis of chronic wound-associated fibroblasts revealed lysosomal dysfunction and dysregulated transforming growth factor (TGF)-ß signaling, which could explain the decrease in proliferation and migration.5
In the 20th century, the positive effects of moist wound care on wound healing were described for the first time and advanced wound dressings made from innovative materials were developed.6,7 Wound dressings based on foams, alginates, hydrogels and hydrocolloids can be regarded as state-of-the-art in wound care.7 There is a wealth of clinical evidence supporting the effectiveness of different wound dressings, although no advanced wound dressing appears to be superior for specific wound etiologies.8–10 Nonetheless, these dressings have been proven to be beneficial compared to traditional gauze dressings.8–10 The following properties of a wound dressing are believed to be critical in promoting wound healing: (i) providing or maintaining a moist environment, (ii) allowing gas exchange, (iii) maintaining an adequate tissue temperature to enhance blood flow and epidermal migration, (iv) offers protection against bacterial infection and (v) is non-adherent, sterile, non-toxic and non-allergic.6,7 Moisture vapor transmission rate (MVTR) and thermal insulation capability are therefore important parameters when evaluating wound dressings.11,12
The hydropolymer gel dressing analyzed here is a sterile, self-adhesive wound dressing with a semi-permeable polyurethane backing. The moisture balance of this combination is achieved through the three-dimensional network of hydrophilic, synthetic polymers, which can absorb or release fluid depending on the hydration status of the wound. Notably, injured skin can lose 20 times more fluid compared to normal skin, pointing to the importance of MVTR and the high fluid capacity of wound dressings such as hydropolymer gel dressings. These conditions allow for cell proliferation and epithelialization, which helps accelerate the healing rate.13
In clinical use, the hydropolymer dressing demonstrated a positive influence on the wound environment in chronic wounds.14 The exact mechanisms why the dressing stimulates the tissue and restarts the wound healing process have not yet been described. It is suggested that the hydropolymer dressing creates a moist environment through vertical fluid uptake while keeping exudate away from the wound edges and stimulating autolytic debridement. The aim of the study was to investigate technical properties of the hydropolymer dressing such as absorption and MVTR, influence on inflammatory proteases, growth factors and temperature for the first time in a comprehensive approach as well as demonstrate the influence of the dressing in the clinical setting.
Materials and Methods
Measurement of Absorption Capacity and MVTR
Absorption and MVTR were measured for the hydropolymer gel dressing (Cutimed® HydroControl, BSN medical GmbH, Hamburg, Germany) and the results evaluated in comparison to a hydrocolloid dressing (Comfeel® Plus Transparent; Coloplast A/S, Humlebæk, Denmark) and a foam dressing (ALLEVYN® Thin; Smith & Nephew, London, United Kingdom). Each wound 10×10 cm dressing was placed in inverted beakers for an incubation period of 24 hours at 37 ± 1° C and 10 ± 1% relative humidity in accordance with DIN EN 13726–1:2002, section 3.3 (n=6). Vertical absorption was measured for the hydropolymer gel dressing. The dressing was placed on a vertical, heated glass plate (37° C) while a colored test solution flowed through the center of the dressing at 1 mL/h. The spread of fluid on the front and back of the dressings was assessed photographically at baseline, after 1 hour, 12 hours and 24 hours.
Binding Assays for MMP-8, MMP-9, PMN Elastase and PDGF-BB
Binding assays were performed as previously reported.15–17 In brief, 8 mm samples (~ 0.5 cm²) of wound dressings were placed in 24-well cell culture plates (n = 4) and treated with human MMP-8 (2 ng/mL), MMP-9 (2 ng/mL), platelet-derived growth factor (PDGF-BB, 1 ng/mL) (all R&D Systems GmbH, Wiesbaden Germany) or PMN elastase (250 ng/mL, Demeditec Diagnostics GmbH, Kiel, Germany) in a final volume of 1 mL protein solution. Glass cover slips served as controls. Samples were incubated for up to 24 hours at 37°C on a plate mixer before collection of supernatants. Bound protein was eluted from the individual samples by shaking in 1 mL phosphate-buffered saline (BioConcept AG, Allschwil, Switzerland) for 1 hour at 37°C. Mediator concentrations were determined by specific ELISA assays as recommended by the manufacturers (R&D Systems GmbH, Wiesbaden Germany, Demeditec Diagnostics GmbH, Kiel Germany). All values are expressed as mean ± SD (standard deviation). One-way analysis of variance was carried out to determine statistical significances (Microsoft Excel 2000). Differences were considered statistically significant at a level of p < 0.05. Asterisks indicate significant deviations from the control (* p < 0.05; ** p < 0.01; *** p < 0.001).
