Back to Journals » International Journal of Women's Health » Volume 16
A Case Report of Consecutive Live Birth Twice Through in vitro Fertilization and Embryo Transfer After Endometrial Carcinoma Fertility Preservation Treatment
Authors Wang J, Fang Y, Chen T, Xin Z, Wu Y, Yang X
Received 25 September 2023
Accepted for publication 25 February 2024
Published 6 March 2024 Volume 2024:16 Pages 395—400
DOI https://doi.org/10.2147/IJWH.S441984
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Jingying Wang,1,2 Ying Fang,1,2 Tong Chen,1,2 Zhimin Xin,1,2 Yumei Wu,3,4,* Xiaokui Yang1,2,*
1Department of Human Reproductive Medicine, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Human Reproductive Medicine, Beijing Maternal and Child Health Care Hospital, Beijing, People’s Republic of China; 3Department of Gynecological Oncology, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing, People’s Republic of China; 4Department of Gynecological Oncology, Beijing Maternal and Child Health Care Hospital, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaokui Yang, Department of Human Reproductive Medicine, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, 251 Yaojiayuan Road, Beijing, 100026, People’s Republic of China, Tel +86 522 766 15, Email [email protected] Yumei Wu, Department of Gynecological Oncology, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, 251 Yaojiayuan Road, Beijing, 100026, People’s Republic of China, Tel +86 522 766 53, Email [email protected]
Abstract: Preserving fertility is a vital concern for young women diagnosed with endometrial carcinoma. The clinical management of such patients is often disappointing. It is rare to have two consecutive successful pregnancies. We present a child-bearing-age woman who underwent fertility preservation therapy due to endometrial carcinoma. Following fertility preservation therapy, she underwent in vitro fertilization and embryo transfer. After receiving her first fresh embryo transfer, she successfully conceived and gave birth to a healthy child. Two years after the first embryo transfer and regular follow-up, she had another frozen embryo transfer of two cleavage embryos and successfully gave birth to another healthy baby. After the delivery of her second child, she underwent surgical treatment for endometrial carcinoma. For endometrial carcinoma patients who intend to preserve fertility, high-quality long-term follow-up and personalized treatment are necessary.
Plain Language Summary: In this case report, we share the story of one young woman who had endometrial cancer but desired to have children. She received fertility-sparing treatment and in vitro fertilization to increase her chances of conceiving. She successfully delivered a healthy child after the first embryo transfer. Two years later, she had another healthy child through a second frozen embryo transfer. Rigorous monitoring showed no cancer recurrence throughout the entire treatment. There are currently few reported cases of a patient with endometrial cancer successfully and safely giving birth twice through assisted reproductive technology. This case report emphasizes that, with personalized treatment and monitoring, endometrial cancer patients can have multiple pregnancies safely. In summary, this case report brings hope to young women with early-stage endometrial cancer who aspire to become mothers. With the right support, they can overcome the challenges of cancer and have their own babies.
Keywords: endometrial carcinoma, fertility preservation, pregnancy outcome, in vitro fertilization-embryo transfer
Introduction
Endometrial carcinoma (EC) is a malignancy that originates from the endometrium, ranking as the fifth most prevalent cancer in females.1 The standard treatment for EC is surgery, which includes total extra-fascial hysterectomy, bilateral salpingo-oophorectomy, and pelvic and para-aortic lymph node dissection.2 Patients with early-stage EC can usually achieve satisfactory survival outcomes after surgery. The 5-year survival rate for these patients is up to 96%.3 Approximately 14–25% of EC patients are premenopausal, and 4–7% of them are under 40 years of age.4,5 These patients often express a strong desire for fertility preservation. Fortunately, patients diagnosed with early-stage and well-differentiated EC usually have a favorable prognosis. However, for women of childbearing age who wish to preserve their fertility, this definitive approach may not be an optimal option.
Previous studies have explored the safety and feasibility of hormonal therapy to preserve fertility in early-stage EC women.6,7 Hormonal therapy includes medroxyprogesterone acetate (MPA), levonorgestrel-releasing intrauterine devices (LNG-IUDs) and more recently, gonadotropin-releasing hormone agonist (GnRH-a).8,9 Hysteroscopic pathological examination is recommended to determine the effectiveness of the medication or the progressing disease.10 While studies have revealed that patients achieving complete remission after fertility-preserving treatments could benefit from assisted reproductive technology (ART),11 the number of reports on consecutive live births through ART in EC patients is limited.
