Back to Journals » Journal of Asthma and Allergy » Volume 17
A Case of Type I Food Allergy Induced by Monosodium Glutamate
Authors Osada R, Oshikata C, Kurihara Y , Terada K, Kodama Y, Yamashita Y, Nakadegawa R, Masumitsu H, Motobayashi Y, Takayasu H, Masumoto N, Manabe S, Zhu Y , Tanaka R, Kaneko T, Sasaki A , Tsurikisawa N
Received 8 December 2023
Accepted for publication 20 February 2024
Published 7 March 2024 Volume 2024:17 Pages 161—165
DOI https://doi.org/10.2147/JAA.S451911
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Reeko Osada,1 Chiyako Oshikata,1– 3 Yuichi Kurihara,4 Kosuke Terada,1 Yuka Kodama,1 Yuga Yamashita,1 Ryo Nakadegawa,1 Hinako Masumitsu,1 Yuto Motobayashi,1 Hirokazu Takayasu,1 Nami Masumoto,1,3 Saki Manabe,1 Yingyao Zhu,4 Ryo Tanaka,4 Takeshi Kaneko,3 Aya Sasaki,5 Naomi Tsurikisawa1– 3
1Department of Respirology, National Hospital Organization Yokohama Medical Center, Yokohama, Japan; 2Department of Allergy and Respirology, Hiratsuka City Hospital, Hiratsuka, Japan; 3Department of Pulmonology, Yokohama City University Graduate School of Medicine, Yokohama, Japan; 4Department of Dermatology, Hiratsuka City Hospital, Hiratsuka, Japan; 5Department of Clinical Laboratory, Tokyo Dental College Ichikawa General Hospital, Ichikawa, Japan
Correspondence: Naomi Tsurikisawa, Department of Respirology, National Hospital Organization Yokohama Medical Center, 3-60-2 Harajuku, Totsuka-ku, Yokohama, 245-8575, Japan, Tel +81-45-851-2621, Fax +81-45-851-3902, Email [email protected]
Abstract: Monosodium glutamate (MSG), a salt form of a non-essential amino acid, is widely used as a food additive, particularly in Asian cuisines, due to its unique flavor-enhancing qualities. Type I allergic reactions to MSG have not previously been reported. Our patient, a 21-year-old woman, was 14 years old when she first noticed swelling of her tongue (but no oral itching, diarrhea, or abdominal pain) after eating various snack foods. Current skin prick testing elicited a weak positive reaction to MSG. We then performed an oral challenge test during which our patient ingested potato snacks. Subsequent histology showed telangiectasia of the buccal mucosa, interstitial edema in the subepithelial submucosa, and mast cell infiltration. Oral mucosal challenge tests using sodium glutamate confirmed oral swelling in this patient. This report is the first to confirm a case of type 1 allergy to MSG by combining pathology findings with the results of challenge testing.
Keywords: food additive, mast cell, monosodium glutamate, food allergy
Introduction
Monosodium glutamate (MSG), a salt form of a non-essential amino acid, is widely used as a food additive, particularly in Asian cuisines, due to its unique flavor-enhancing qualities. First reported in 1968 as Chinese restaurant syndrome,1 some people have described symptoms including a burning sensation at the back of the neck and on the forearms and chest; headache; chest pain; numbness at the back of the neck and radiating to the arms and back; nausea; and palpitations after consuming MSG. Using results from an evaluation conducted by the American Federation of Experimental Biology Societies, the United States Food and Drug Administration reaffirmed the safety of MSG as a food additive in 1995.2
MSG is intentionally added to foods as a flavoring agent. One of its components, glutamic acid is found naturally in almost all foods, including vegetables, meat, fish, and breast milk. In general, protein-rich foods such as breast milk and meat contain large amounts of protein-bound glutamate, whereas vegetables (particularly peas, tomatoes, and potatoes), fruits, and mushrooms contain high concentrations of free glutamate. In addition, glutamate is particularly abundant in some cheeses, including parmesan.2
The symptoms complex due to MSG consumption is considered to begin within one hour of ingestion of more than 3 g of MSG as an oral bolus. Typically, a dose of MSG of 2.5 g or less causes no symptoms in nonallergic people; larger doses can cause a burning sensation, facial pressure, headache, and drowsiness, but these symptoms usually dissipate within 4 h.3 However, the information was not communicated accurately overseas, where the use of MSG remained restricted. Descriptions of MSG-induced asthma, urticaria, angioedema, and rhinitis have prompted suggestions that MSG causes an allergic reaction in patients presenting with these conditions. However, none of the reports of MSG-induced asthma have proven reproducible, and MSG-induced asthma was not replicated in a double-blind placebo-controlled study.4 Our current report is the first to confirm a case of type 1 allergy to MSG by combining pathology findings with the results of challenge testing.