Scratch Wound Assay
The scratch wound assay was performed as described by Wiegand et al18 Briefly, HaCaT keratinocytes (provided by Prof. Fusenig, University Heidelberg, Germany) were cultured in Dulbecco’s modified Eagle’s Medium (DMEM) (BioConcept AG) supplemented with 1% antibiotic-antimycotic solution (PELOBiotech GmbH, Planegg, Germany) and 10% fetal calf serum (PAN-Biotech GmbH, Aidenbach, Germany) at 37°C and 5% CO2 atmosphere. Use of the keratinocyte cell lines was approved by the institutional ethics committee (4739–03/16). A total of 40.000 cells/cm² were seeded into 6-well slides and cultured for 48 hours to confluence. Cell monolayers were scratched with a sterile pipette tip and loose cells were removed by rinsing with medium prior to the experiment. Samples of 4 cm diameter were prepared from the hydropolymer gel dressing and cotton gauze pads (Fuhrmann GmbH, Much, Germany) and equilibrated in DMEM before application. The samples were directly placed onto the cells (n = 4), while supernatant was adjusted beforehand to pH 7.4. Cells were incubated for up to 48 hours followed by hematoxylin and eosin staining. Scratch width was determined using VHX 950F software (KEYENCE DEUTSCHLAND GmbH, Neu-Isenburg, Germany). Progression of healing, designated as wound area in [%], was calculated comparing scratch width at each time point to the initial scratch width at baseline. All values are expressed as mean ± SD (standard deviation). One-way analysis of variance was carried out to determine statistical significances (Microsoft Excel 2000). Differences were considered statistically significant at a level of p < 0.05. Asterisks indicate significant deviations from the control (* p < 0.05; ** p < 0.01; *** p < 0.001).
Determination of Thermal Insulation
Temperature profiles beneath the hydropolymer gel dressing were measured via temperature sensors covering an agar surface (MHE agar plates of 5 mm thickness and 10 cm diameter, Biomerieux, Marcy l’Etoile, France) on a heating block at 32.5°C, representing the skin wound temperature. Agar plates were covered with the hydropolymer gel dressing or cotton gauze pads, either dry or prewetted with 10 mL and 2 mL water, respectively. Temperature sensors (TC Direct, Germany) were placed under the samples and recorded the temperature every 5 seconds for 3 hours to assess heat transfer (n = 5).
Case Report of Hydropolymer Gel Dressing Performance
A Case report was collected in everyday clinical practice at the Herdecke Community Hospital in 2021. Treatment of a 74-year-old male patient, who had been suffering from venous leg ulcers on the lateral malleolus for 6 weeks as a result of a traumatic accident, is briefly described. The written informed consent was provided by the patient to have the case details and any accompanying images published.
Clinical Study on Pain
Between March and September 2017, a prospective, monocentric, observational study investigating hydropolymer gel dressings for wound pain was conducted at the Christliches Klinikum Melle (Melle, Germany). The design and conduct of the observational study were in accordance with the Declaration of Helsinki. All procedures performed in the study with human participants were reviewed and were in accordance with the ethical standards of the institution. The observational study was conducted in 2016 based on MDD 93/42 EEC and consideration of the German Medical Device Regulation §23 Patient Consent. Written informed consent was obtained from all participants. The responsible PI, had the additional qualification of good clinical practice to ensure the implementation and documentation according to the MDD.