Here, we present a case report of a woman who underwent fertility preservation therapy due to EC and successfully achieved deliveries twice through in vitro fertilization and embryo transfer (IVF-ET). She received hormone therapy and regular endometrium evaluations, and no signs of recurrence was observed throughout the process. After two successful deliveries, she received the standard surgical treatment for EC, including total extra-fascial hysterectomy, bilateral salpingo-oophorectomy, and pelvic and para-aortic lymph node assessment. To our knowledge, there are few reported cases of patients with EC achieving consecutive two successful pregnancies through IVF-ET. The timeline of the entire treatment procedure is outlined in Figure 1.
 |
Figure 1 Timeline of the whole treatment procedure. |
Case Presentation
The Patient
A 27-year-old female patient was diagnosed with EC through biopsy in Beijing Obstetrics and Gynecology Hospital. Prior to hysteroscopy, the patient underwent magnetic resonance imaging (MRI) imaging, which revealed heterogeneous endometrial thickening with distinct demarcation between the endometrium and myometrium. Subsequently, hysteroscopy was arranged to take lesion tissue for pathology evaluation in April 2017. During hysteroscopy, an endometrial polyp of 4 mm in diameter was observed in the uterine cavity, along with uneven thickening of the endometrium and abnormally rich blood supply. Postoperative pathology revealed atypical endometrial hyperplasia with a portion of highly differentiated endometrioid carcinoma.
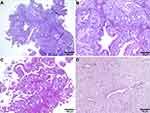
As the patient desired to preserve fertility, a six-month course of GnRH-a and MPA was administered after hysteroscopy. In October 2017, the patient underwent another hysteroscopy to evaluate the therapeutic effect. Postoperative pathology revealed that atypical endometrial hyperplasia was still present. The patient continued MPA administration for 11 months and underwent regular endometrial examinations. Hysteroscopy was performed in February 2018, May 2018, and September 2018 during the treatment. The final endometrial pathology revealed glandular atrophy and interstitial degeneration, which was consistent with the effect of drug treatment. (Shown in Figure 2)
Controlled Ovarian Stimulation
Before initiating the controlled ovarian stimulation cycle, the patient’s endometrial condition was evaluated by both gynecologic oncologists and reproductive physicians. She received a measurement of serum levels of hormones (follicle-stimulating hormone, progesterone, estradiol, luteinizing hormone). The patient’s ovarian reserve was evaluated by measuring the serum level of anti-Mullerian hormone (AMH), which was found to be 1.75 ng/mL, indicating normal ovarian reserve. A GnRH-a ultra-long protocol was selected for controlled ovarian stimulation. On the third day of the menstrual cycle, a single dose of 3.75 mg of the long-acting GnRH-a triptorelin (Ferring GmbH, Decapeptyl®, Germany) was administered. After 28 days, exogenous human menopausal gonadotropins (Livzon Group Pharmaceutical Factory, Zhuhai, Guangdong, China) was given at a dose of 225 IU per day for 10 days.
When serum levels of estradiol reached 644.58 pg/mL, and the ultrasound-measured follicular diameters were 2.15 cm, 1.7 cm, and 1.66 cm, a subcutaneous injection of recombinant human chorionic gonadotropin 250 μg (Merck Serono Inc., Geneva, Switzerland) was administered to trigger ovulation. Four oocytes were retrieved 36 hours after the trigger and all oocytes were fertilized.
Fresh/Frozen Embryo Transfer
Two embryos were transferred after transvaginal ultrasound-guided ovum pick-up, and the remaining two embryos were cryopreserved. A successful singleton pregnancy was confirmed 28–35 days after embryo transfer. The patient had a natural delivery with no complications. She declined surgical intervention due to a desire for another pregnancy. Thus, regular endometrial monitoring and the administration of MPA were arranged for her to prevent recurrence.