Case
The 21-year-old woman whose case we present was diagnosed with allergic rhinoconjunctivitis when she was 17. Beginning when she was 14 years old, she noticed that her tongue swelled after eating various snack foods but had no oral itching, diarrhea, or abdominal pain. The frequency of swelling of the tongue increased once she turned 20. Swelling of the tongue did not occur after eating either homemade meals without additives, items from conveyor-belt sushi restaurants, or at any of the meals in restaurants during a trip to Europe. In contrast, her tongue swelled after she ate at a chain family restaurant and after having fast-food hamburgers, French fries, potato snacks, parmesan cheese, and consommé-flavored (but not light-salt) potato chips. In addition, she did not develop any adverse symptoms after ingesting potatoes prepared otherwise than mentioned or after eating any type of fruit.
We performed a skin prick test and measured antigen-specific serum immunoglobulin E (IgE) levels. Total IgE (IU/mL) and antigen-specific IgE (IU/mL) in serum were measured by using enzyme-linked immunosorbent assay (ELISA) according to the nephelometry method (BN II, Dade Behring Inc, Deerfield, IL).5 Our patient’s total serum IgE level was 36.0 IU/mL (normal, ≤173 IU/mL), with antigen-specific serum IgE levels of 0.15 IU/mL each for house dust and Japanese cedar pollen (positive reaction defined as specific IgE > 0.35 IU/mL) but undetectable for wheat, potato, and pollens other than cedar. We then measured wheals and flares at 15 min after skin pricking with these antigens;6 we defined a positive allergen reaction as eliciting a mean wheal diameter ≥3 mm larger than that produced by the negative control (saline)7,8 or at least 50% larger than that due to the positive control (histamine).9 We obtained sodium glutamate as MSG (Ajinomoto, Ajinomoto Co., Inc, Tokyo, Japan). Our patient had a positive reaction to 100 mg/mL of MSG (wheal size, 4×4 mm; saline, 0×0 mm; histamine, 6×5 mm) but negative reactions to 10 mg and 1 mg/mL of MSG, potato, wheat, gluten, and omega-5-gliadin. We confirmed the lack of nonspecific reaction (0 × 0 mm) to 100 mg/mL of MSG on the skin of six people without MSG sensitivity.
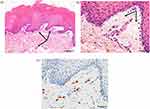
After obtaining written informed consent from our patient, we then performed an oral challenge test during which she ingested potato snacks that contained MSG. In the oral challenge test, this patient continued to consume snack foods until oral symptoms appeared. After ingesting 4 sticks (1.5 g each), she experienced only a tingling tongue; after 23 sticks (ie, about 20 min after starting to eat), she had a sore throat, painful tongue, itchy palate, and buccal mucosal swelling and bleeding. Because of severe pain, she could not ingest any more than 31 sticks; at this point (ie, about 30 min after she began eating), we obtained a biopsy of the buccal mucosa. Routine histology showed telangiectasia of the buccal mucosa (Figure 1a) and interstitial edema in the subepithelial submucosa (Figure 1b). In addition, c-kit immunohistochemistry revealed mast cell infiltration (Figure 1c). Given these findings, we diagnosed a type I allergic reaction in our patient and confirmed that her oral bleeding was from biting her tongue, which became edematous due to her allergic reaction.