Inclusion criteria were men and women ≥18 years, a written consent to participate in the study and dry to moderately exuding, uninfected, superficial wounds. Exclusion criteria were not willing or capable to consent into the study participation or an allergy against one of the product components. 26 patients were enrolled. Patients were scheduled for three consecutive visits (initial visit, dressing change and last visit) and wound pain level was assessed using a visual analogue scale for pain level before application, 15 minutes and 6 hours after application. The dressing was changed and the final visit was made after a maximum of 7 days, depending on the investigator’s decision. In addition, comfort, atraumatic removal, pain on removal, and adverse events were monitored. Descriptive and inferential statistical methods were used for the analysis. Dependent t-tests were performed to compare pain scores at different time points throughout the study. In addition, a sub-analysis was performed distinguishing between patients with a baseline pain score < 4 and a baseline pain score ≥ 4. Mann–Whitney U-tests were performed to compare subgroups.
Results
Absorption Capacity
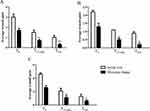
Absorption capacity and MVTR are key characteristics of wound dressings that provide information on the ability to maintain a moist wound environment. The hydropolymer gel dressing showed high absorption and MVTR capacity compared to the comparison dressings (Figure 1A). For the hydropolymer gel dressing, the vertical absorption test further demonstrated that the fluid retained safely (Figure 1B).
Binding of Proteases and Stabilization of Growth Factors
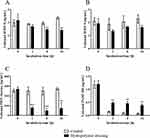
In order to assess the influence of the wound dressing on the molecular status of the wound, the ability of the hydropolymer gel to bind and eliminate inflammatory proteases was examined. Figure 2A and B shows a high binding capacity of the proteases MMP-8 and MMP-9 to the hydropolymer gel dressing samples. The bound proteases were efficiently sequestered and the concentration of free proteases was significantly reduced over time (p<0.01). This effect was even more pronounced for PMN elastase (Figure 2C), which was significantly reduced as early as 1 h and with a persistent effect up to 24 h (p<0.01). Furthermore, the hydropolymer gel dressing was able to stabilize the growth factor PDGF-BB under the test conditions (Figure 2D), thereby efficiently increasing the PDGF concentration in the artificial wound exudate solution (p<0.01).
Scratch Assay
In vitro wound healing was evaluated by a scratch assay using keratinocytes. Mechanical scratching of confluent cell monolayers allows the measurement of cell migration at the wound edges. The assay was further performed specifically to simulate conditions that result in the cells “dry-out” when the applied dressing is unable to maintain a humid environment. The results are shown in Figure 3. It was noted that the scratches covered with cotton gauze (Figure 3A, left panel) exhibited no tendency to heal and the cells presented a damaged, rounded appearance. In contrast, treating the scratches with the hydropolymer gel dressing (Figure 3A, right panel) resulted in a significantly increased rate of scratch healing compared to cotton gauze (Figure 3B, p < 0.001).
Thermal Insulation
Wound dressings play an important part by protecting and insulating the wound to improve healing. Coverage by the hydropolymer gel dressing was shown to increase the temperature on the artificial wound bed over time (Figure 4, blue lines). The insulating function was best in a pre-wetted state. Application of dry gauze pads resulted in only a slight increase, while the temperature under the pre-wetted cotton gauze pad was not much higher than on the bare agar surface (room temperature of 25°C) (Figure 4, grey lines).
Case Report
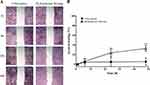
Initially, the 6-months old ulcus cruris venosum was treated by the patient with ointment containing dexpanthenol. At the first visit, the wound measured 40×20 mm and the wound bed was partially covered with fibrin (Figure 5A). The wound was in a low-exuding state, while the wound edges showed signs of edema. There was no further evidence of wound infection. The wound was cleansed prior to application of the hydropolymer gel dressing. Within 4 weeks, wound conditions improved visibly, characterized a dry wound bed and areas of healthy granulation tissue (Figure 5B). The patient reported easy application even under compression.