In May 2021, the patient returned to the department of human reproductive medicine and was administered with 3.75 mg of GnRH-a. Twenty-eight days after the GnRH-a administration, she underwent hormone replacement therapy (HRT) for endometrial preparation. When the endometrial thickness reached 8.5 mm, dydrogestrone (Abbott, Illinois, United States) 20 mg per day and progesterone sustained-release vaginal gel (Merck Serono Inc., Geneva, Switzerland) 90 mg per day were administered to transform the proliferative endometrium into secretory endometrium and luteal support. Following embryo transfer, a singleton pregnancy was confirmed by ultrasound. At 40 weeks of gestation, the patients eventually delivered a healthy infant, with a birth weight of 3546 g and Apgar score of 9-10-10.
Follow-Up Evaluation and Treatment
After five-month postpartum follow-up, the patient presented at the gynecological oncology department and finally chose the standard surgical intervention. In August 2022, a total extra-fascial hysterectomy, bilateral salpingectomy, ovarian biopsy, and pelvic and para-aortic lymph node assessment were performed after obtaining the patient’s consent and a thorough evaluation by the medical team. The postoperative pathology report indicated that the endometrium was in a proliferative phase, and no malignant cells were detected in the entire uterus, bilateral fallopian tubes, ovaries, pelvic lymph nodes, or peritoneal lavage fluid.
Discussion
With the increasing incidence of EC among young women, clinicians are now focusing on the development of fertility-preserving management strategies to help eligible patients achieve pregnancy after therapy. In terms of treatment, the standard approach for EC is total extra-fascial hysterectomy, bilateral salpingo-oophorectomy, and lymph node assessment of the pelvic and para-aortic regions to optimize survival.12 However, this modality of treatment may not be the optimal choice for young women with the desire to preserve their fertility. In line with this perspective, it is crucial to consider alternative, individualized treatment regimens and stringent monitoring for EC patients, especially those desiring fertility preservation.13 This case report indicated EC patients can safely achieve pregnancies for multiple times through individualized treatment regimens and rigorous monitoring.
Based on the patient’s cancer staging, progestin therapy was arranged as fertility preservation treatment. Progestin treatment has shown potential benefits for maintaining fertility in early-stage EC patients.14 GnRH-a has been explored as alternative to long-term oral administration of progesterone, either alone or in combination with MPA.15 GnRH-a exhibits a beneficial impact not only in the preservation of fertility in EC treatment but also in facilitating ovarian stimulation and embryo implantation during the later stages of the reproductive process. Endometrial sampling such as hysteroscopy every 3–6 months are mandatory during MPA administration.16,17 Patients should be informed of the potential hazards of conservative treatment, such as the risk of underestimating the stage of a more advanced cancer, the persistence or progression of the disease.
A crucial goal for couples undergoing fertility-sparing treatment is the achievement of a successful pregnancy. The limited time window between disease remission and recurrence makes IVF a favorable option for achieving pregnancy in the short term. IVF has been proven to be effective in reducing treatment duration for EC patients.18 Recent studies have demonstrated that women who undergoing IVF have better pregnancy outcomes than those who conceive naturally.19–21 These factors emphasize the importance of exploring IVF as a method for overcoming fertility obstacles in these patients. The introduction of IVF-ET immediately after complete remission would be beneficial for patients with a higher age of permission to be pregnant.22 In addition, IVF-ET does not increase the risk of recurrence.23,24 Nevertheless, evidence suggests that patients may choose IVF three months after achieving complete remission.25 In this case report, controlled ovulation stimulation was performed immediately on patients after complete remission to minimize the time window between disease remission and recurrence.
To reduce the possibility of relapse, most patients can consider hysterectomy after the birth of their first child. However, for patients seeking a second pregnancy, IVF-ET and frozen embryo transfer become a viable option if overall monitoring indicates no recurrence. Between two pregnancies, the patient underwent regular check-ups to monitor for any signs of recurrence and received hormone therapy. Recent studies show progestin treatment results in cancer regression rates ranging from 76% to 82%, with a relapse rate of approximately 25%.26 The temporary consideration of conservative measures is necessary to facilitate pregnancy, followed by specific consultations for surgery.27 Ensuring that the patient does not experience any recurrence during the follow-up period is an essential precondition for second pregnancies in EC patients.
This case report has several limitations. While it discusses current research and statistics, it does not address the potential economic and time costs associated with various medications or methods for fertility preservation. These considerations can be significant sources of concern for patients and healthcare systems.
Conclusion
In conclusion, the primary goal of treatment in EC patients who wish to preserve fertility is to balance the patient’s reproductive desires with effective cancer management. By providing tailored, comprehensive care and close monitoring, clinicians can help these patients achieve their fertility goals while also ensuring optimal outcomes in the prognosis of EC patients.