To further characterize our patient’s food allergy, we performed oral mucosal challenge tests using common components of snack foods, specifically powdered fats and oils, salt containing umami seasoning, and a commercial product that is 97.5% sodium glutamate (Ajinomoto), with saline as a control. We performed a single-blind oral mucosal challenge test using saline and confirmed a negative reaction. Powdered fats and oils did not elicit any adverse responses, but both the umami-seasoned salt and commercial 100 mg/mL MSG product caused pain in the oral cavity, swelling of the tongue, and facial erythema (flushing), with the MSG product causing stronger reactions. We used the dose of 100 mg/mL MSG because it yielded a reaction in the skin-prick test, whereas 10 and 1 mg/mL did not. We therefore further characterized our patient’s food allergy as a type 1 allergic reaction to MSG.
Many foods contain MSG, and it is difficult to avoid consuming it when eating out. Our patient learned to be careful not to bite her tongue when she experiences oral discomfort during meals. Since her diagnosis of MSG allergy, our patient has not had any more episodes of oral bleeding, and her quality of life has improved.
Discussion
First categorized by Gell and Coombs, hypersensitivity reactions are now classified according to nine types: antibody-mediated (types I–III), cell-mediated (IVa–c), tissue-driven mechanisms (V, VI), and direct response to chemicals (VII).10 Type I (IgE-mediated) reactions occur in patients with allergic rhinitis, conjunctivitis, asthma, atopic dermatitis, pollen-induced food and drug allergies and allergies to house dust mites and other animals, various foods, materials (eg, latex), and drugs.10 The allergen-specific IgE in type I reactions is produced by mast cells and basophils. Although our patient had considerable numbers of mast cells in the subepithelial submucosa, skin-prick testing elicited only a token IgE reaction. Because food allergies also manifest through non-IgE-dependent mechanisms,11 we consider that allergy to food additives may develop through a type I allergy mechanism with low IgE reactivity. In the case we present, our patient experienced no allergic symptoms after ingesting MSG-containing snacks before the age of 13, and she developed hay fever when she was 17 years old; these features suggest that her sensitization to MSG was acquired after provocation. This congenital sensitization likely explains her low total IgE level and modest responses to skin prick tests.
Reported allergic reactions to MSG include urticaria, angioedema, allergic rhinitis, bronchial asthma, and exercise-induced anaphylaxis, but these reports are associated with discrepancies in the time between MSG intake and appearance of the allergic reaction and a lack of reproducibility in provocation tests using a placebo.4,12 The evidence to support MSG as a cause of urticaria or angioedema was insufficient, because reported cases have been limited by inadequate blinding, small sample sizes, and the potentially confounding withdrawal of antihistamines before MSG challenges. The possibility that MSG may induce acute rhinitis symptoms and contribute to chronic rhinitis has been raised. Future studies addressing a possible relationship between the ingestion of MSG and the development of rhinitis will need to differentiate MSG allergic rhinitis from incidentally occurring chronic rhinitis.
In regard to diagnosing food allergy, a food provocation test under double-blind placebo-controlled conditions is considered the gold standard.13 We instead opted to perform a single-blind placebo-controlled challenge provocation test because we anticipated that our patient would experience oral swelling and severe pain after ingesting food containing MSG. Because we had previously confirmed that the placebo test using saline was negative, we decided that a single-blind design was our best option in terms of patient safety during the provocation test. In addition, we quickly responded to and treated the severe allergic reaction that occurred during the provocation test.
The actual dose of MSG in the snack food that caused our patient’s symptoms was unknown, so she continued to eat the snack food during the provocation test until symptoms actually appeared. The dose of 100 mg/mL MSG used in the oral challenge test was determined according to the prick test threshold, but we consider that this dose exceeds that when MSG is used as an actual seasoning. Our patient ingested the MSG-containing food for 20 to 30 min before symptoms began to appear. We therefore consider it likely that the symptoms caused by a 3-g oral bolus differ from those due to consumption of commercially available foods.