Assessment of Pain Reduction in a Clinical Setting
Perceived pain can be alleviated by a cooling effect. The ability to provide pain relief by cooling was evaluated in a clinical trial. Twenty-six patients (median age: 65 years) were followed for a median wear-time of 9 days. Most patients were male (69.2%). Half of the patients (N = 13) were treated for lacerations or abrasions. The dressing was applied to donor sites in 5 patients and to superficial ulcers in another 4 patients. Wound exudate was mostly low (n = 23), while 2 patients showed no signs of exudate, and 1 patient had a moderately exuding wound. None of them had decreased pain sensitivity. Wound pain prior to initial dressing application was 3.0 (± 0.3). The results of the pain assessment are shown in Figure 6. Pain was rated significantly lower after application of the hydropolymer gel dressing (Figure 6A) for 15 minutes (difference after 15 minutes: 1.6 ± 0.2; p < 0.05) and 6 hours (difference after 6 hours: 2.0 ± 0.3; p < 0.05). A sub-analysis distinguishing between patients who rated their initial wound pain < 4 (Figure 6B) and those who rated their initial wound pain ≥ 4 (Figure 6C) showed that the effect was more pronounced in patients with higher initial pain scores. Overall, 73.0% of the patients rated the pain-relieving properties of the dressing as “very good” or “good”. Most patients (80.7%) further rated the cooling effect of the dressing as “good” or “satisfactory”. Moreover, a 100% of the patients rated the wearing comfort of the dressing to be “very good” or “good”. At the final visit, dressing removal was atraumatic in 90.2% of patients. No skin reaction occurred in any of the patients during the study period.
Discussion
There might not be an ideal wound dressings for all wounds, however, the following characteristics are crucial for adequate support: (i) providing or maintaining a moist environment, (ii) allowing gas exchange, (iii) maintaining appropriate tissue temperature to improve blood flow and epidermal migration, (iv) providing protection against bacterial infection as well as (v) being non-adherent, sterile, non-toxic and non-allergic.6,7 The wound environment and thus the wound healing process are influenced by a variety of factors. Key factors include the amount and composition of wound exudate, which varies between acute and chronic wound etiologies.19,20 The fluid represents blood depleted of most red cells and platelets21–23 and is normally produced in the initial healing process, helping to create a moist wound environment, removing devitalized tissue and providing nutrients to support epithelialization.21
A moist wound environment, such as that created by modern dressings, supports wound healing by supporting cell proliferation and migration.13 Xu et al reported on appropriate MVTRs to maintain optimal wound hydration to support proliferation and regular function of epidermal cells and fibroblasts.24 Hydrogels are characterized by a hydrophilic, cross-linked 3D network of natural or synthetic polymers with a high-water content. The structure and function of hydrogels is similar to the natural extracellular matrix (ECM). Hydrogels create a moist environment on the wound surface, feature good absorption capacity for tissue exudates and possess oxygen permeability [Qi et al 2022, Zhang et al 2022]. Therefore, hydrogel dressings are also suitable for necrotic wounds25 by increasing the moisture content of the necrotic tissue and thus facilitating autolytic debridement.26 The presented case report on a chronic venous leg ulcer demonstrates the necessity of establishing a humid milieu for a chronically dry wound to ensure an optimal wound healing environment, which could be achieved by application of the hydrogel dressing. On the other hand, hydrogel dressings can also absorb up to 1000 g/g of liquid, making them suitable dressings for highly exuding wounds.27 Here, it was demonstrated that the absorption capacity of the hydropolymer gel dressing was significantly higher compared to a hydrocolloid or foam dressing making it suitable for appropriate exudate management. The scratch wound test showed that the application of the hydropolymer gel dressing improved the cell repair processes, while cotton gauze negatively influenced the regeneration of the cell monolayers. Cell damage under cotton gauze was attributed to desiccation of cells due to moisture loss, as cotton gauze samples slowly dried out over time under the experimental conditions. In contrast, it could be shown for the first time that the hydropolymer gel dressing maintains a humid environment, which supported wound closure in the test. Previously, it could be demonstrated that wound dressings are capable of inducing growth factor gene expression in keratinocytes and fibroblasts in vitro [Morgner et al 2022]. While moisturizing, the hydropolymer may further support wound healing through gas-induced osmosis, which oxygenates the wound and could increase collagen production. This would indirectly support epithelization; however, further analysis evaluating oxygenation are needed to validate the hypothesis.