Abbreviations
IVF, in vitro fertilization; EC, endometrial carcinoma; MPA, medroxyprogesterone acetate; LNG-IUDs, levonorgestrel-releasing intrauterine devices; GnRH-a, gonadotropin-releasing hormone agonist; ART, assisted reproductive technologies; IVF-ET, in vitro fertilization and embryo transfer; MRI, magnetic resonance imaging; AMH, anti-Mullerian hormone; HRT, hormone replacement therapy.
Data Sharing Statement
Please contact correspondence author for data requests.
Ethics Approval and Consent to Participate
This case report was reviewed and approved by the Ethics Committee of the Beijing Obstetrics and Gynecology Hospital, Capital Medical University, 2018-KY-015-01. Written informed consent was obtained from the participants.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Author Contributions
JYW collected the patient data and wrote the first draft of the article. YF interpreted patient follow-up data and edited drafts. TC gathered materials and interpreted the patient data. YMW reviewed the patient’s surgery procedure, drafted figures, and reviewed the case report. XKY and ZMX critically reviewed and revised the manuscript. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This case report was supported by the Beijing Municipal Administration of Hospitals Clinical Medicine Development (grant number ZYLX201830) and Beijing Hospitals Authority’ Ascent Plan (grant number DFL20191401). The funders had no role in data collection, patient follow-up, or the preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Oaknin A, Bosse TJ, Creutzberg CL, et al. Endometrial cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(9):860–877. doi:10.1016/j.annonc.2022.05.009
2. Abu-Rustum N, Yashar C, Arend R, et al. Uterine neoplasms, version 1.2023, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2023;21(2):181–209.
3. Pergialiotis V, Zachariou E, Vlachos DE, et al. Tumor free distance from serosa and survival rates of endometrial cancer patients: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2023;286:16–22. doi:10.1016/j.ejogrb.2023.05.001
4. Son J, Carr C, Yao M, et al. Endometrial cancer in young women: prognostic factors and treatment outcomes in women aged ≤40 years. Int J Gynecol Cancer. 2020;30(5):631–639. doi:10.1136/ijgc-2019-001105
5. Bourou MZ, Matsas A, Vrekoussis T, Mastorakos G, Valsamakis G, Panoskaltsis T. Conservative treatment of endometrial cancer in women of reproductive age (Review). Mol Clin Oncol. 2023;19(1):55. doi:10.3892/mco.2023.2651
6. Fan Z, Li H, Hu R, Liu Y, Liu X, Gu L. Fertility-preserving treatment in young women with grade 1 presumed stage IA endometrial adenocarcinoma: a meta-analysis. Int J Gynecol Cancer. 2018;28(2):385–393. doi:10.1097/IGC.0000000000001164
7. Quick CM, May T, Horowitz NS, Nucci MR. Low-grade, low-stage endometrioid endometrial adenocarcinoma: a clinicopathologic analysis of 324 cases focusing on frequency and pattern of myoinvasion. Int J Gynecol Pathol. 2012;31(4):337–343. doi:10.1097/PGP.0b013e31823ff422
8. Concin N, Matias-Guiu X, Vergote I, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Radiother Oncol. 2021;154:327–353. doi:10.1016/j.radonc.2020.11.018
9. Rodolakis A, Biliatis I, Morice P, et al. European Society of Gynecological Oncology Task Force for Fertility Preservation: clinical recommendations for fertility-sparing management in young endometrial cancer patients. Int J Gynecol Cancer. 2015;25(7):1258–1265. doi:10.1097/IGC.0000000000000493
10. Laganà AS, La Rosa VL, Rapisarda AM, Vitale SG. Comment on: “Needs and priorities of women with endometrial and cervical cancer”. J Psychosom Obstet Gynaecol. 2017;38(1):85–86. doi:10.1080/0167482X.2016.1244186
11. Park JY, Nam JH. Progestins in the fertility-sparing treatment and retreatment of patients with primary and recurrent endometrial cancer. Oncologist. 2015;20(3):270–278. doi:10.1634/theoncologist.2013-0445