Anaphylaxis due to poly-γ-glutamic acid reportedly develops relatively late (ie, 5 to 14 h) after the ingestion of fermented soybeans.14 Although natto and MSG have similar structural formulas, their sensitization pathways seem to differ.15 We speculate that, given the widespread use of this food additive, MSG allergy may be more prevalent than appreciated, with many cases unreported. In the case we report here, the skin prick test for MSG was positive, challenge testing led to the appearance of mast cells in our patient’s oral submucosa, and the reproducibility of her symptoms supported the diagnosis of a type I allergy to MSG. In addition, this report is the first to describe the use of pathology findings with the results of challenge testing to confirm MSG type 1 allergy.
Approval
Institutional approval of the manuscript was not required to publish the case details.
Conclusion
We here present a patient who showed oral allergy induced through the ingestion of MSG. This report is the first to confirm a type 1 allergy to MSG by combining pathology findings with the results of challenge testing.
Consent for Publication
Written informed consent was obtained from this patient.
Funding
The work was not funded by a grant or any other external source of financial support.
Disclosure
No author has any conflict of interest to disclose for this work.
References
1. Kwok RHM. Chinese-restaurant syndrome. N Engl J Med. 1968;278:796.
2. Raiten DJ, Talbot JM, Fisher KD. Analysis of adverse reactions to monosodium glutamate (MSG). Federation of American Societies for Experimental Biology Publisher, American Institute of Nutrition; 1995;p1–119.
3. Geha RS, Beiser A, Ren C, et al. Multicenter, double-blind, placebo-controlled, multiple-challenge evaluation of reported reactions to monosodium glutamate. J Allergy Clin Immunol. 2000;106:973–980. doi:10.1067/mai.2000.110794
4. Williams AN, Woessner KM. Monosodium glutamate ‘allergy’: menace or myth? Clin Exp Allergy. 2009;39:640–646. doi:10.1111/j.1365-2222.2009.03221.x
5. Hamilton RG, Williams PB. Specific IgE testing task force of the American Academy of Allergy, Asthma & Immunology; American college of Allergy, Asthma and Immunology: allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100:S1–S148.
6. Aas K, Backman A, Belin L, Weeke B. Standardization of allergen extracts with appropriate methods. The combined use of skin prick testing and radio-allergosorbent tests. Allergy. 1978;33:130–137. doi:10.1111/j.1398-9995.1978.tb01522.x
7. Adinoff AD, Rosloniec DM, McCall LL, Nelson HS. Immediate skin test reactivity to Food and Drug Administration-approved standardized extracts. J Allergy Clin Immunol. 1990;86:766–774. doi:10.1016/S0091-6749(05)80181-2
8. Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107:891–896. doi:10.1067/mai.2001.114708
9. Malling HJ. Skin prick testing and the use of histamine references. Allergy. 1984;39:596–601. doi:10.1111/j.1398-9995.1984.tb01979.x
10. Jutel M, Agache I, Zemelka-Wiacek M, et al. Nomenclature of allergic diseases and hypersensitivity reactions: adapted to modern needs: an EAACI position paper. Allergy. 2023;78:2851–2874. doi:10.1111/all.15889
11. Cianferoni A. Non-IgE mediated food allergy. Curr Pediatr Rev. 2020;16:95–105. doi:10.2174/1573396315666191031103714
12. Trent JS, Tassin S. A case of possible monosodium glutamate-dependent, exercise-induced anaphylaxis. Cureus. 2019;11:e5345.
13. Olivier CE. Food Allergy. J Allergy Therapy. 2013;S3(004):1–7.
14. Inomata N, Osuna H, Kawano K, et al. Late-onset anaphylaxis after ingestion of Bacillus Subtilis-fermented soybeans (Natto): clinical review of 7 patients. Allergol Int. 2007;56:257–261. doi:10.2332/allergolint.O-06-460
15. Inomata N, Chin K, Nagashima M, Ikezawa Z. Late-onset anaphylaxis due to poly (γ-glutamic acid) in the soup of commercial cold Chinese noodles in a patient with allergy to fermented soybeans (natto). Allergol Int. 2011;60:393–396. doi:10.2332/allergolint.10-CR-0267
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.