The availability of molecular oxygen is essential in wound tissue during collagen synthesis and maturation of procollagen into stable triple helix collagen.28 Often, extracellular matrix deposition is insufficient in chronic wounds due to deficient collagen remodeling and production by fibroblast. At the same time, increased concentrations of proteases, including MMPs and PMN elastase, excessively degrade the extracellular matrix.28 Several studies have shown that exudates from non-healing wounds contain elevated levels of proteases including MMPs and PMN elastase.29–31 The excessive action of elastase further leads to significantly reduced levels of growth factors and protease inhibitors,32 leaving MMP-2 (gelatinase A) uncontrolled to cleave collagen, elastin and fibronectin eventually causing extracellular matrix destruction.31 State-of-the-art concepts of modern wound management focus on reducing these inflammatory mediators and establishing a moist wound environment. Therefore, absorbent capacity and the ability to entrap inflammatory proteases are important functions for dressings to be used on chronic wounds. The hydropolymer gel dressing decreased the concentration of the proteases MMP-8, MMP-9 and PMN-elastase in vitro by safely entrapping the biomolecules in the absorbed exudate via an osmotic effect. It is assumed that inhibition of pro-inflammatory protease activity and its sequestration protects growth factors and newly formed granulation tissue.33 This property is also described for other wound dressings such as alginates and superabsorbent wound dressings with polyacrylate.15–17,34 Clinical evidence for the beneficial effects on MMP-inhibiting wound dressings with regard to wound closure, wound size reduction, healing time and healing rate exists [Dissemond et al 2020]. Current developments in wound management further involve the delivery of growth factors to the wound.35 For example, a PDGF-containing gel showed simultaneous biomimetic and chemotactic effects and promoted wound healing in excision wounds.36 It was further found that the hydropolymer gel dressing stabilizes the growth factor PDGF in addition to eliminating pro-inflammatory proteases, which would benefit the wound healing process.
The optimal wound environment is further determined by tissue temperature. It has been shown that a temperature below 28°C impedes the wound healing process due to disturbed cell metabolism.12,37 Accordingly, it has been found that the center of chronic wounds is often relatively hypothermic, delaying any healing progress.12,38,39 In addition to physical treatments to increase the wound temperature, wound dressings play an important role in ensuring the protection and insulation of the wound to improve healing progression. However, this feature seems to be rarely addressed in performance studies on wound dressings. In this novel experimental approach, it was demonstrated that the hydropolymer gel dressing offered superior insulation properties compared to cotton gauze pads. Interestingly, pre-moistened cotton gauze reduced wound temperature, while moisture was insulated and warmed by the hydropolymer gel dressing.
Another aspect of wound management is the management of wound-related pain, as this is also essential for optimal wound healing. Dressings can contribute to pain and trauma by mechanically detaching the stratum corneum from underlying epidermal and/or dermal cells, but also by mechanically stimulating underlying inflammatory tissue and damaging granulation tissue. Therefore, advanced wound dressings should prevent wound-related pain during dressing changes. A reduction of the skin temperature can have a soothing effect on painful and inflamed skin areas. Due to the water content and water activity, such a cooling effect of the hydropolymer gel dressing is to be expected.40 In accordance, a significant reduction of the wound pain perceived by patients could be demonstrated for the hydropolymer gel dressing. The observed effect was above the smallest reasonable reduction as described.41 A sub-analysis showed that the effect was greater in patients with higher initial pain. The cooling effect is due to the high water content and subsequent exposure and evaporation of the water in the hydrogel layer. It is hypothesized that this effect also has an impact on the inflammatory process,42 but there is no evidence to date for the hydropolymer gel dressing. Nevertheless, the clinical data together with reports in the literature43 showed that this analgesic effect lasted up to 6 hours and was dependent on the initial pain. Other studies have shown that the cooling effect of hydrogel dressings reduces pain, particularly in burns.43 The cooling effect can be enhanced by air movement; therefore, the dressing should remain uncovered. The temperature differences on the skin can be up to 4 degrees (considering an analgesic threshold of 28 °C and 32 °C skin temperature).43
Comparing the properties of the hydropolymeric gel dressing to the ideal wound dressing discussed above, it can be concluded that the dressing provides and maintains a moist environment, allows gas exchange, isolates the wound and maintains an appropriate tissue temperature while protecting the wound from external influences. Clinical evidence also confirmed that the hydropolymer gel dressing allowed for atraumatic dressing changes as it was non-adherent and no potential for toxicity and allergic reactions was reported.