12. Fukui Y, Taguchi A, Adachi K, et al. Polycystic ovarian morphology may be a positive prognostic factor in patients with endometrial cancer who achieved complete remission after fertility-sparing therapy with progestin. Asian Pac J Cancer Prev. 2017;18(11):3111–3116. doi:10.22034/APJCP.2017.18.11.3111
13. Boardman L, Novetsky AP, Valea F. Management of endometrial intraepithelial neoplasia or atypical endometrial hyperplasia: ACOG Clinical Consensus No. 5. Obstet Gynecol. 2023;142(3):735–744. doi:10.1097/AOG.0000000000005297
14. Terzic M, Norton M, Terzic S, Bapayeva G, Aimagambetova G. Fertility preservation in endometrial cancer patients: options, challenges and perspectives. Ecancermedicalscience. 2020;14:
15. Wang Y, Yang JX. Fertility-preserving treatment in women with early endometrial cancer: the Chinese experience. Cancer Manag Res. 2018;10:6803–6813. doi:10.2147/CMAR.S188087
16. Giampaolino P, Cafasso V, Boccia D, et al. Fertility-sparing approach in patients with endometrioid endometrial cancer grade 2 Stage IA (FIGO): a qualitative systematic review. Biomed Res Int. 2022;2022:4070368. doi:10.1155/2022/4070368
17. Wang F, Yu A, Xu H, et al. Fertility preserved hysteroscopic approach for the treatment of stage IA endometrioid carcinoma. Int J Gynecol Cancer. 2017;27(9):1919–1925. doi:10.1097/IGC.0000000000001109
18. Yuan F, Hu Y, Han X, Li Q, Hashmi MF. Metformin in combination with progesterone improves the pregnancy rate for patients with early endometrial cancer. Contrast Media Mol Imaging. 2022;2022:1961016. doi:10.1155/2022/1961016
19. Tai W, Hu L, Wen J. Maternal and neonatal outcomes after assisted reproductive technology: a retrospective cohort study in China. Front Med. 2022;9:837762. doi:10.3389/fmed.2022.837762
20. Gallos ID, Yap J, Rajkhowa M, Luesley DM, Coomarasamy A, Gupta JK. Regression, relapse, and live birth rates with fertility-sparing therapy for endometrial cancer and atypical complex endometrial hyperplasia: a systematic review and metaanalysis. Am J Obstet Gynecol. 2012;207(4):266.e1–266.12. doi:10.1016/j.ajog.2012.08.011
21. Park J-Y, Seong SJ, Kim T-J, et al. Pregnancy outcomes after fertility-sparing management in young women with early endometrial cancer. Obstet Gynecol. 2013;121(1):136–142. doi:10.1097/AOG.0b013e31827a0643
22. Inoue O, Hamatani T, Susumu N, et al. Factors affecting pregnancy outcomes in young women treated with fertility-preserving therapy for well-differentiated endometrial cancer or atypical endometrial hyperplasia. Reprod Biol Endocrinol. 2016;14(1):2. doi:10.1186/s12958-015-0136-7
23. Siristatidis C, Sergentanis TN, Kanavidis P, et al. Controlled ovarian hyperstimulation for IVF: impact on ovarian, endometrial and cervical cancer--a systematic review and meta-analysis. Hum Reprod Update. 2013;19(2):105–123. doi:10.1093/humupd/dms051
24. Vaugon M, Peigné M, Phelippeau J, Gonthier C, Koskas M. IVF impact on the risk of recurrence of endometrial adenocarcinoma after fertility-sparing management. Reprod Biomed Online. 2021;43(3):495–502. doi:10.1016/j.rbmo.2021.06.007
25. Song Z, Liu H, Zhou R, et al. The optimal time for the initiation of in vitro fertilization and embryo transfer among women with atypical endometrial hyperplasia and endometrial carcinoma receiving fertility-sparing treatment. Arch Gynecol Obstet. 2022;305(5):1215–1223. doi:10.1007/s00404-021-06320-3
26. Qin Y, Yu Z, Yang J, et al. Oral progestin treatment for early-stage endometrial cancer: a systematic review and meta-analysis. Int J Gynecol Cancer. 2016;26(6):1081–1091. doi:10.1097/IGC.0000000000000723
27. Ronsini C, Mosca L, Iavarone I, et al. Oncological outcomes in fertility-sparing treatment in stage IA-G2 endometrial cancer. Front Oncol. 2022;12:965029. doi:10.3389/fonc.2022.965029
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.