Conclusion
Our data showed that the hydropolymer gel dressing conveys a tissue boost to the wound by providing a moist wound environment and depleting inflammatory proteases via an osmotic mode of action. In addition, the wound temperature is stabilized by the dressing, which enables effective cell metabolism. The result of the observational study showed that the cooling effect of the dressing and the associated pain relief in the patients is achieved through a high MVTR and the quality of life is improved. Together, these factors provide a beneficial environment for wound healing and restarting stagnating wounds as well as providing optimal conditions for acute wounds.
Abbreviations
DIN-EN, Deutsches Institut für Normung-Europäische Norm (German institute for standardization-European standard); MHE, Mueller Hinton E; MMP, matrix metalloproteinase; MVTR, moisture vapor transmission rate; PDGF, platelet-derived growth factor; PMN, polymorphonuclear; RONS, reactive oxygen and nitrogen species; SD, standard deviation.
Acknowledgments
The authors would like to thank Ms. Bianka Morgner for excellent technical assistance for the scratch wound assays. The authors would further like to express their gratitude to BSN medical GmbH for providing financial support for manuscript editing.
Funding
The in vitro study and the case report were financially supported by BSN medical GmbH. The observational study was sponsored by BSN medical GmbH.
Disclosure
Author CW received travel grants from BSN medical GmbH to attend wound healing conferences. The authors report no other conflicts of interest in this work.
References
1. Braund R, Hook S, Medlicott NJ. The role of topical growth factors in chronic wounds. Curr Drug Deliv. 2007;4(3):195–204. doi:10.2174/156720107781023857
2. Han G, Ceilley R. Chronic wound healing: a review of current management and treatments. Adv Ther. 2017;34(3):599–610. doi:10.1007/s12325-017-0478-y
3. Eming SA, Hammerschmidt M, Krieg T, Roers A. Interrelation of immunity and tissue repair or regeneration. Semin Cell Dev Biol. 2009;20(5):517–527. doi:10.1016/j.semcdb.2009.04.009
4. Patel S, Maheshwari A, Chandra A. Biomarkers for wound healing and their evaluation. J Wound Care. 2016;25(1):46–55. doi:10.12968/jowc.2016.25.1.46
5. Berberich B, Thriene K, Gretzmeier C, et al. Proteomic profiling of fibroblasts isolated from chronic wounds identifies disease-relevant signaling pathways. J Invest Dermatol. 2020;140(11):2280–2290. doi:10.1016/j.jid.2020.02.040
6. Dhivya S, Padma VV, Santhini E. Wound dressings - A review. Biomedicine. 2015;5(4):22. doi:10.7603/s40681-015-0022-9
7. Ghomi ER, Khalili S, Khorasani SN, Neisiany R, Ramakrishna S. Wound dressings: current advances and future directions. J Appl Polym Sci. 2019;136:47738. doi:10.1002/app.47738
8. Dumville JC, O’Meara S, Deshpande S, Speak K. Alginate dressings for healing diabetic foot ulcers. Cochrane Database Syst Rev. 2013;2013(6):CD009110. doi:10.1002/14651858.CD009110.pub3
9. O’Meara S, Martyn-St James M, Adderley UJ. Alginate dressings for venous leg ulcers. Cochrane Database Syst Rev. 2015;2015(8):CD010182.
10. Everett E, Mathioudakis N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci. 2018;1411(1):153–165. doi:10.1111/nyas.13569
11. Mennini N, Greco A, Bellingeri A, De Vita F, Petrella F. Quality of wound dressings: a first step in establishing shared criteria and objective procedures to evaluate their performance. J Wound Care. 2016;25(8):428–437. doi:10.12968/jowc.2016.25.8.428
12. Hoffmann G. Wassergefiltertes Infrarot A (wIRA) zur Verbesserung der Wundheilung. GMS Krankenhaushyg Interdiszip. 2006;1(1):Doc20.
13. Kamoun EA, Kenawy ES, Chen X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J Adv Res. 2017;8(3):217–233. doi:10.1016/j.jare.2017.01.005
14. Tickle J. Using an innovative dressing to balance wound moisture. J Commun Nurs. 2017;31(3):30.
15. Wiegand C, Hipler UC. A superabsorbent polymer-containing wound dressing efficiently sequesters MMPs and inhibits collagenase activity in vitro. J Mater Sci Mater Med. 2013;24(10):2473–2478. doi:10.1007/s10856-013-4990-6
16. Wiegand C, Abel M, Ruth P, Hipler UC. Superabsorbent polymer-containing wound dressings have a beneficial effect on wound healing by reducing PMN elastase concentration and inhibiting microbial growth. J Mater Sci Mater Med. 2011;22(11):2583–2590. doi:10.1007/s10856-011-4423-3
17. Wiegand C, Heinze T, Hipler UC. Comparative in vitro study on cytotoxicity, antimicrobial activity, and binding capacity for pathophysiological factors in chronic wounds of alginate and silver-containing alginate. Wound Repair Regen. 2009;17(4):511–521. doi:10.1111/j.1524-475X.2009.00503.x
18. Wiegand C, Abel M, Hipler UC, Elsner P. Effect of non-adhering dressings on promotion of fibroblast proliferation and wound healing in vitro. Sci Rep. 2019;9(1):4320. doi:10.1038/s41598-019-40921-y
19. Gibson D, Cullen B, Legerstee R, Harding K, Schultz G. MMPs made easy. Wounds Int. 2009;1:1–6.
20. Amato B, Coretti G, Compagna R, et al. Role of matrix metalloproteinases in non-healing venous ulcers. Int Wound J. 2015;12(6):641–645. doi:10.1111/iwj.12181
21. Boateng JS, Matthews KH, Stevens HN, Eccleston GM. Wound healing dressings and drug delivery systems: a review. J Pharm Sci. 2008;97(8):2892–2923. doi:10.1002/jps.21210
22. Wiegand C, Tittelbach J, Hipler UC, Elsner P. Clinical efficacy of dressings for treatment of heavily exuding chronic wounds. Chronic Wound Care Manag Res. 2015;2:101–111. doi:10.2147/CWCMR.S60315
23. Hindhede A, Meuleneire F. A clinical case-series evaluation of a superabsorbent dressing on exuding wounds. J Wound Care. 2012;21(11):574–580. doi:10.12968/jowc.2012.21.11.574
24. Xu R, Xia H, He W, et al. Controlled water vapor transmission rate promotes wound-healing via wound re-epithelialization and contraction enhancement. Sci Rep. 2016;6:24596. doi:10.1038/srep24596
25. Sahiner N, Sagbas S, Sahiner M, Silan C, Aktas N, Turk M. Biocompatible and biodegradable poly(Tannic Acid) hydrogel with antimicrobial and antioxidant properties. Int J Biol Macromol. 2016;82:150–159. doi:10.1016/j.ijbiomac.2015.10.057
26. Schultz GS, Sibbald RG, Falanga V, et al. Wound bed preparation: a systematic approach to wound management. Wound Repair Regen. 2003;11(Suppl 1):S1–28. doi:10.1046/j.1524-475X.11.s2.1.x
27. Chen Y, Zhang Y, Wang F, et al. Preparation of porous carboxymethyl chitosan grafted poly (acrylic acid) superabsorbent by solvent precipitation and its application as a hemostatic wound dressing. Mater Sci Eng C Mater Biol Appl. 2016;63:18–29. doi:10.1016/j.msec.2016.02.048
28. Eisenbud DE. Oxygen in wound healing: nutrient, antibiotic, signaling molecule, and therapeutic agent. Clin Plast Surg. 2012;39(3):293–310. doi:10.1016/j.cps.2012.05.001
29. Barrick B, Campbell EJ, Owen CA. Leukocyte proteinases in wound healing: roles in physiologic and pathologic processes. Wound Repair Regen. 1999;7(6):410–422. doi:10.1046/j.1524-475X.1999.00410.x
30. Trengove NJ, Stacey MC, MacAuley S, et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen. 1999;7(6):442–452. doi:10.1046/j.1524-475X.1999.00442.x
31. Yager DR, Nwomeh BC. The proteolytic environment of chronic wounds. Wound Repair Regen. 1999;7(6):433–441. doi:10.1046/j.1524-475X.1999.00433.x
32. He C, Hughes MA, Cherry GW, Arnold F. Effects of chronic wound fluid on the bioactivity of platelet-derived growth factor in serum-free medium and its direct effect on fibroblast growth. Wound Repair Regen. 1999;7(2):97–105. doi:10.1046/j.1524-475X.1999.00097.x
33. Hollister C, Li VW. Using angiogenesis in chronic wound care with becaplermin and oxidized regenerated cellulose/collagen. Nurs Clin North Am. 2007;42(3):457–465. doi:10.1016/j.cnur.2007.05.002
34. Eming S, Smola H, Hartmann B, et al. The inhibition of matrix metalloproteinase activity in chronic wounds by a polyacrylate superabsorber. Biomaterials. 2008;29(19):2932–2940. doi:10.1016/j.biomaterials.2008.03.029
35. Jin Q, Ma PX, Giannobile WV. Platelet-derived growth factor delivery via nanofibrous scaffolds for soft-tissue repair. Adv Skin Wound Care. 2010;1:375–381.
36. Judith R, Nithya M, Rose C, Mandal AB. Application of a PDGF-containing novel gel for cutaneous wound healing. Life Sci. 2010;87(1–2):1–8. doi:10.1016/j.lfs.2010.05.003
37. Hoffmann G. Principles and working mechanisms of water-filtered infrared-A (wIRA) in relation to wound healing. GMS Krankenhaushyg Interdiszip. 2007;2(2):Doc54.
38. Hartel M, Hoffmann G, Wente MN, Martignoni ME, Büchler MW, Friess H. Randomized clinical trial of the influence of local water-filtered infrared A irradiation on wound healing after abdominal surgery. Br J Surg. 2006;93(8):952–960. doi:10.1002/bjs.5429
39. Mercer J, de Werd L. The effect of water-filtered infrared-A (wIRA) irradiation on skin temperature and skin blood flow as evaluated by infrared thermography and scanning laser Doppler imaging. Thermology Int. 2015;15(3):89–94.
40. Jones L. Thermal touch in Prescott T. In: Ahissar E, Izhikevich E, editors. Scholarpedia of Touch. Paris: Atlantis Press; 2016:257–262.
41. Campbell WI, Patterson CC. Quantifying meaningful changes in pain. Anaesthesia. 1998;53(2):121–125. doi:10.1046/j.1365-2044.1998.00294.x
42. Jandera V, Hudson DA, de Wet PM, Innes PM, Rode H. Cooling the burn wound: evaluation of different modalites. Burns. 2000;26(3):265–270. doi:10.1016/S0305-4179(99)00133-3
43. Coats TJ, Edwards C, Newton R, Staun E. The effect of gel burns dressings on skin temperature. Emerg Med J. 2002;19(3):224–225. doi:10.1136/emj.19.3.224
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.